Green Tea Waste as an Efficient Adsorbent for Methylene Blue: Structuring of a Novel Adsorbent Using Full Factorial Design
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Instrumentation and Software
2.3. Preparation of the Tested Adsorbents
2.4. Preparation of the Dye Samples
2.5. Full Factorial Design (24-FFD)
2.6. Point of Zero Charge (pHPZC)
2.7. Equilibrium and Kinetics Studies
3. Results and Discussion
3.1. Preliminary Screening of the Adsorption Performance
3.2. Design Analysis
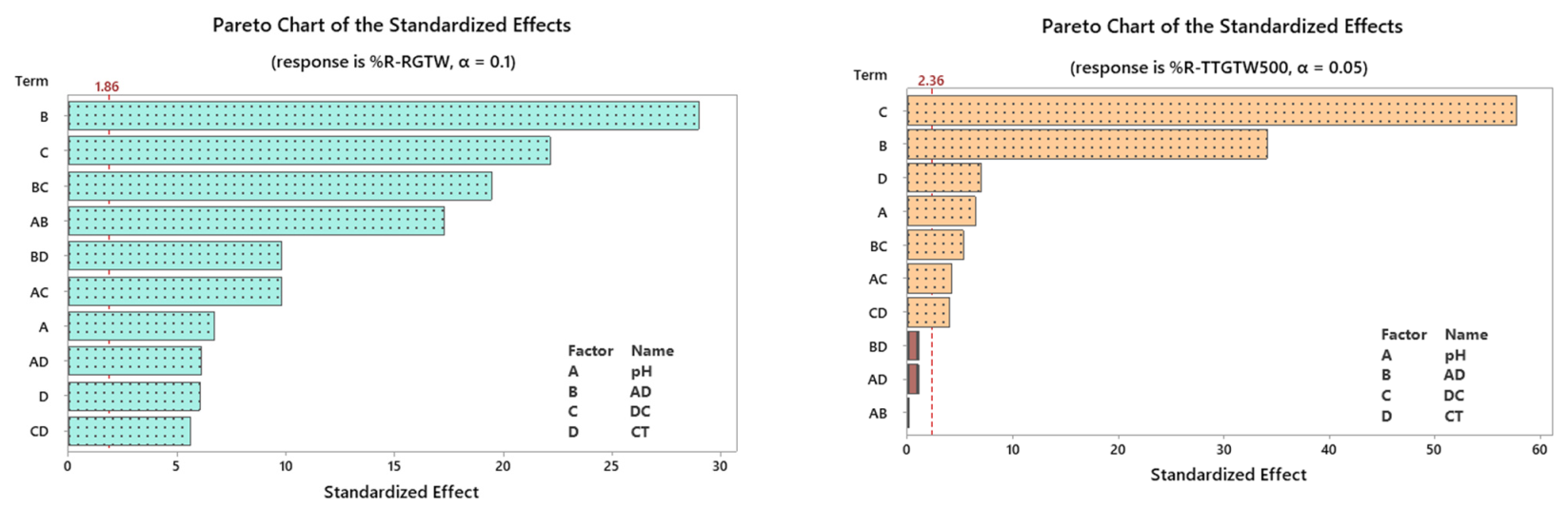
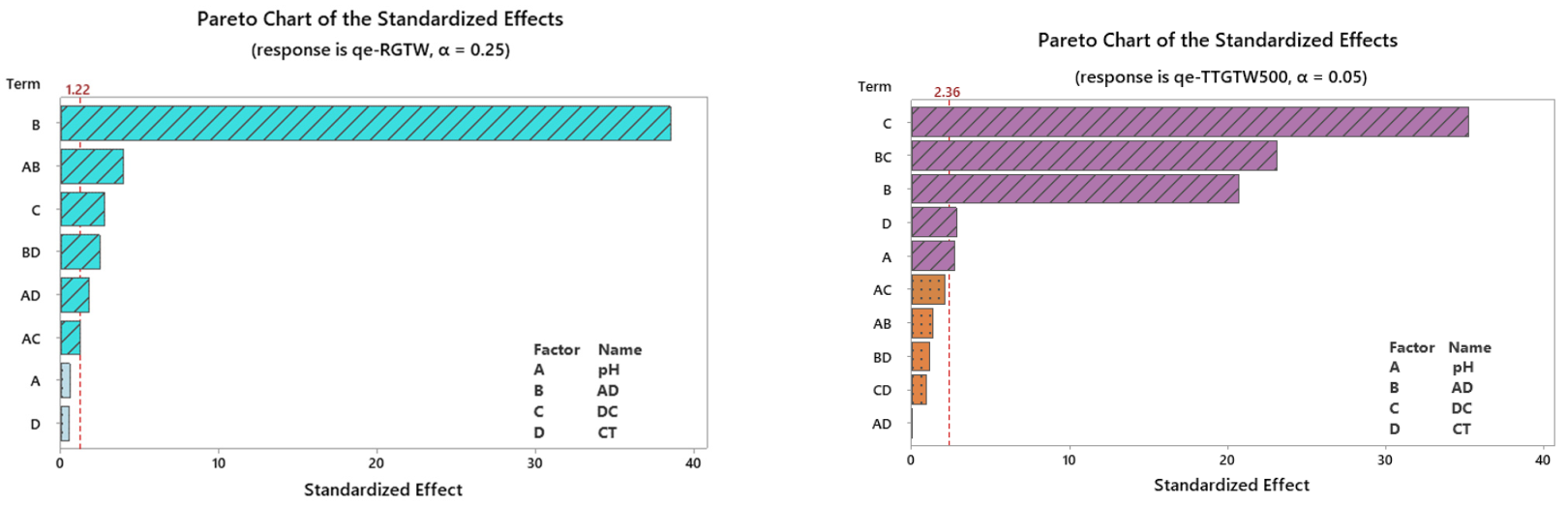
3.2.1. Quality Charts and Analysis of Variance (ANOVA) Testing
3.2.2. Contour and Surface Plots
3.2.3. Response Optimization
3.3. Characterization of GTW Samples
3.3.1. FT-IR Analysis and Proposed Adsorption Mechanism
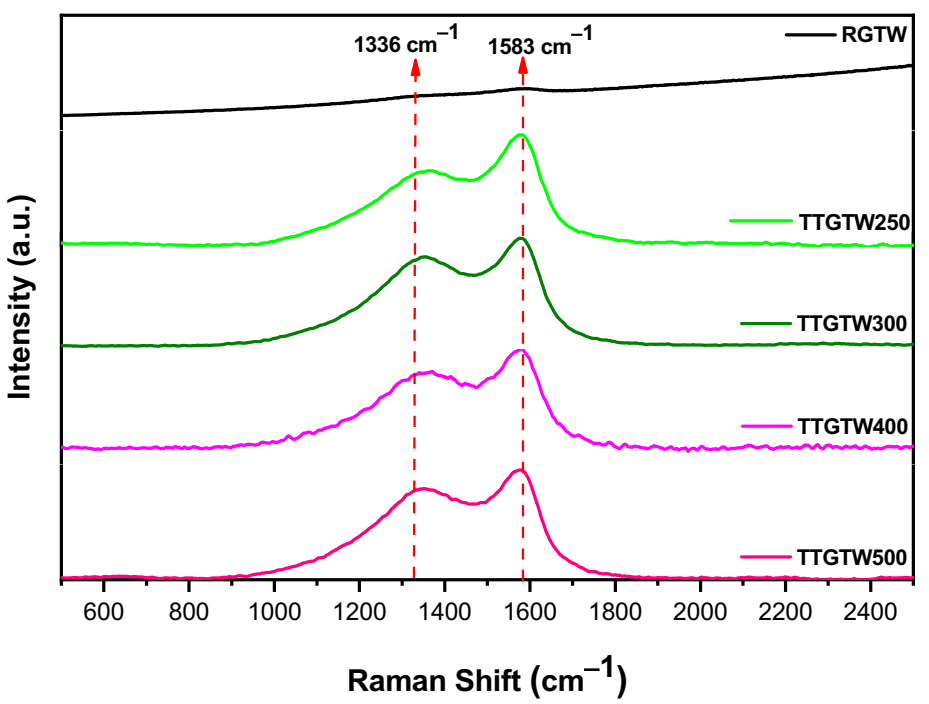
3.3.2. Raman Analysis
3.3.3. CHN Analysis
3.3.4. TGA Analysis of RGTW
- In the temperature range from 25 to 100 °C, the adsorbed water molecules were lost, followed by the loss of crystalline water at ~200 °C, which represented 6.14% of the sample;
- In this step, >80% of the sample was decomposed between 200 and 600 °C; two major peaks at 350.97 °C could be observed in addition to three shoulders at 250.68, 313.97, and 421.40 °C, which could be attributable to the decomposition of the organic materials present in RGTW and the conversion to carbonaceous material.
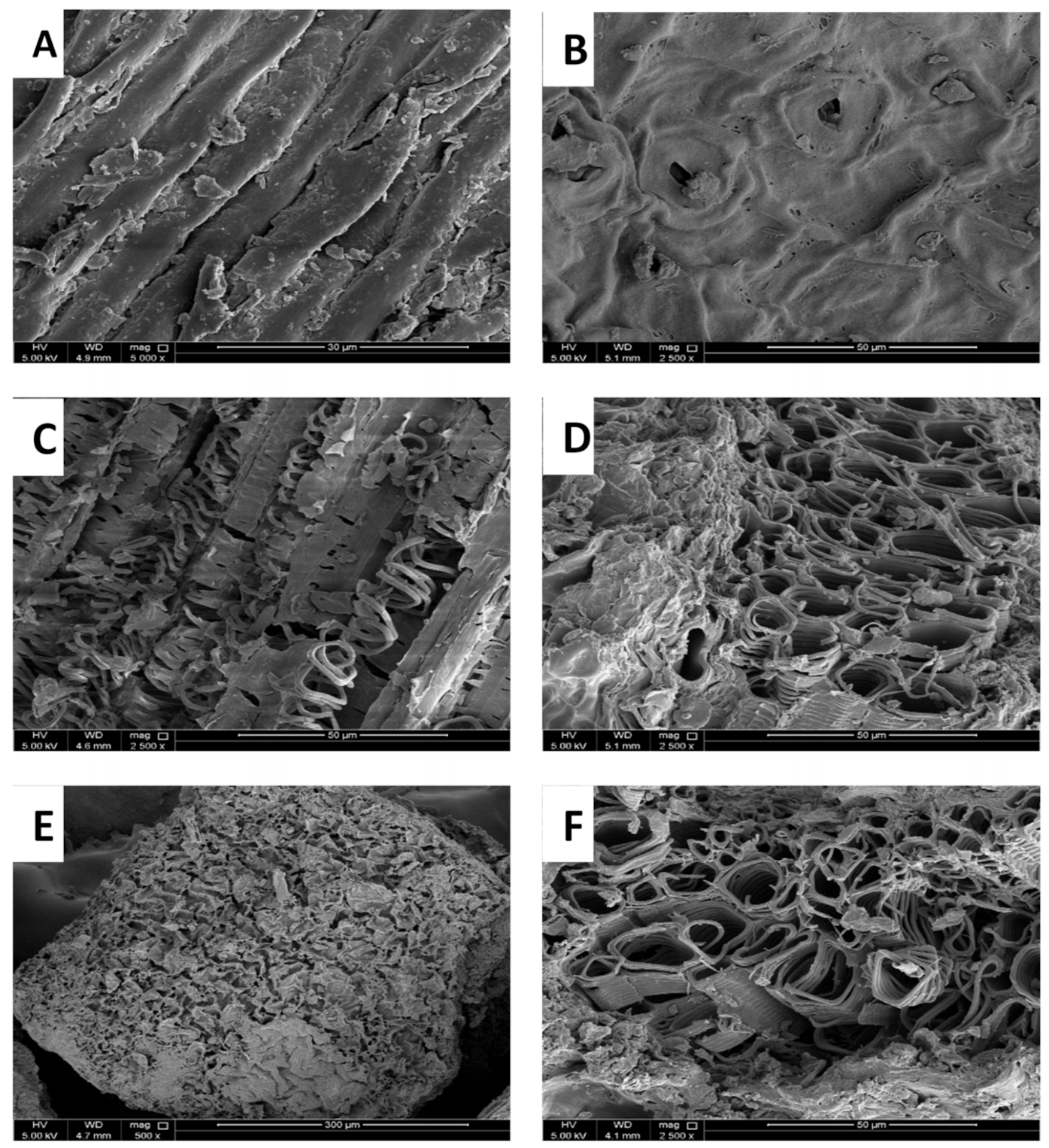
3.3.5. SEM Analysis
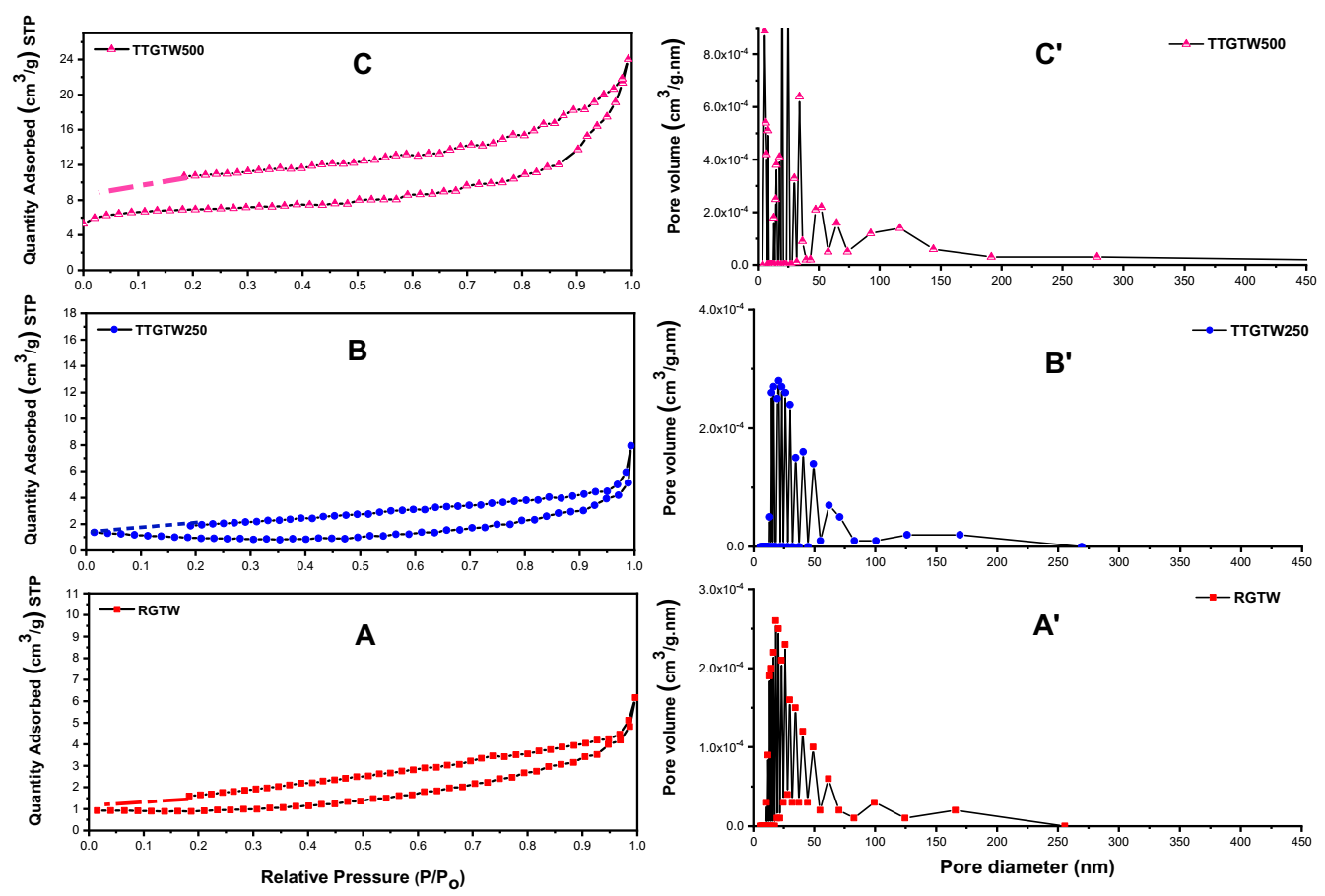
3.3.6. BET Analysis
3.4. Equilibrium and Kinetics Studies of the Adsorption of MB onto RGTW and TTGTW500
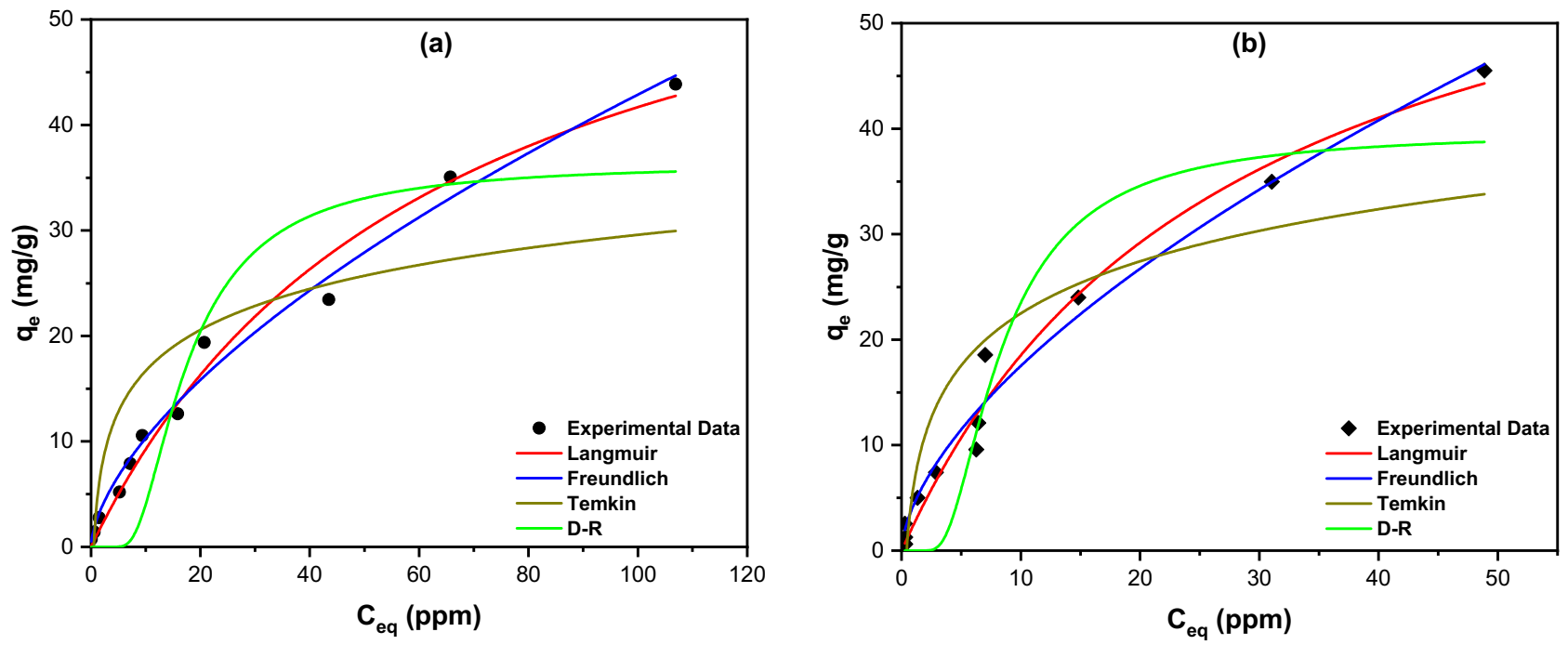
3.4.1. Equilibrium Studies
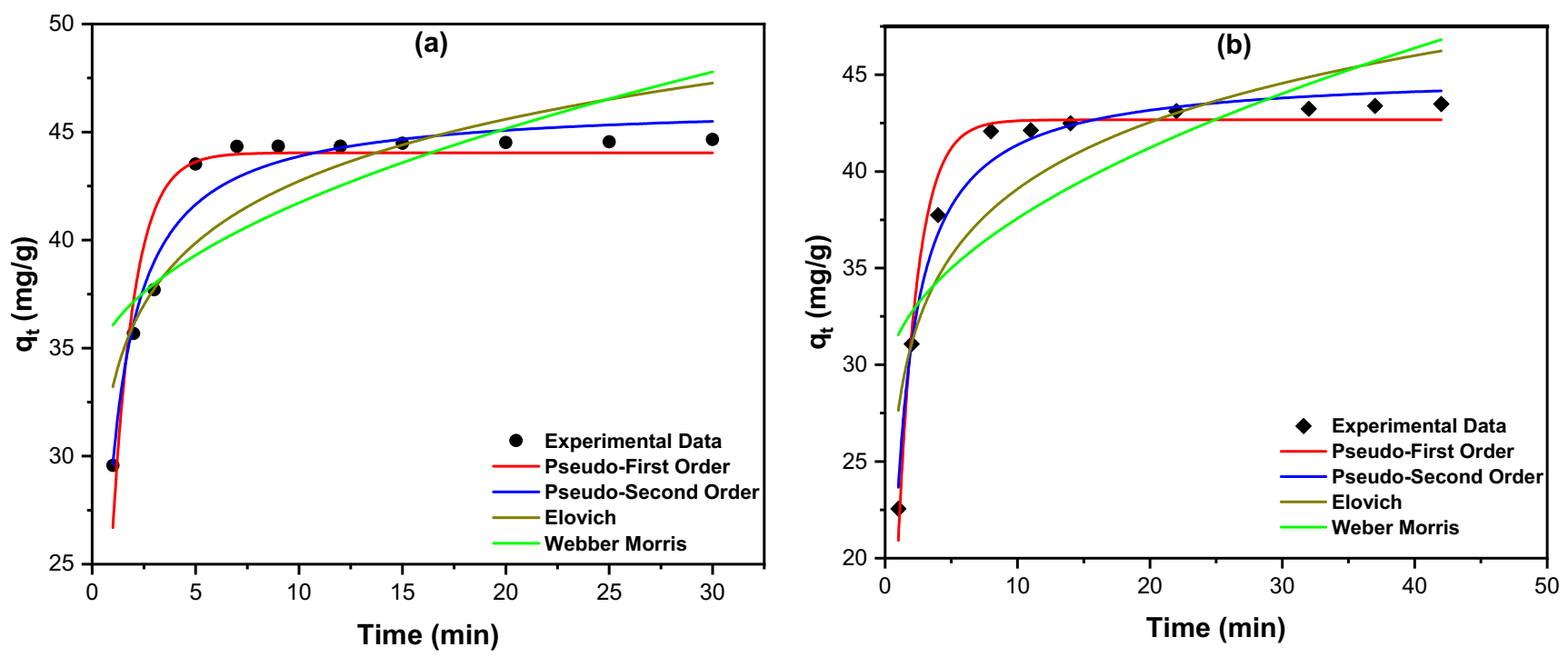
3.4.2. Kinetics Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Owa, F.D. Water Pollution: Sources, Effects, Control and Management. Med. J. Soc. Sci. 2013, 4, 58–65. [Google Scholar] [CrossRef]
- Pukšec, T.; Leahy, P.; Foley, A.; Markovska, N.; Duić, N. Sustainable development of energy, water and environment systems 2016 (SDEWES2016). Renew Sustain. Energy Rev. 2018, 82, 1685–1690. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Khan, A.A.; Kumari, S.; Hussain, S. Super adsorbent Ni–Co–S/SDS Nanocomposites for Ultrahigh Removal of Cationic, Anionic Organic Dyes and Toxic Metal Ions: Kinetics, Isotherm and Adsorption Mechanism. ACS Sustain. Chem. Eng. 2019, 7, 4165–4176. [Google Scholar] [CrossRef]
- Eren, M.S.A.; Arslanoğlu, H.; Çiftçi, H. Production of microporous Cu-doped BTC (Cu-BTC) metal-organic framework composite materials, superior adsorbents for the removal of methylene blue (Basic Blue 9). J. Environ. Chem. Eng. 2020, 8, 104247. [Google Scholar] [CrossRef]
- Moharrami, P.; Motamedi, E. Application of cellulose nanocrystals prepared from agricultural wastes for synthesis of starch-based hydrogel nanocomposites: Efficient and selective nanoadsorbent for removal of cationic dyes from water. Bioresour. Technol. 2020, 313, 123661. [Google Scholar] [CrossRef]
- Hou, Y.; Yan, S.; Huang, G.; Yang, Q.; Huang, S.; Cai, J. Fabrication of N-doped carbons from waste bamboo shoot shell with high removal efficiency of organic dyes from water. Bioresour. Technol. 2020, 303, 122939. [Google Scholar] [CrossRef]
- Paredes-Laverde, M.; Salamanca, M.; Diaz-Corrales, J.D.; Flórez, E.; Silva-Agredo, J.; Torres-Palma, R.A. Understanding the removal of an anionic dye in textile wastewaters by adsorption on ZnCl2 activated carbons from rice and coffee husk wastes: A combined experimental and theoretical study. J. Environ. Chem. Eng. 2021, 9, 105685. [Google Scholar] [CrossRef]
- Xia, L.; Zhou, S.; Zhang, C.; Fu, Z.; Wang, A.; Zhang, Q.; Wang, Y.; Liu, X.; Wang, X.; Xu, W. Environment-friendly Juncus effusus-based adsorbent with a three-dimensional network structure for highly efficient removal of dyes from wastewater. J. Clean. Prod. 2020, 259, 120812. [Google Scholar] [CrossRef]
- El-Shafie, A.S.; Hassan, S.S.; Akther, N.; El-Azazy, M. Watermelon rinds as cost-efficient adsorbent for acridine orange: A response surface methodological approach. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- He, H.J.; Xiang, Z.H.; Chen, X.J.; Chen, H.; Huang, H.; Wen, M.; Yang, C.P. Biosorption of Cd(II) from synthetic wastewater using dry biofilms from biotrickling filters. Int. J. Environ. Sci. Technol. 2018, 15, 1491–1500. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; He, H.; Zhang, J.; Liu, J.; Wang, D.; Huang, L.; Tu, Z. Preparation, Performances and Mechanisms of Co@AC Composite for Herbicide Atrazine Removal in Water. Water 2021, 13, 240. [Google Scholar] [CrossRef]
- El-Gendy, A.; El-Shafie, A.S.; Issa, A.; Al-Meer, S.; Al-Saad, K.; El-Azazy, M. Carbon-based materials (CBMS) for determination and remediation of antimicrobials in different substrates: Wastewater and infant foods as examples. In Carbon-Based Material for Environmental Protection and Remediation; Bartoli, M., Frediani, M., Rosi, L., Eds.; IntechOpen: London, UK, 2020; pp. 103–122. [Google Scholar] [CrossRef]
- Antony, J.; Roy, R.K. Improving the processes quality using statistical design of experiments: A case study. Qual. Assur. 1999, 6, 87–95. [Google Scholar] [CrossRef]
- Mi, H.; Yi, L.; Wu, Q.; Xia, J.; Zhang, B. Preparation and optimization of a low-cost adsorbent for heavy metal ions from red mud using fraction factorial design and Box-Behnken response methodology. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127198. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [Green Version]
- Kaison, C. World tea production and trade: Current and future development. In FAO Intergovernmental Group on Tea; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Dattner, C.; Boussabba, S. The Book of Green Tea; Universe Books; Simon & Schuster, King Street East: Toronto, CA, USA, 2003; p. 13. ISBN 978-0-7893-0853-5. [Google Scholar]
- Abd El-Atya, A.M.; Choia, J.H.; Rahmana, M.M.; Kima, S.-W.; Tosunc, A.; Shim, J.-H. Residues and contaminants in tea and tea infusions: A review. Food Addit. Contam. A 2014, 31, 1794–1804. [Google Scholar] [CrossRef]
- Ahmad, M.; Khan, M.A.; Farooq, U.; Athar, M. Carbonized green tea dredge, a potential adsorbent for removal of remazol brilliant yellow dye. J. Mater. Environ. Sci. 2012, 3, 149–156. [Google Scholar]
- Weng, C.-H.; Lin, Y.-T.; Chena, Y.-J.; Sharma, Y.C. Spent green tea leaves for decolourisation of raw textile industry wastewater. Color. Technol. 2013, 129, 298–304. [Google Scholar] [CrossRef]
- Singh, K.K.; Senapati, K.K.; Sarma, K.C. Synthesis of superparamagnetic Fe3O4 nanoparticles coated with green tea polyphenols and their use for removal of dye pollutant from aqueous solution. J. Environ. Chem. Eng. 2017, 5, 2214–2221. [Google Scholar] [CrossRef]
- Indolean, C.; Burcă, S.; Măicăneanu, A. Adsorptive Removal of Malachite Green from Model Aqueous Solutions by Chemically Modified Waste Green Tea Biomass. Acta Chim. Slov. 2017, 64, 513–521. [Google Scholar] [CrossRef] [Green Version]
- Plachtová, P.; Medříková, Z.; Zbořil, R.; Tuček, J.; Varma, R.S.; Maršálek, B. Iron and Iron Oxide Nanoparticles Synthesized with Green Tea Extract: Differences in Ecotoxicological Profile and Ability to Degrade Malachite Green. ACS Sustain. Chem. Eng. 2018, 6, 8679–8687. [Google Scholar] [CrossRef]
- Elazazy, M.S.; Ganesh, K.; Sivakumar, V.; Huessein, Y.H.A. Interaction of p-synephrine with p-chloranil: Experimental design and multiple response optimization. RSC Adv. 2016, 6, 64967–64976. [Google Scholar] [CrossRef]
- Al-Saad, K.; Issa, A.A.; Idoudi, S.; Shomar, B.; Al-Ghouti, M.A.; Al-Hashimi, N.; El-Azazy, M. Smart synthesis of trimethyl ethoxysilane (TMS) functionalized core–shell magnetic nanosorbents Fe3O4@SiO2: Process optimization and application for extraction of pesticides. Molecules 2020, 25, 4827. [Google Scholar] [CrossRef]
- Ferro-García, M.A.; Rivera-Utrilla, J.; Bautista-Toledo, I.; Moreno-Castilla, C. Adsorption of humic substances on activated carbon from aqueous solutions and their effect on the removal of Cr (III) ions. Langmuir 1998, 14, 1880–1886. [Google Scholar] [CrossRef]
- Box, G.E.; Hunter, W.G.; Hunter, J.S. Statistics for Experimenters: Design, Innovation, and Discovery, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 1–672. [Google Scholar]
- Wasewar, K.L.; Atif, M.; Prasad, B.; Mishra, I.M. Batch adsorption of zinc on tea factory waste. Desalination 2009, 244, 66–67. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Morad, N.; Tan, K.A. Magnetic nanoparticle (Fe3O4) impregnated onto tea waste for the removal of nickel(II) from aqueous solution. J. Hazard. Mater. 2011, 186, 160–168. [Google Scholar] [CrossRef]
- Tounsadi, H.; Khalidi, A.; Abdennouri, M.; Barka, N. Biosorption potential of Diplotaxis harra and Glebionis coronaria L. biomasses for the removal of Cd(II) and Co(II) from aqueous solutions. J. Environ. Chem. Eng. 2015, 3, 822–830. [Google Scholar] [CrossRef]
- Eroglu, H.; Yapici, S.; Nuhoğlu, C.; Varoğlu, E. An environmentally friendly process; Adsorption of radionuclide Tl-201 on fibrous waste tea. J. Hazard. Mater. 2009, 163, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Malkoc, E.; Nuhoglu, Y. Removal of Ni(II) ions from aqueous solutions using waste of tea factory: Adsorption on a fixed-bed column. J. Hazard. Mater. 2006, 135, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Ma, Z.; Xue, Y.; Ma, M.; Xu, S.; Qian, L.; Zhang, Q. Sorption of lead (II), cadmium (II), and copper (II)ions from aqueous solutions using tea waste. Ind. Eng. Chem. Res. 2014, 53, 3629–3635. [Google Scholar] [CrossRef]
- Efimov, M.N.; Vasilev, A.A.; Muratov, D.G.; Baranchikov, A.E.; Karpacheva, G.P. IR radiation assisted preparation of KOH-activated polymer-derived carbon for methylene blue adsorption. J. Environ. Chem. Eng. 2019, 7, 103514. [Google Scholar] [CrossRef]
- Dotto, G.L.; Santos, J.M.N.; Rodrigues, I.L.; Rosa, R.; Pavan, F.A.; Lima, E.C. Adsorption of Methylene Blue by ultrasonic surface modified chitin. J. Colloid. Interface Sci. 2015, 446, 133–140. [Google Scholar] [CrossRef]
- Ramakrishna, K.R.; Viraraghavan, T. Use of slag for dye removal. Waste Manag. 1998, 17, 483–488. [Google Scholar] [CrossRef]
- He, X.; Male, K.B.; Nesterenko, P.N.; Brabazon, D.; Paull, B.; Luong, J.H. Adsorption and desorption of methylene blue on porous carbon monoliths and nanocrystalline cellulose. ACS Appl. Mater. Interfaces 2013, 5, 8796–8804. [Google Scholar] [CrossRef] [Green Version]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, X.; Gu, Y.; Liu, S.; Liu, Y.; Hu, X.; Li, J.; Zhou, Y.; Liu, S.; He, Y. Rice waste biochars produced at different pyrolysis temperatures for arsenic and cadmium abatement and detoxification in sediment. Chemosphere 2020, 250, 126268. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1052–1069. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The adsorption of gases on glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Wang, J. Comparison of linearization methods for modeling the Langmuir adsorption isotherm. J. Mol. Liq. 2019, 296, 111850. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.O.; Léon, J.J.L.; de Matos, T.N. Elucidation of mechanism involved in adsorption of Pb(II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Moussavi, G.; Barikbin, B. Biosorption of chromium (VI) from industrial wastewater onto pistachio hull waste biomass. Chem. Eng. J. 2010, 162, 893–900. [Google Scholar] [CrossRef]
- Sarma, G.K.; Khan, A.; El-Toni, A.M.; Rashid, M.H. Shape-tunable CuO-Nd(OH)3 nanocomposites with excellent adsorption capacity in organic dye removal and regeneration of spent adsorbent to reduce secondary waste. J. Hazard. Mater. 2019, 380, 120838. [Google Scholar] [CrossRef]
- Sun, B.; Yuan, Y.; Li, H.; Li, X.; Zhang, C.; Guo, F.; Liu, X.; Wang, K.; Zhao, X. Waste-cellulose-derived porous carbon adsorbents for methyl orange removal. Chem. Eng. J. 2019, 371, 55–63. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]




| Adsorbent | Dye(s) | Design of the Experiment | Kinetic Model | Isotherm Model | Surface Area (m2/g) | qe/qmax (mg/g) | %R | Ref |
|---|---|---|---|---|---|---|---|---|
| Raw green tea waste (RGTW) Thermally treated green tea waste at 500 °C (TTGTW500) | Methylene blue (MB) | Multivariate analysis, 2k-FFD | PSO * | Freundlich | 3.84 30.71 | 68.28 69.01 | 96.58 98.07 | Current approach |
| Green tea dredge (carbonized green tea waste (CGT)) | Remazol brilliant yellow | Univariate analysis | PSO * | Langmuir | ND * | 40.65 | ND ** | [20] |
| Green tea leaf powder (GTLP) | Raw wastewater (90–95% reactive dye, 5–10% dispersive dyes) | Univariate analysis | ND ** | Modified Freundlich isotherm and intraparticle diffusion | 1.99 | 775 ADMI g−1 *** | ND ** | [21] |
| Superparamagnetic Fe3O4 nanoparticles coated with green tea polyphenols (GTP): (Fe3O4@GTPs NPs) | Methylene blue | Univariate analysis | PSO * | Langmuir | 126.79 | 7.25 | 95% | [22] |
| Waste green tea (WGT), untreated Six chemical treatments were used (four acidic—H3PO4, H2SO4, HCl, and tartaric acid—one oxidant—H2O2—and one basic—NaOH) | Malachite green | Univariate analysis | ND ** | ND ** | ND ** | ND ** | 89–95% | [23] |
| Iron-based nanoparticles with extract from green tea (GTFe) Superparamagnetic iron oxide nanoparticles (smGT) derived from GTFe | Malachite green | Univariate analysis | ND ** | ND ** | ND ** | ND ** | 93% | [24] |
| Independent Factors | Levels | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −1, Low | 0, Ct Pt | +1, High | |||||||||||
| pH (pH, A, pH unit) | 5.00 | 7.00 | 9.00 | ||||||||||
| Adsorbent (GTW) dose (AD, B, mg/50 mL) | 50 | 150 | 250 | ||||||||||
| MB dye concentration (DC, C, ppm) | 10 | 20 | 30 | ||||||||||
| Contact time (CT, D, min) | 5 | 32.5 | 60 | ||||||||||
| Run | * Blk | Variables | RGTW | TTGTW500 | |||||||||
| %R | qe | %R | qe | ||||||||||
| pH | AD | DC | CT | Obs. | Pred. | Obs. | Pred. | Obs. | Pred. | Obs. | Pred. | ||
| 01 | 1 | + | + | + | − | 90.79 | 91.67 | 2.26 | 2.31 | 25.07 | 25.63 | 0.63 | 0.56 |
| 02 | 1 | 0 | 0 | 0 | 0 | 92.90 | 93.73 | 3.91 | 3.63 | 52.30 | 51.80 | 2.40 | 2.33 |
| 03 | 1 | + | + | − | + | 82.52 | 83.10 | 2.24 | 2.25 | 95.26 | 96.67 | 2.38 | 2.45 |
| 04 | 1 | + | − | − | − | 89.66 | 89.93 | 11.06 | 10.40 | 58.84 | 58.37 | 7.33 | 7.30 |
| 05 | 1 | − | + | − | − | 96.53 | 97.58 | 2.41 | 2.43 | 96.02 | 94.17 | 2.40 | 2.27 |
| 06 | 1 | − | − | − | + | 88.32 | 87.99 | 10.97 | 9.73 | 62.73 | 63.04 | 7.74 | 7.91 |
| 07 | 1 | 0 | 0 | 0 | 0 | 94.60 | 93.73 | 3.50 | 3.63 | 53.40 | 51.80 | 2.60 | 2.33 |
| 08 | 1 | + | − | + | + | 79.09 | 78.44 | 9.89 | 9.72 | 3.20 | 4.58 | 0.31 | 0.55 |
| 09 | 1 | − | − | + | − | 36.00 | 36.07 | 4.27 | 4.55 | 5.33 | 5.20 | 0.67 | 0.70 |
| 10 | 1 | − | + | + | + | 92.62 | 92.89 | 2.31 | 2.31 | 46.09 | 47.47 | 1.15 | 1.29 |
| 11 | 2 | + | + | + | + | 89.89 | 89.59 | 2.24 | 2.21 | 40.24 | 37.96 | 1.00 | 0.87 |
| 12 | 2 | − | + | + | − | 89.94 | 89.05 | 2.36 | 2.32 | 36.08 | 36.41 | 0.91 | 0.97 |
| 13 | 2 | + | + | − | − | 92.05 | 90.94 | 2.38 | 2.35 | 89.76 | 90.42 | 2.24 | 2.37 |
| 14 | 2 | + | − | + | − | 69.34 | 69.31 | 8.58 | 7.97 | 0.000 | 0.000 | 0.00 | 0.00 |
| 15 | 2 | − | − | − | − | 79.74 | 79.43 | 5.99 | 5.67 | 60.65 | 62.19 | 7.51 | 7.54 |
| 16 | 2 | 0 | 0 | 0 | 0 | 94.01 | 93.73 | 3.91 | 3.63 | 51.05 | 51.80 | 2.12 | 2.33 |
| 17 | 2 | 0 | 0 | 0 | 0 | 93.40 | 93.73 | 3.30 | 3.63 | 50.45 | 51.80 | 2.20 | 2.33 |
| 18 | 2 | − | − | + | + | 60.19 | 61.03 | 6.11 | 5.10 | 12.19 | 11.12 | 1.52 | 1.29 |
| 19 | 2 | − | + | − | + | 96.58 | 96.08 | 2.41 | 2.43 | 98.07 | 97.86 | 2.44 | 2.37 |
| 20 | 2 | + | − | − | + | 91.61 | 91.92 | 11.41 | 15.63 | 62.91 | 61.53 | 7.84 | 7.66 |
| Adsorbent | %R | qe (mg/g) |
|---|---|---|
| RGTW | 93.05 | 2.41 |
| TTGTW250 | 87.28 | 2.26 |
| TTGTW300 | 28.16 | 0.73 |
| TTGTW400 | 35.77 | 0.87 |
| TTGTW500 | 57.90 | 1.50 |
| R2 % | R2—adj % | R2—pred % |
|---|---|---|
| 99.70 | 99.28 | 97.51 |
| 99.36 | 98.78 | 98.18 |
| 99.85 | 99.59 | 98.24 |
| 99.69 | 99.16 | 96.97 |
| Optimum Conditions | Maximum %R | Maximum qe (mg/g) | d-Value | |||
|---|---|---|---|---|---|---|
| pH | AD (mg/50 mL) | DC (ppm) | CT (min) | |||
| RGTW | ||||||
| 5.0 | 250.0 | 10.0 | 5.0 | 97.58 | 1.0000 | |
| 9.0 | 50.0 | 10.0 | 60.0 | 15.63 | 1.0000 | |
| TTGTW500 | ||||||
| 5.0 | 250.0 | 10.0 | 60.0 | 97.86 | 0.9979 | |
| 5.0 | 50.0 | 10.0 | 60.0 | 7.91 | 1.0000 | |
| Sample Code | N (%) | C (%) | H (%) |
|---|---|---|---|
| RGTW | 4.094 | 46.151 | 6.435 |
| TTGTW250 | 5.694 | 55.885 | 6.287 |
| TTGTW300 | 5.958 | 60.186 | 5.138 |
| TTGTW400 | 5.253 | 64.551 | 3.890 |
| TTGTW500 | 6.378 | 72.723 | 2.952 |
| Parameters | RGTW | TTGTW250 | TTGTW300 | TTGTW400 | TTGTW500 |
|---|---|---|---|---|---|
| Langmuir SA (m2/g) | 3.84 | 4.51 | 6.61 | 8.94 | 30.71 |
| Total pore volume (cm3/g) | 0.0096 | 0.0113 | 0.0124 | 0.017 | 0.037 |
| Average pore radius (°A) | 74.2 | 87.5 | 54.0 | 41.2 | 33.7 |
| Isotherm | Equations (Nonlinear Forms) | Parameters | RGTW | TTGTW500 |
|---|---|---|---|---|
| Langmuir | (mg/g) | 68.28 | 69.01 | |
| (L·mole−1) | 0.0157 | 0.0367 | ||
| R2 | 0.9833 | 0.9796 | ||
| Freundlich | 0.619 | 0.610 | ||
| (mole/g)(L/mole)1/n | 2.476 | 4.298 | ||
| R2 | 0.9878 | 0.9809 | ||
| Temkin | (J/mole) | 442.3 | 347.6 | |
| (L/mole) | 1.969 | 2.347 | ||
| R2 | 0.7139 | 0.8108 | ||
| D-R | . exp (−β.) | 7 × 10−9 | 1.8 × 10−8 | |
| (kJ/mole) | 8.45 | 5.27 | ||
| (mg/g) | 36.34 | 39.67 | ||
| R2 | 0.8535 | 0.9041 |
| Model | Parameter | RGTW | TTGTW500 |
|---|---|---|---|
| Pseudo-first order (PFO) = k1(qe−qt) | K1 (min−1) | 0.931 | 0.674 |
| qe (mg/g) | 44.04 | 42.66 | |
| R2 | 0.9011 | 0.9788 | |
| Pseudo-second order (PSO) = k2(qe−qt)2 where K2 is the rate constant (g·mg−1·min−1) | K2 (g·mg−1·min−1) | 0.038 | 0.024 |
| qe (mg/g) | 46.35 | 45.12 | |
| R2 | 0.9619 | 0.9861 | |
| Elovich model qt = where qt is the adsorbed quantity at time t, while α and β are the initial sorption concentration rate (mg·g−1·min−1) and desorption constant (g/mg), respectively | α | 1.27 × 104 | 1.28 × 104 |
| β | 0.241 | 0.201 | |
| R2 | 0.7921 | 0.8238 | |
| Weber–Morris intraparticle diffusion model , where KI is the intraparticle diffusion rate constant (mg·g−1·min−0.5) and C is the boundary thickness effect | KI | 2.622 | 2.769 |
| C | 33.44 | 38.76 | |
| R2 | 0.5877 | 0.6084 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Azazy, M.; El-Shafie, A.S.; Al-Shaikh Yousef, B. Green Tea Waste as an Efficient Adsorbent for Methylene Blue: Structuring of a Novel Adsorbent Using Full Factorial Design. Molecules 2021, 26, 6138. https://doi.org/10.3390/molecules26206138
El-Azazy M, El-Shafie AS, Al-Shaikh Yousef B. Green Tea Waste as an Efficient Adsorbent for Methylene Blue: Structuring of a Novel Adsorbent Using Full Factorial Design. Molecules. 2021; 26(20):6138. https://doi.org/10.3390/molecules26206138
Chicago/Turabian StyleEl-Azazy, Marwa, Ahmed S. El-Shafie, and Bayan Al-Shaikh Yousef. 2021. "Green Tea Waste as an Efficient Adsorbent for Methylene Blue: Structuring of a Novel Adsorbent Using Full Factorial Design" Molecules 26, no. 20: 6138. https://doi.org/10.3390/molecules26206138
APA StyleEl-Azazy, M., El-Shafie, A. S., & Al-Shaikh Yousef, B. (2021). Green Tea Waste as an Efficient Adsorbent for Methylene Blue: Structuring of a Novel Adsorbent Using Full Factorial Design. Molecules, 26(20), 6138. https://doi.org/10.3390/molecules26206138








