High Performance Near-Infrared Emitters with Methylated Triphenylamine and Thiadiazolo[3,4-g]quinoxaline-Based Fluorophores
Abstract
:1. Introduction
2. Results and Discussion
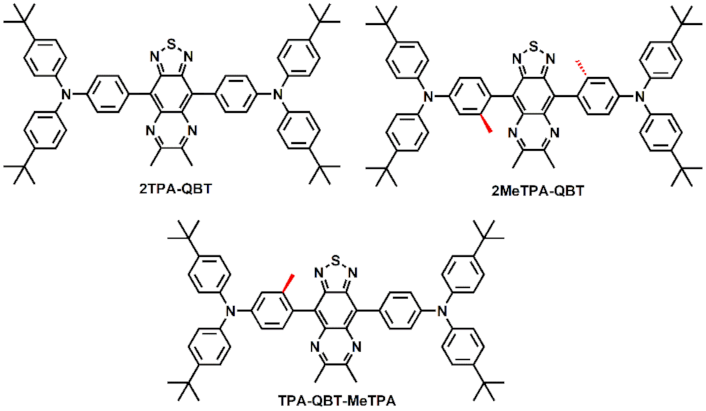
2.1. Synthesis and Thermal Stability
2.2. Electrochemical Properties
2.3. Photophysical Properties
2.4. Theoretical Calculations
2.5. Electroluminescence Properties
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, X.Q.; Hu, Y.; Yu, Y.J.; Tian, Q.S.; Shen, W.S.; Yang, W.Y.; Jiang, Z.Q.; Liao, L.S. Over 800 nm Emission via Harvesting of Triplet Excitons in Exciplex Organic Light-Emitting Diodes. J. Phys. Chem. Lett. 2021, 12, 6034–6040. [Google Scholar] [CrossRef]
- Babu Kajjam, A.; Vaidyanathan, S. Acenaphthene-imidazole based red-to-NIR Emissive Homoleptic and Heteroleptic Ir(III) complexes for OLEDs: Combined experimental and theoretical approach. Inor. Chim. Acta 2021, 519, 120268. [Google Scholar] [CrossRef]
- Yang, K.; Wang, L.; Zhang, Q. Preparation and properties of a flexible night vision imaging system filter for avionic LED displays. J. Mater. Sci.-Mater. Electron. 2015, 26, 2222–2229. [Google Scholar] [CrossRef]
- Strobl, M.; Mayr, T.; Klimant, I.; Borisov, S.M. Photostable upconverting and downconverting pH sensors based on combination of a colorimetric NIR indicator and stable inorganic phosphors as secondary emitters. Sens. Actuators B-Chem. 2017, 245, 972–979. [Google Scholar] [CrossRef]
- Xiang, H.; Cheng, J.; Ma, X.; Zhou, X.; Chruma, J.J. Near-infrared phosphorescence: Materials and applications. Chem. Soc. Rev. 2013, 42, 6128–6185. [Google Scholar] [CrossRef]
- Qian, G.; Zhong, Z.; Luo, M.; Yu, D.; Zhang, Z.; Wang, Z.Y.; Ma, D. Simple and Efficient Near-Infrared Organic Chromophores for Light-Emitting Diodes with Single Electroluminescent Emission above 1000 nm. Adv. Mater. 2009, 21, 111–116. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.-L.; Gao, Y.; Wang, L.; Zhang, S.; Zhao, L.; Lu, P.; Yang, B.; Su, S.-J.; Ma, Y. Efficient Near-Infrared (NIR) Organic Light-Emitting Diodes Based on Donor-Acceptor Architecture: An Improved Emissive State from Mixing to Hybridization. Chem. Mater. 2008, 20, 6208–6216. [Google Scholar] [CrossRef]
- Xiong, W.; Meng, F.; Tan, H.; Wang, Y.; Wang, P.; Zhang, Y.; Tao, Q.; Su, S.; Zhu, W. Dinuclear platinum complexes containing aryl-isoquinoline and oxadiazole-thiol with an efficiency of over 8.8%: In-depth investigation of the relationship between their molecular structure and near-infrared electroluminescent properties in PLEDs. J. Mater. Chem. C 2016, 4, 6007–6015. [Google Scholar] [CrossRef]
- Leppenen, N.V.; Lanco, L.; Smirnov, D.S. Quantum Zeno effect and quantum nondemolition spin measurement in a quantum dot–micropillar cavity in the strong coupling regime. Phys. Rev. B 2021, 103, 045413. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, S.; Wang, R.; Li, W.; Shen, F.; Yang, B.; Ma, Y. Highly efficient near-infrared organic light-emitting diode based on a butterfly-shaped donor-acceptor chromophore with strong solid-state fluorescence and a large proportion of radiative excitons. Angew. Chem. Int. Ed. 2014, 53, 2119–2123. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Peng, J.; Cao, Y.; Roncali, J. Solution-processable single-material molecular emitters for organic light-emitting devices. Chem. Soc. Rev. 2011, 40, 3509–3524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, C.; Liu, D.; Yu, J.; Tan, H.; Zhu, M.; Zhang, B.; Liu, Y.; Wang, Y.; Zhu, W. Boosting Efficiency of Near-Infrared Emitting Iridium(III) Phosphors by Administrating Their π–π Conjugation Effect of Core–Shell Structure in Solution-Processed OLEDs. Adv. Opt. Mater. 2020, 8, 2000154. [Google Scholar] [CrossRef]
- Chaaban, M.; Chi, Y.C.; Worku, M.; Zhou, C.; Lin, H.; Lee, S.; Ben-Akacha, A.; Lin, X.; Huang, C.; Ma, B. Thiazol-2-thiolate-Bridged Binuclear Platinum(II) Complexes with High Photoluminescence Quantum Efficiencies of up to Near Unity. Inorg. Chem. 2020, 59, 13109–13116. [Google Scholar] [CrossRef]
- Kajjam, A.B.; Vaidyanathan, S. Structural Mimics of Phenyl Pyridine (ppy)—Substituted, Phosphorescent Cyclometalated Homo and Heteroleptic Iridium(III) Complexes for Organic Light Emitting Diodes—An Overview. Chem. Rec. 2018, 18, 293–349. [Google Scholar] [CrossRef]
- Chaskar, A.; Chen, H.F.; Wong, K.T. Bipolar host materials: A chemical approach for highly efficient electrophosphorescent devices. Adv. Mater. 2011, 23, 3876–3895. [Google Scholar] [CrossRef]
- Cebrian, C.; Mauro, M. Recent advances in phosphorescent platinum complexes for organic light-emitting diodes. Beilstein. J. Org. Chem. 2018, 14, 1459–1481. [Google Scholar] [CrossRef]
- Ganesan, P.; Hung, W.Y.; Tso, J.Y.; Ko, C.L.; Wang, T.H.; Chen, P.T.; Hsu, H.F.; Liu, S.H.; Lee, G.H.; Chou, P.T.; et al. Functional Pyrimidinyl Pyrazolate Pt(II) Complexes: Role of Nitrogen Atom in Tuning the Solid-State Stacking and Photophysics. Adv. Funct. Mater. 2019, 29, 1900923. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, C.; Batagoda, T.; Coburn, C.; Thompson, M.E.; Forrest, S.R. Hot excited state management for long-lived blue phosphorescent organic light-emitting diodes. Nat. Commun. 2017, 8, 15566. [Google Scholar] [CrossRef]
- Tuong Ly, K.; Chen-Cheng, R.-W.; Lin, H.-W.; Shiau, Y.-J.; Liu, S.-H.; Chou, P.-T.; Tsao, C.-S.; Huang, Y.-C.; Chi, Y. Near-infrared organic light-emitting diodes with very high external quantum efficiency and radiance. Nat. Photonics 2016, 11, 63–68. [Google Scholar] [CrossRef]
- Tang, X.; Lee, Y.-T.; Feng, Z.; Ko, S.Y.; Wu, J.W.; Placide, V.; Ribierre, J.-C.; D’Aléo, A.; Adachi, C. Color-Tunable Low-Threshold Amplified Spontaneous Emission from Yellow to Near-Infrared (NIR) Based on Donor–Spacer–Acceptor–Spacer–Donor Linear Dyes. Angew. Chem. Int. Ed. 2016, 55, 5739–5744. [Google Scholar] [CrossRef]
- Yang, Z.; Mao, Z.; Xie, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M.P. Recent advances in organic thermally activated delayed fluorescence materials. Chem. Soc. Rev. 2017, 46, 915–1016. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hu, Y.; Zhang, Y.-X.; Lin, J.-D.; Wang, Y.-K.; Jiang, Z.-Q.; Liao, L.-S.; Lee, S.-T. Over 10% EQE Near-Infrared Electroluminescence Based on a Thermally Activated Delayed Fluorescence Emitter. Adv. Funct. Mater. 2017, 27, 1700986. [Google Scholar] [CrossRef]
- Tang, X.; Li, X.-L.; Liu, H.; Gao, Y.; Shen, Y.; Zhang, S.; Lu, P.; Yang, B.; Su, S.-J.; Ma, Y. Efficient near-infrared emission based on donor-acceptor molecular architecture: The role of ancillary acceptor of cyanophenyl. Dye Pigm. 2018, 149, 430–436. [Google Scholar] [CrossRef]
- Ganesan, P.; Chen, D.-G.; Chen, W.-C.; Gnanasekaran, P.; Lin, J.-A.; Huang, C.-Y.; Chen, M.-C.; Lee, C.-S.; Chou, P.-T.; Chi, Y. Methoxy substituents activated carbazole-based boron dimesityl TADF emitters. J. Mater. Chem. C 2020, 8, 4780–4788. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Li, H.; Chen, R.; Jin, L.; Yuan, K.; Li, H.; Lu, P.; Yang, B.; Huang, W. Breaking the Efficiency Limit of Fluorescent OLEDs by Hybridized Local and Charge-Transfer Host Materials. J. Phys. Chem. Lett. 2018, 9, 5240–5245. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, Z.; Li, W.; Mao, Z.; Zhao, J.; Zhang, Y.; Wu, Y.C.; Jiao, S.; Liu, Y.; Chi, Z. Nondoped Red Fluorophores with Hybridized Local and Charge-Transfer State for High-Performance Fluorescent White Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2019, 11, 39026–39034. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hu, Z.; Zhou, B.; Zhong, C.; Sun, Z.; Sun, H. Accurate Prediction for Dynamic Hybrid Local and Charge Transfer Excited States from Optimally Tuned Range-Separated Density Functionals. J. Phys. Chem. C 2019, 123, 5616–5625. [Google Scholar] [CrossRef]
- Song, J.; Lee, H.; Jeong, E.G.; Choi, K.C.; Yoo, S. Organic Light-Emitting Diodes: Pushing Toward the Limits and Beyond. Adv. Mater. 2020, 32, 1907539. [Google Scholar] [CrossRef]
- Liu, T.; Xie, G.; Zhong, C.; Gong, S.; Yang, C. Boosting the Efficiency of Near-Infrared Fluorescent OLEDs with an Electroluminescent Peak of Nearly 800 nm by Sensitizer-Based Cascade Energy Transfer. Adv. Funct. Mater. 2018, 28, 1706088. [Google Scholar] [CrossRef]
- Li, C.; Duan, R.; Liang, B.; Han, G.; Wang, S.; Ye, K.; Liu, Y.; Yi, Y.; Wang, Y. Deep-Red to Near-Infrared Thermally Activated Delayed Fluorescence in Organic Solid Films and Electroluminescent Devices. Angew. Chem. Int. Ed. 2017, 56, 11525–11529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, X.; Zhou, C.; Su, Q.; Chen, S.; Song, J.; Wong, W.-Y. High-efficiency organic electroluminescent materials based on the D–A–D type with sterically hindered methyl groups. J. Mater. Chem. C 2020, 8, 6851–6860. [Google Scholar] [CrossRef]
- Qian, G.; Zhong, Z.; Luo, M.; Yu, D.; Zhang, Z.; Ma, D.; Wang, Z.Y. Synthesis and Application of Thiadiazoloquinoxaline-Containing Chromophores as Dopants for Efficient Near-Infrared Organic Light-Emitting Diodes. J. Phys. Chem. C 2009, 113, 1589–1595. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical considerations for determining absolute frontier orbital energy levels of conjugated polymers for solar cell applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, Z.; Yu, M.; Zhang, X.; Xu, Y.; Lv, P.; Chu, S.; Liu, C.; Lai, W.Y.; Huang, W. Iridium(III)-Complexed Polydendrimers for Inkjet-Printing OLEDs: The Influence of Solubilizing Steric Hindrance Groups. J. Mater. Chem. C 2019, 7, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.; Song, J.; Chen, Z.; He, J.; Wang, X.; Liu, H.; Chen, S.; Qu, J.; Wong, W.-Y. Achieving High-Performance Solution-Processed Deep-Red/Near-Infrared Organic Light-Emitting Diodes with a Phenanthroline-Based and Wedge-Shaped Fluorophore. Adv. Electron. Mater. 2019, 5, 1800677. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, S.-T.; Gao, Y.; Yang, X.; Liu, H.; Li, W.; Yang, B. Efficient and stable deep-blue narrow-spectrum electroluminescence based on hybridized local and charge-transfer (HLCT) state. Dye Pigm. 2021, 193, 109482. [Google Scholar] [CrossRef]
- Lippert, V.E. Spektroskopische Bestimmung des Dipolmomentes aromatischer Verbindungen im ersten angeregten Singulettzustand. Electrochemistry 1957, 61, 962–975. [Google Scholar]
- Bozkurt, E.; Gul, H.I.; Mete, E. Solvent and substituent effect on the photophysical properties of pyrazoline derivatives: A spectroscopic study. J. Photochem. Photobiol. A 2018, 352, 35–42. [Google Scholar] [CrossRef]
- Kumar Satpati, A.; Kumbhakar, M.; Kumar Maity, D.; Pal, H. Photophysical investigations of the solvent polarity effect on the properties of coumarin-6 dye. Chem. Phys. Lett. 2005, 407, 114–118. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman; Montgomery, J.R.J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revisionc.02; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
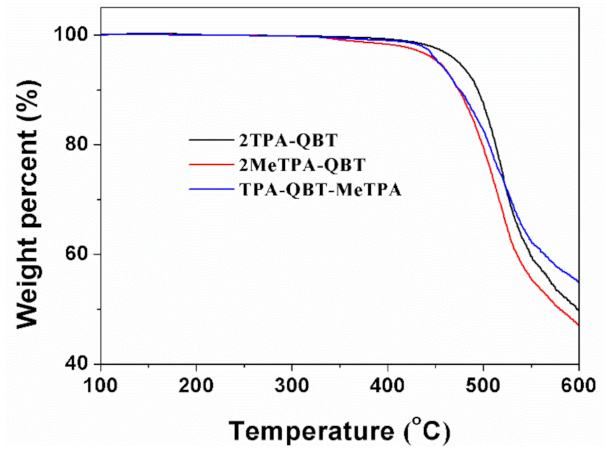
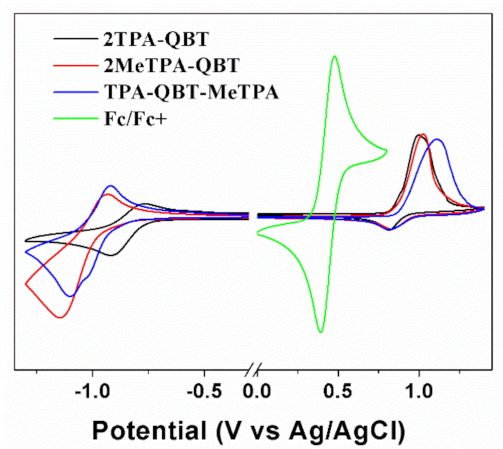
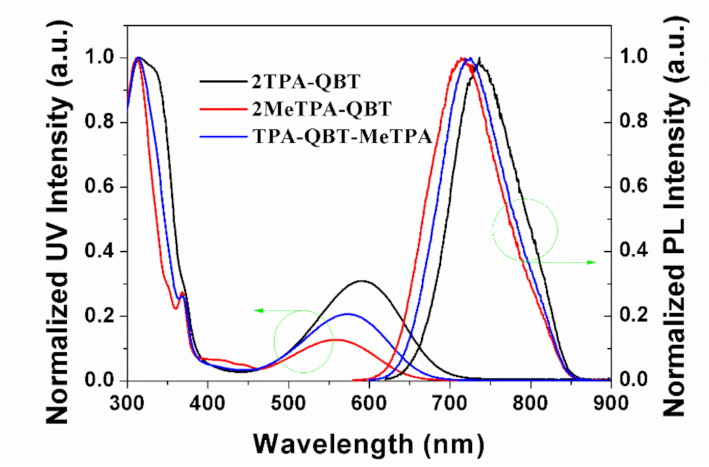

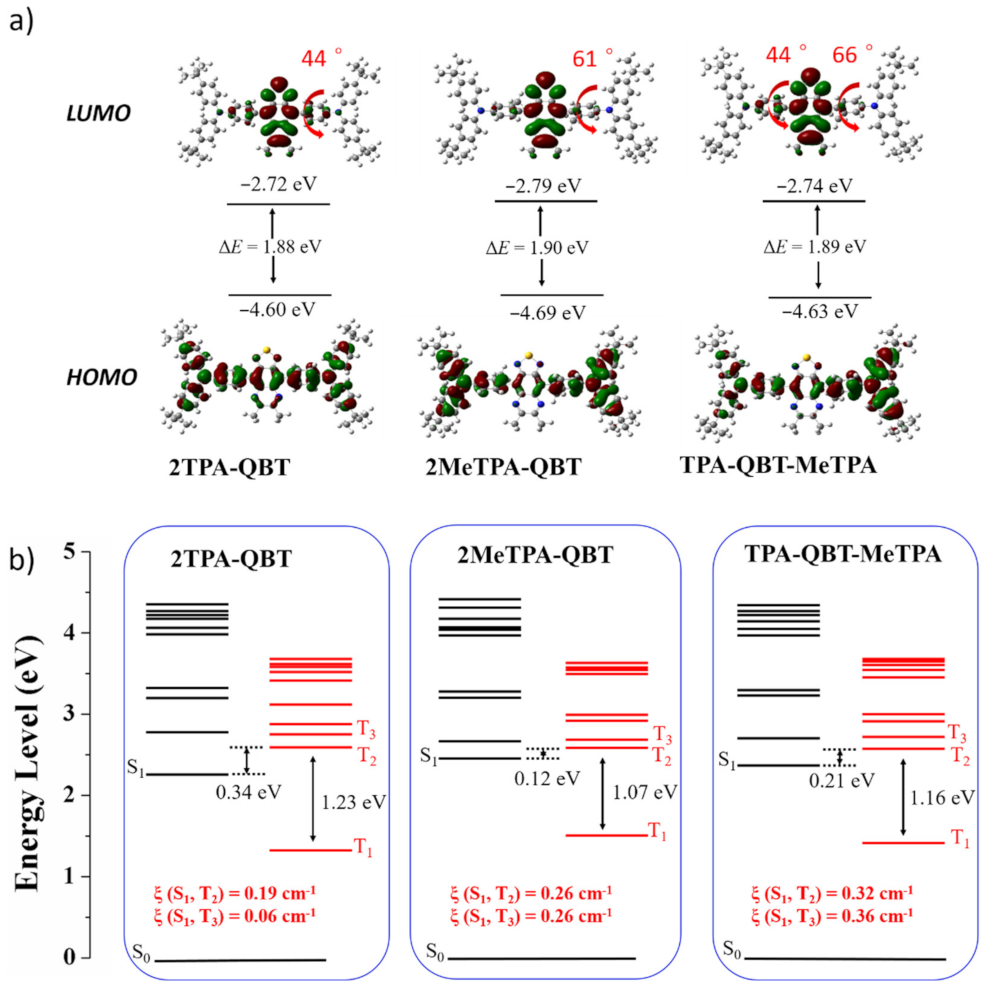
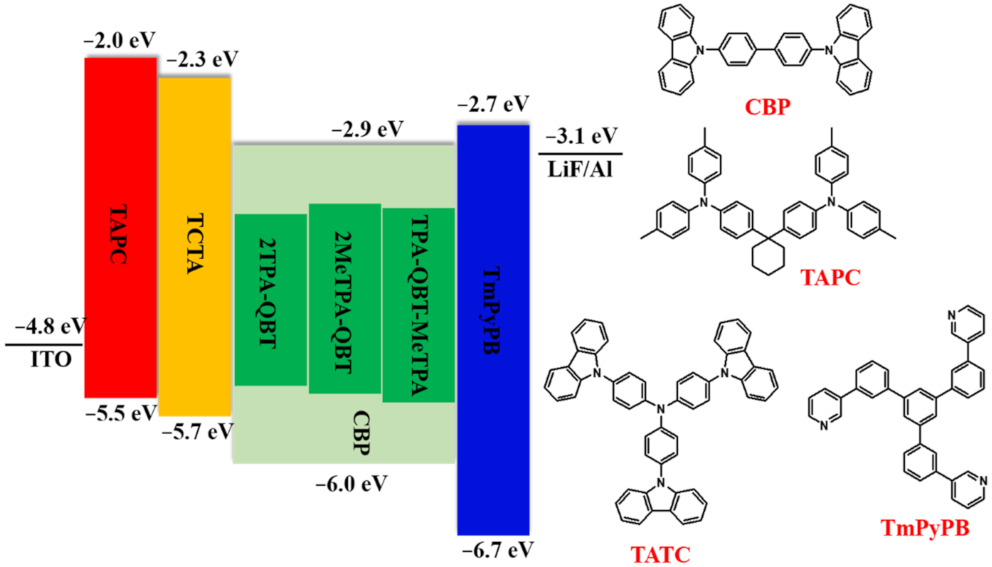
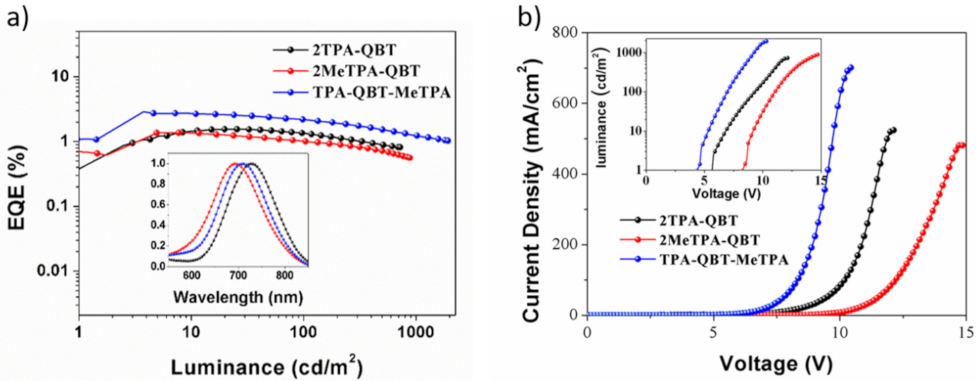
| Emitters | Eoxa/V | Ereda/V | EHOMOb/eV | ELUMOb/eV | Ecv/eV | Td/°C |
|---|---|---|---|---|---|---|
| 2TPA-QBT | 0.81 | −0.73 | −5.17 | −3.63 | 1.54 | 473 |
| 2MeTPA-QBT | 0.82 | −0.88 | −5.18 | −3.48 | 1.70 | 453 |
| TPA-QBT-MeTPA | 0.85 | −0.80 | −5.21 | −3.56 | 1.65 | 453 |
| Emitters | λabs sol/nm a | λabs film/nm b | λem sol/nm a | λem film/nm b | /eV c | ΦF/% sold | τsol/ns |
|---|---|---|---|---|---|---|---|
| 2TPA-QBT | 589, 314 | 609 | 736 | 780 | 1.62 | 26 | 6a/3b |
| 2MeTPA-QBT | 557, 312 | 574 | 714 | 732 | 1.72 | 38 | 5a/6b |
| TPA-QBT-MeTPA | 557, 312 | 585 | 724 | 748 | 1.68 | 34 | 7a/3b |
| Emitters | λEL (nm) a | Von (V) b | EQE (%) c | Lmax (cd/m2) d | CIE e |
|---|---|---|---|---|---|
| 2TPA-QBT | 718 | 5.6 | 1.58 | 783 | (0.70, 0.31) |
| 2MeTPA-QBT | 693 | 8.3 | 1.33 | 839 | (0.70, 0.29) |
| TPA-QBT-MeTPA | 707 | 4.4 | 3.02 | 1875 | (0.69, 0.30) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wu, C.; Zhu, M.; Miao, J. High Performance Near-Infrared Emitters with Methylated Triphenylamine and Thiadiazolo[3,4-g]quinoxaline-Based Fluorophores. Molecules 2021, 26, 6386. https://doi.org/10.3390/molecules26216386
Zhang Y, Wu C, Zhu M, Miao J. High Performance Near-Infrared Emitters with Methylated Triphenylamine and Thiadiazolo[3,4-g]quinoxaline-Based Fluorophores. Molecules. 2021; 26(21):6386. https://doi.org/10.3390/molecules26216386
Chicago/Turabian StyleZhang, Youming, Chengjun Wu, Minrong Zhu, and Jingsheng Miao. 2021. "High Performance Near-Infrared Emitters with Methylated Triphenylamine and Thiadiazolo[3,4-g]quinoxaline-Based Fluorophores" Molecules 26, no. 21: 6386. https://doi.org/10.3390/molecules26216386





