Effects of Side-Chain Engineering with the S Atom in Thieno[3,2-b]thiophene-porphyrin to Obtain Small-Molecule Donor Materials for Organic Solar Cells
Abstract
:1. Introduction
2. Results and Discussions
2.1. Optical Absorption Properties of Solutions and Films
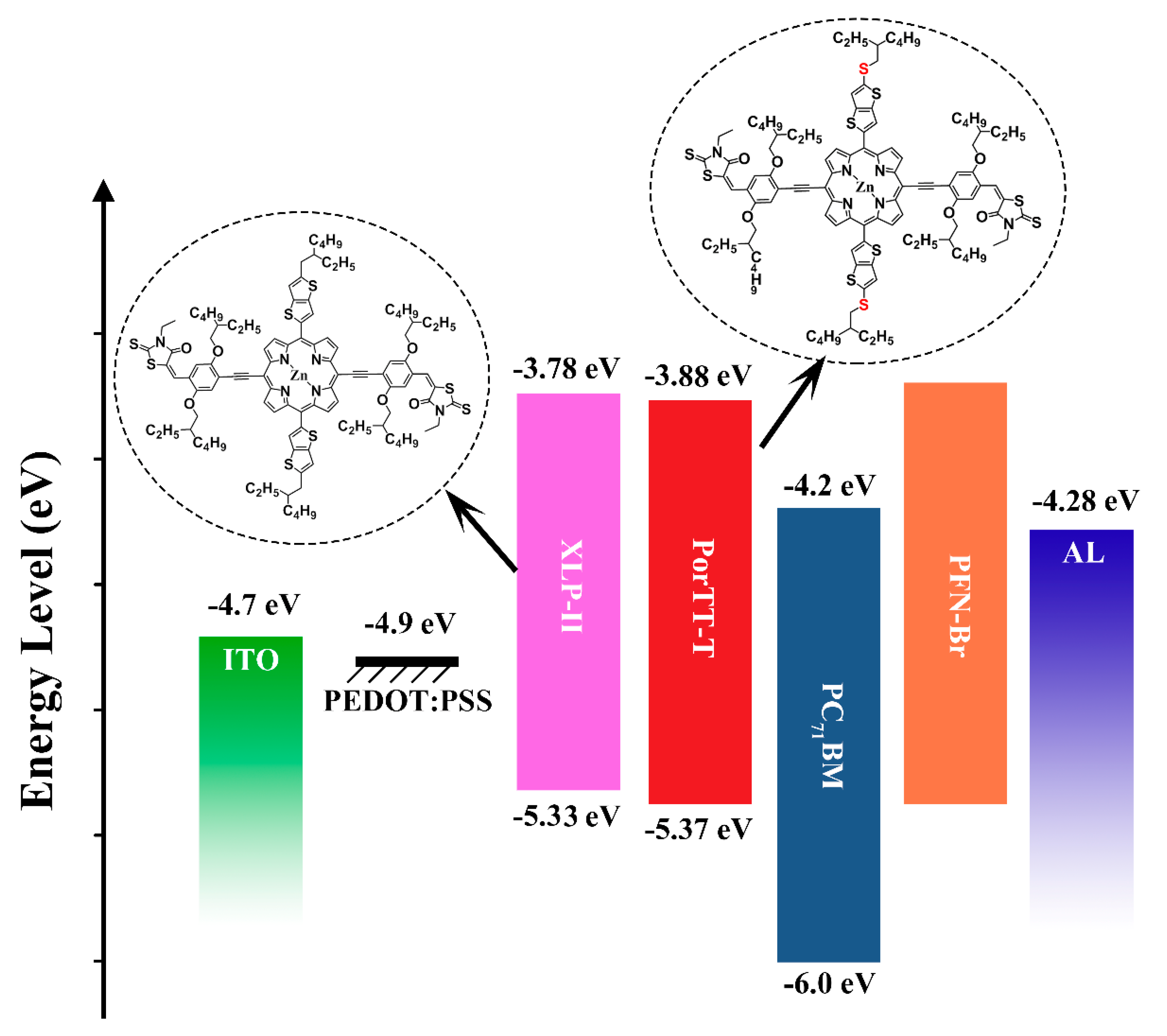
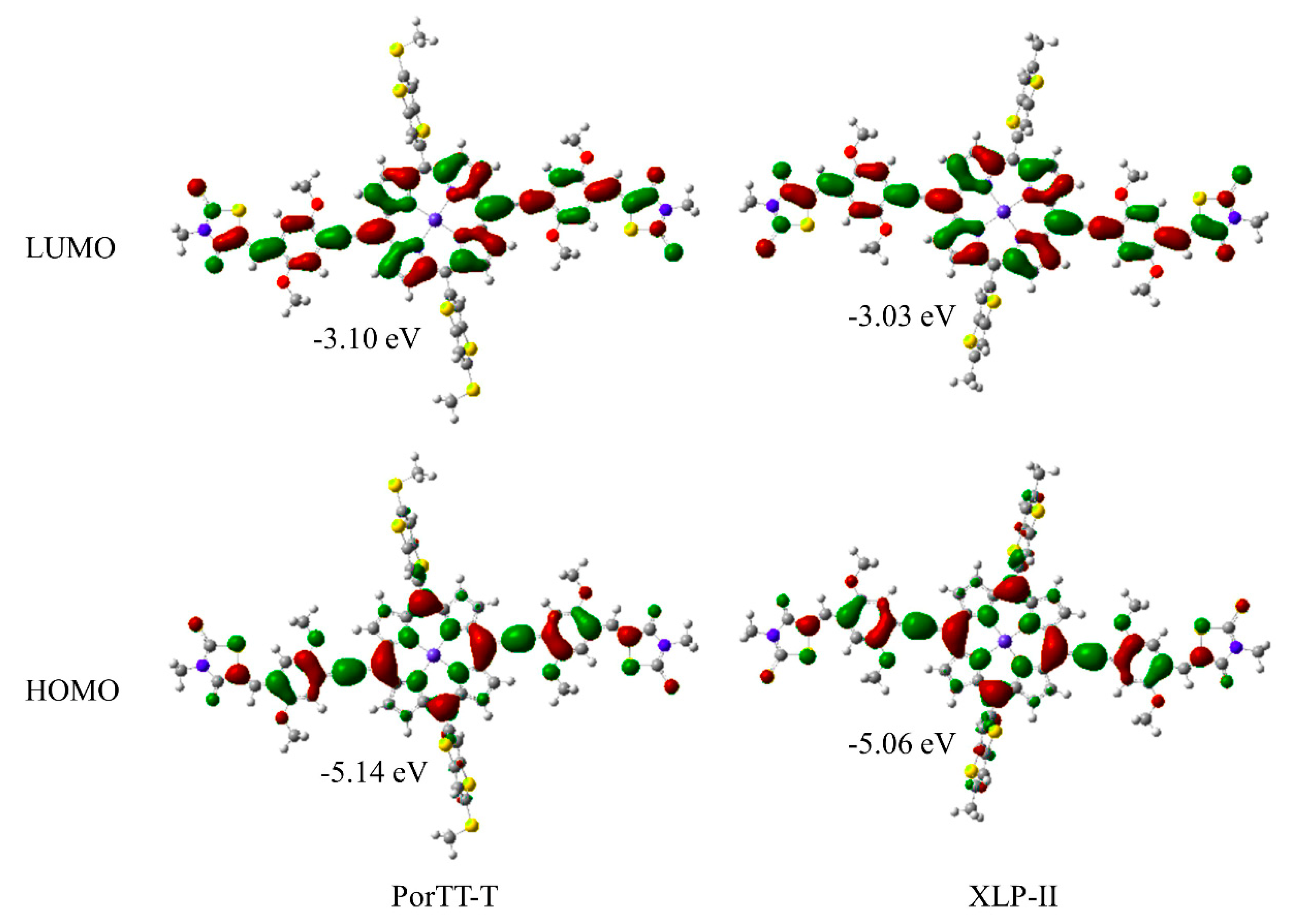
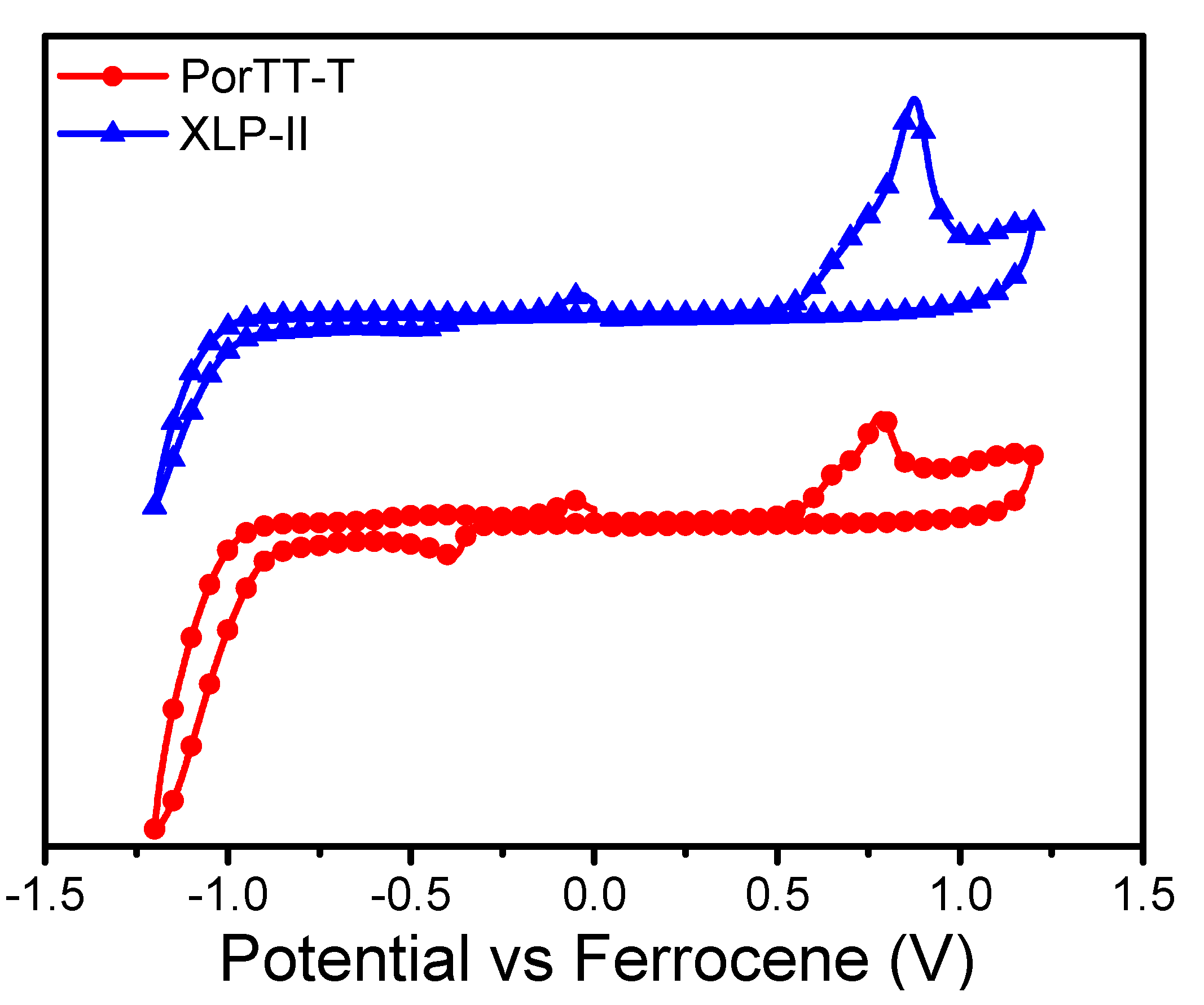
2.2. DFT Calculations and Electrochemical Properties
2.3. OSC Performance
3. Material and Method
3.1. Synthesis
3.2. Characterizations
3.3. Device Fabrication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cheng, Z.; Fang, W.; Zhao, T.; Fang, S.; Bi, J.; Liang, S.; Li, L.; Yu, Y.; Wu, L. Efficient visible-light-driven photocatalytic hydrogen evolution on phosphorus-doped covalent triazine-based frameworks. ACS Appl. Mater. Interfaces 2018, 10, 41415–41421. [Google Scholar] [CrossRef]
- Ma, X.; Wang, J.; An, Q.; Gao, J.; Hu, Z.; Xu, C.; Zhang, X.; Liu, Z.; Zhang, F. Highly efficient quaternary organic photovoltaics by optimizing photogenerated exciton distribution and active layer morphology. Nano Energy 2020, 70, 104496. [Google Scholar] [CrossRef]
- Wadsworth, A.; Hamid, Z.; Kosco, J.; Gasparini, N.; McCulloch, I. The bulk heterojunction in organic photovoltaic, photodetector, and photocatalytic applications. Adv. Mater. 2020, 32, 2001763. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Sun, R.; Zhong, C.; Liu, T.; Zhang, G.; Zou, Y.; Jiao, X.; Min, J.; Yang, C. Altering alkyl-chains branching positions for boosting the performance of small-molecule acceptors for highly efficient nonfullerene organic solar cells. Sci. China Chem. 2020, 63, 361–369. [Google Scholar] [CrossRef]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photonics 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Huang, P.; Liu, Y.; Zhang, K.; Yuan, L.; Li, D.; Hou, G.; Dong, B.; Zhou, Y.; Song, B.; Li, Y. Catechol derivatives as dopants in PEDOT: PSS to improve the performance of p–i–n perovskite solar cells. J. Mater. Chem. A 2017, 5, 24275–24281. [Google Scholar] [CrossRef]
- Yao, H.; Chen, Y.; Qin, Y.; Yu, R.; Cui, Y.; Yang, B.; Li, S.; Zhang, K.; Hou, J. Design and synthesis of a low bandgap small molecule acceptor for efficient polymer solar cells. Adv. Mater. 2016, 28, 8283–8287. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Tu, B.; Gao, K.; Liang, T.; Zhu, X.; Liu, B.; Duan, W.; Peng, X.; Fang, Q.; Geng, Y.; et al. Unravelling the self-assembly of diketopyrrolopyrrole-based photovoltaic molecules. Langmuir 2018, 34, 11952–11959. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, F.; He, Q.; Huo, L.; Wu, Y.; Parker, T.C.; Ma, W.; Sun, Y.; Wang, C.; Zhu, D.; et al. High-performance electron acceptor with thienyl side chains for organic photovoltaics. J. Am. Chem. Soc. 2016, 138, 4955–4961. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Ding, Z.; Miao, J.; Wang, J.; Dou, C.; Meng, B.; Liu, J.; Wang, L. Small molecular donor/polymer acceptor type organic solar cells: Effect of molecular weight on active layer morphology. Macromolecules 2019, 52, 8682–8689. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, Z.; Miao, J.; Xin, J.; Ma, W.; Dou, C.; Liu, J.; Wang, L. Morphology of small molecular donor/polymer acceptor blends in organic solar cells: Effect of the π–π stacking capability of the small molecular donors. J. Mater. Chem. C 2019, 7, 10521–10529. [Google Scholar] [CrossRef]
- Holliday, S.; Ashraf, R.S.; Wadsworth, A.; Baran, D.; Yousaf, S.A.; Nielsen, C.B.; Tan, C.H.; Dimitrov, S.D.; Shang, Z.; Gasparini, N.; et al. High-efficiency and air-stable P3HT-based polymer solar cells with a new non-fullerene acceptor. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Wu, Y.; Yang, H.; Dong, Y.; Cui, C.; Li, Y. The effect of alkylthio side chains in oligothiophene-based donor materials for organic solar cells. Mol. Syst. Des. Eng. 2018, 3, 131–141. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Kan, B.; Zhang, Q.; Li, M.; Wan, X.; Ni, W.; Long, G.; Wang, Y.; Yang, X.; Feng, H.; Chen, Y. Solution-processed organic solar cells based on dialkylthiol-substituted benzodithiophene unit with efficiency near 10%. J. Am. Chem. Soc. 2014, 136, 15529–15532. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Kan, B.; Wan, X.; Liu, F.; Ni, W.; Feng, H.; Russell, T.P.; Chen, Y. A solution-processed high performance organic solar cell using a small molecule with the thieno[3,2-b]thiophene central unit. Chem. Commun. 2015, 51, 15268–15271. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, Y.; Zhang, J.; Wang, Z.; Zhu, L.; Fang, J.; Xia, B.; Wang, Z.; Lu, K.; Ma, W.; et al. Fluorination-enabled optimal morphology leads to over 11% efficiency for inverted small-molecule organic solar cells. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wan, X.; Long, G. High performance photovoltaic applications using solution-processed small molecules. Acc. Chem. Res. 2013, 46, 2645–2655. [Google Scholar] [CrossRef]
- Gupta, V.; Lai, L.F.; Datt, R.; Chand, S.; Heeger, A.J.; Bazan, G.C.; Singh, S.P. Dithienogermole-based solution-processed molecular solar cells with efficiency over 9%. Chem. Commun. 2016, 52, 8596–8599. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Hsu, B.B.; Lorbach, A.; Qi, L.; Heeger, A.J.; Baza, G.C. Design and properties of intermediate-sized narrow band-gap conjugated molecules relevant to solution-processed organic solar cells. J. Am. Chem. Soc. 2014, 136, 5697–5708. [Google Scholar] [CrossRef]
- Wang, J.-L.; Liu, K.-K.; Yan, J.; Wu, Z.; Liu, F.; Xiao, F.; Chang, Z.; Wu, H.; Cao, Y.; Russell, T.P. Series of multifluorine substituted oligomers for organic solar cells with efficiency over 9% and fill factor of 0.77 by combination thermal and solvent vapor annealing. J. Am. Chem. Soc. 2016, 138, 7687–7697. [Google Scholar] [CrossRef]
- Gao, K.; Miao, J.; Xiao, L.; Deng, W.; Kan, Y.; Liang, T.; Wang, C.; Huang, F.; Peng, J.; Cao, Y.; et al. Multi-Length-Scale Morphologies Driven by Mixed Additives in Porphyrin-Based Organic Photovoltaics. Adv. Mater. 2016, 28, 4727–4733. [Google Scholar] [CrossRef]
- Caliò, L.; Follana-Berná, J.; Kazim, S.; Madsen, M.; Rubahn, H.-G.; Sastre-Santos, Á.; Ahmad, S. Cu (ii) and Zn (ii) based phthalocyanines as hole selective layers for perovskite solar cells. Sustain. Energy Fuels 2017, 1, 2071–2077. [Google Scholar] [CrossRef]
- Tanaka, T.; Osuka, A. Chemistry of meso-aryl-substituted expanded porphyrins: Aromaticity and molecular twist. Chem. Rev. 2017, 117, 2584–2640. [Google Scholar] [CrossRef]
- Kesters, J.; Verstappen, P.; Kelchtermans, M.; Lutsen, L.; Vanderzande, D.; Maes, W. Porphyrin-based bulk heterojunction organic photovoltaics: The rise of the colors of life. Adv. Energy Mater. 2015, 5, 1500218. [Google Scholar] [CrossRef]
- Zhou, X.; Tang, W.; Bi, P.; Yan, L.; Wang, X.; Wong, W.-K.; Hao, X.; Ong, B.S.; Zhu, X. Chemically driven supramolecular self-assembly of porphyrin donors for high-performance organic solar cells. J. Mater. Chem. A 2018, 6, 14675–14680. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Tsang, S.-W.; Du, X.; Zhou, J.; Tao, Y.; Ding, J. Alkyl side chain impact on the charge transport and photovoltaic properties of benzodithiophene and diketopyrrolopyrrole-based copolymers. J. Phys. Chem. C 2011, 115, 18002–18009. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Fan, H.; Liu, Y.; Hu, W.; Li, Y.; Zhan, X. A copolymer of benzodithiophene with TIPS side chains for enhanced photovoltaic performance. Macromolecules 2011, 44, 9173–9179. [Google Scholar] [CrossRef]
- Yasuda, T.; Sakamoto, K. Influence of the alkyl chain lengths in perylenetetracarboxylic diimide (PTCDI) derivatives on the photovoltaic properties of planar organic solar cells. Org. Electron. 2018, 62, 429–433. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, X.; Liu, Y.; Zuo, Y.; Li, Z.; He, G.; Long, G.; Ni, W.; Li, C.; Su, X.; et al. Small molecules based on benzo [1, 2-b: 4, 5-b′] dithiophene unit for high-performance solution-processed organic solar cells. J. Am. Chem. Soc. 2012, 134, 16345–16351. [Google Scholar] [CrossRef] [PubMed]
- Kan, B.; Zhang, Q.; Wan, X.; Ke, X.; Wang, Y.; Feng, H.; Zhang, M.; Chen, Y. Oligothiophene-based small molecules with 3,3′-difluoro-2,2′-bithiophene central unit for solution-processed organic solar cells. Org. Electron. 2016, 38, 172–179. [Google Scholar] [CrossRef]
- Ni, W.; Li, M.; Wan, X.; Zuo, Y.; Kan, B.; Feng, H.; Zhang, Q.; Chen, Y. A new oligobenzodithiophene end-capped with 3-ethyl-rhodanine groups for organic solar cells with high open-circuit voltage. Sci. China Chem. 2015, 58, 339–346. [Google Scholar] [CrossRef]
- Xie, L.; Tang, W.; He, X.; Liu, Z.; Zhai, F.; Yuan, Z.; Zhou, X.; Yan, L.; Wang, X. Structure influence of alkyl chains of thienothiophene-porphyrins on the performance of organic solar cells. J. Mater. Chem. C 2021, revised. [Google Scholar]
- Hadmojo, W.T.; Lee, U.-H.; Yim, D.; Kim, H.W.; Jang, W.-D.; Yoon, S.C.; Jung, I.H.; Jang, S.Y. High-performance near-infrared absorbing n-type porphyrin acceptor for organic solar cells. ACS Appl. Mater. Interfaces 2018, 10, 41344–41349. [Google Scholar] [CrossRef]
- Bin, H.; Angunawela, I.; Qiu, B.; Colberts, F.J.; Li, M.; Dyson, M.J.; Wienk, M.M.; Ade, H.; Li, Y.; Janssen, R.A.J. Precise control of phase separation enables 12% efficiency in all small molecule solar cells. Adv. Energy Mater. 2020, 10, 2001589. [Google Scholar] [CrossRef]
- Dai, S.; Li, T.; Wang, W.; Xiao, Y.; Lau, T.K.; Li, Z.; Liu, K.; Lu, X.; Zhan, X. Enhancing the performance of polymer solar cells via core engineering of NIR-absorbing electron acceptors. Adv. Mater. 2018, 30, 1706571. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, W.; Yin, H.; Wang, Z.; Zheng, K.; Xie, L.; Wang, X.; So, S.K.; Liu, F.; Zhu, X. Tuning electronic properties of molecular acceptor-π-porphyrin-π-acceptor donors via π-linkage structural engineering. Org. Electron. 2019, 73, 146–151. [Google Scholar] [CrossRef]
- Savikhin, V.; Babics, M.; Neophytou, M.; Liu, S.; Oosterhout, S.D.; Yan, H.; Gu, X.; Beaujuge, P.M.; Toney, M.F. Impact of polymer side chain modification on OPV morphology and performance. Chem. Mater. 2018, 30, 7872–7884. [Google Scholar] [CrossRef] [Green Version]
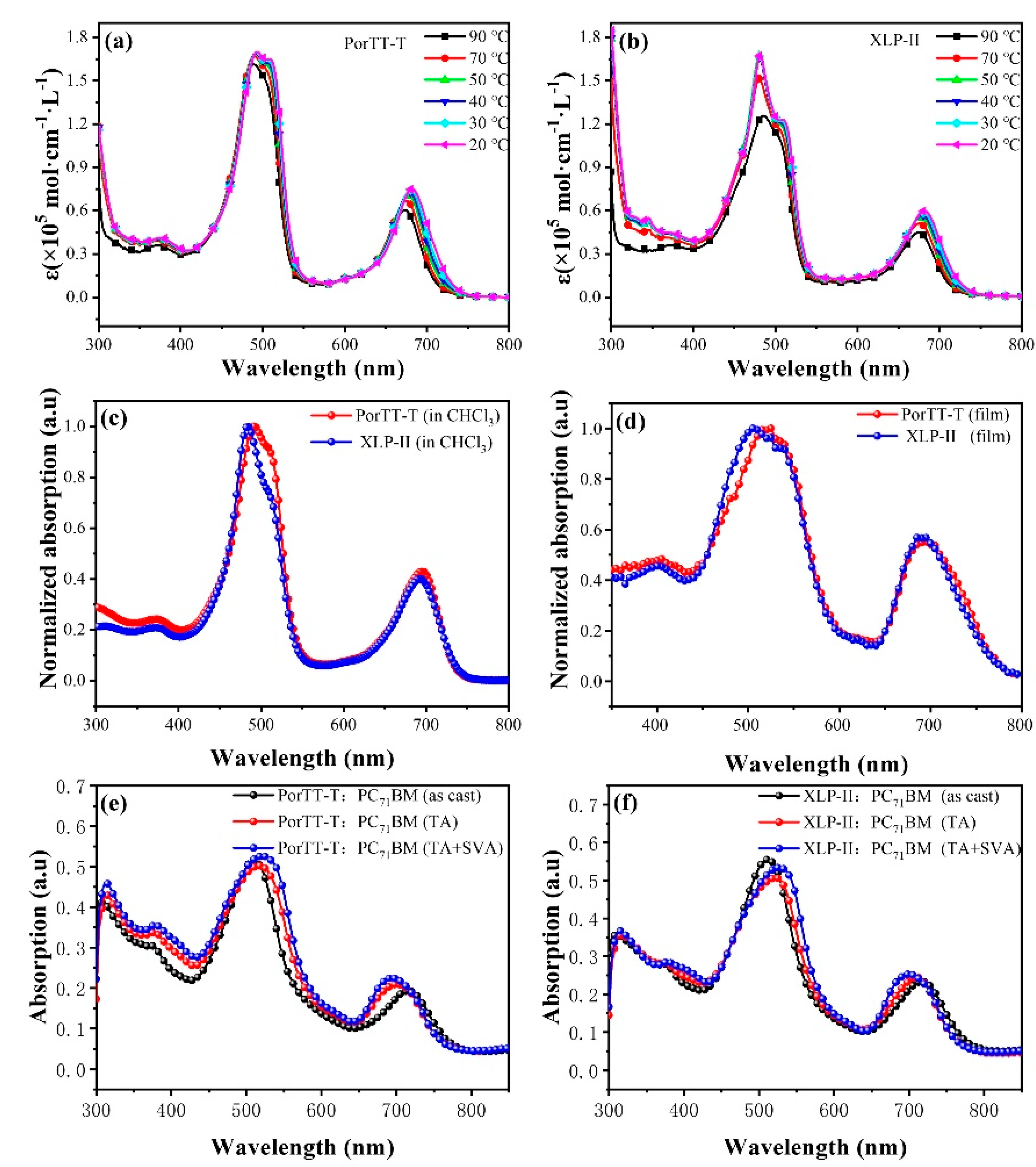



| Temperature (°C) | PorTT-T | XLP-II | ||
|---|---|---|---|---|
| Q-Band (nm) (L/mol·cm) | B-Band (nm) (L/mol·cm) | Q-Band (nm) (L/mol·cm) | B-Band (nm) (L/mol·cm) | |
| 20 | 493 (1.70), 508 (1.65) | 681 (0.75) | 481 (1.68), 508 (1.23) | 681 (0.60) |
| 30 | 492 (1.69), 506 (1.64) | 679 (0.74) | 481 (1.67), 505 (1.22) | 679 (0.58) |
| 40 | 491 (1.68), 504 (1.62) | 678 (0.73) | 480 (1.67), 505 (1.22) | 678 (0.56) |
| 50 | 490 (1.68), 503 (1.61) | 677 (0.71) | 480 (1.67), 502 (1.21) | 677 (0.55) |
| 70 | 489 (1.67), 498 (1.61) | 675 (0.67) | 480 (1.51), 505 (1.16) | 675 (0.51) |
| 90 | 488 (1.61) | 673 (0.60) | 484 (1.26) | 674 (0.45) |
| Samples | λmax (Solution) (nm) | λmax (Film) (nm) | λonset-edge (nm) | Egopt (nm) |
|---|---|---|---|---|
| PorTT-T | 492, 504, 695 | 524,694 | 772 | 1.60 |
| XLP-II | 483, 502, 692 | 505,690 | 770 | 1.61 |
| Samples | Eonsetox (V) | EHOMO (eV) | ELUMO (eV) | EonsetRe (V) | EHOMOcal (eV) | Egapcal (eV) | EcalLUMO (eV) |
|---|---|---|---|---|---|---|---|
| PorTT-T | 0.57 | −5.37 | −3.88 | −0.92 | −5.14 | 1.96 | −3.10 |
| XLP-II | 0.53 | −5.33 | −3.78 | −1.02 | −5.06 | 2.03 | −3.03 |
| Donors | The Cathode | TA (Temperature/Time) | SVA (Solvent/Time) | Voc (V) | Jsc (mAcm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|---|---|
| PorTT-T:PC71BM | PFN-Br/Al | No | No | 0.845 | 7.15 | 34.1 | 2.06 |
| PFN-Br/Al | 100 °C/10 min | No | 0.938 | 9.15 | 33.7 | 2.89 | |
| PFN-Br/Al | 100 °C/10 min | CF/30 s | 0.886 | 12.03 | 49.9 | 5.32 | |
| LiF/Ca/Al | 100 °C/8 min | DCM/20 s | 0.662 | 8.44 | 32.1 | 1.98 | |
| LiF/Ca/Al | 100 °C/8 min | DCM/20 s | 0.68 | 11.04 | 34.55 | 2.6 | |
| LiF/Ca/Al | 100 °C/8 min | CF/20 s | 0.75 | 11.32 | 38.45 | 3.25 | |
| LiF/Ca/Al | 100 °C/8 min | CS2/20 s | 0.76 | 10.64 | 39.9 | 3.22 | |
| LiF/Ca/Al | 100 °C/10 min | CS2/20 s | 0.74 | 10.27 | 39.35 | 2.98 | |
| LiF/Ca/Al | 100 °C/10 min | CS2/30 s | 0.74 | 9.2 | 37.8 | 2.58 | |
| LiF/Ca/Al | 100 °C/10 min | CS2/40 s | 0.77 | 7.85 | 36.05 | 2.18 | |
| LiF/Ca/Al | 100 °C/10 min | CF/20 s | 0.80 | 11.27 | 44.5 | 3.99 | |
| LiF/Ca/Al | 100 °C/10 min | CF/30 s | 0.82 | 11.16 | 47.28 | 4.30 | |
| LiF/Ca/Al | 100 °C/10 min | CF/40 s | 0.82 | 11.13 | 49.7 | 4.56 | |
| LiF/Ca/Al | 100 °C/10 min | CF/50 s | 0.83 | 10.44 | 51.55 | 4.49 | |
| LiF/Ca/Al | 100 °C/15 min | CF/40 s | 0.83 | 10.72 | 50.2 | 4.48 | |
| LiF/Ca/Al | 90 °C/10 min | CF/40 s | 0.84 | 10.90 | 50.82 | 4.64 | |
| LiF/Ca/Al | 110 °C/10 min | CF/40 s | 0.82 | 10.85 | 50.2 | 4.47 | |
| XLP-II:PC71BM | PFN-Br/Al | No | No | 0.894 | 5.76 | 27.2 | 1.40 |
| PFN-Br/Al | 100 °C/10 min | No | 0.863 | 8.03 | 32.6 | 2.26 | |
| PFN-Br/Al | 100 °C/10 min | CF/30s | 0.847 | 7.51 | 49.3 | 3.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Tang, W.; Liu, Z.; Tang, W.; Yuan, Z.; Qin, Y.; Yan, L.; Zhu, X.; Zhu, W.; Wang, X. Effects of Side-Chain Engineering with the S Atom in Thieno[3,2-b]thiophene-porphyrin to Obtain Small-Molecule Donor Materials for Organic Solar Cells. Molecules 2021, 26, 6134. https://doi.org/10.3390/molecules26206134
Xie L, Tang W, Liu Z, Tang W, Yuan Z, Qin Y, Yan L, Zhu X, Zhu W, Wang X. Effects of Side-Chain Engineering with the S Atom in Thieno[3,2-b]thiophene-porphyrin to Obtain Small-Molecule Donor Materials for Organic Solar Cells. Molecules. 2021; 26(20):6134. https://doi.org/10.3390/molecules26206134
Chicago/Turabian StyleXie, Liuping, Wei Tang, Zhixin Liu, Wencheng Tang, Zihao Yuan, Yinbin Qin, Lei Yan, Xunjin Zhu, Weiguo Zhu, and Xingzhu Wang. 2021. "Effects of Side-Chain Engineering with the S Atom in Thieno[3,2-b]thiophene-porphyrin to Obtain Small-Molecule Donor Materials for Organic Solar Cells" Molecules 26, no. 20: 6134. https://doi.org/10.3390/molecules26206134
APA StyleXie, L., Tang, W., Liu, Z., Tang, W., Yuan, Z., Qin, Y., Yan, L., Zhu, X., Zhu, W., & Wang, X. (2021). Effects of Side-Chain Engineering with the S Atom in Thieno[3,2-b]thiophene-porphyrin to Obtain Small-Molecule Donor Materials for Organic Solar Cells. Molecules, 26(20), 6134. https://doi.org/10.3390/molecules26206134







