Development of Ecofriendly Derivative Spectrophotometric Methods for the Simultaneous Quantitative Analysis of Remogliflozin and Vildagliptin from Formulation
Abstract
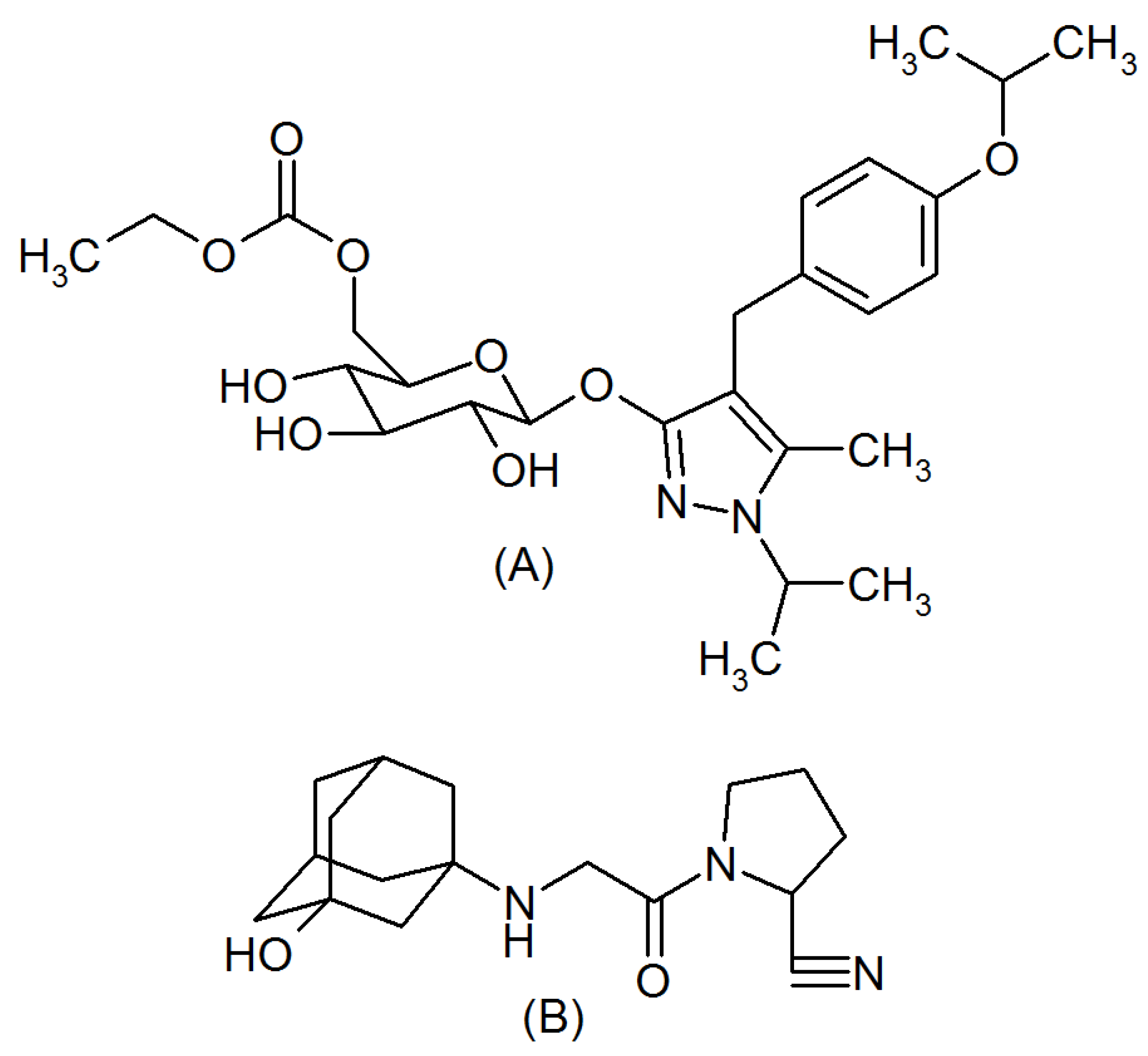
:1. Introduction
2. Results and Discussion
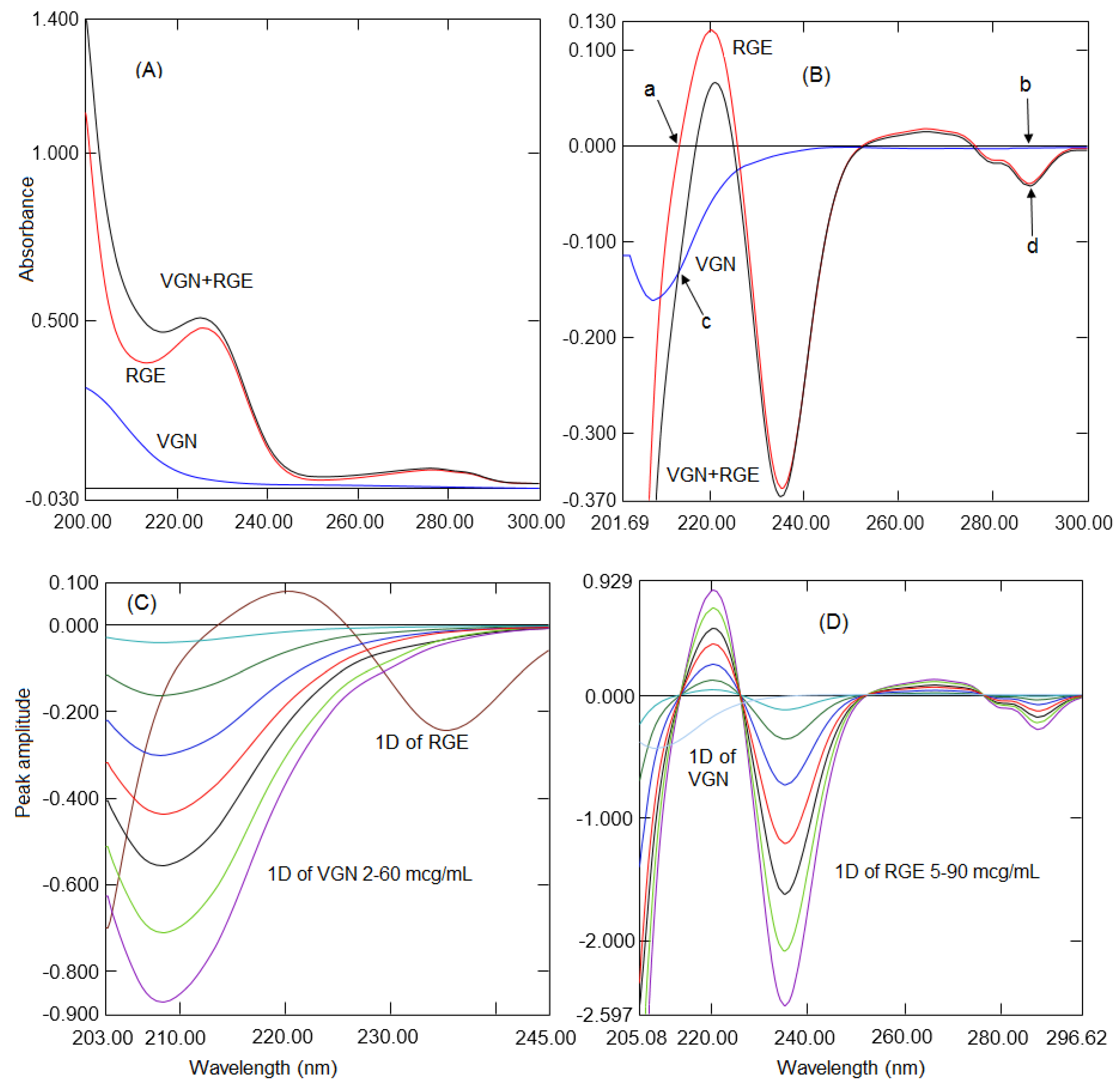
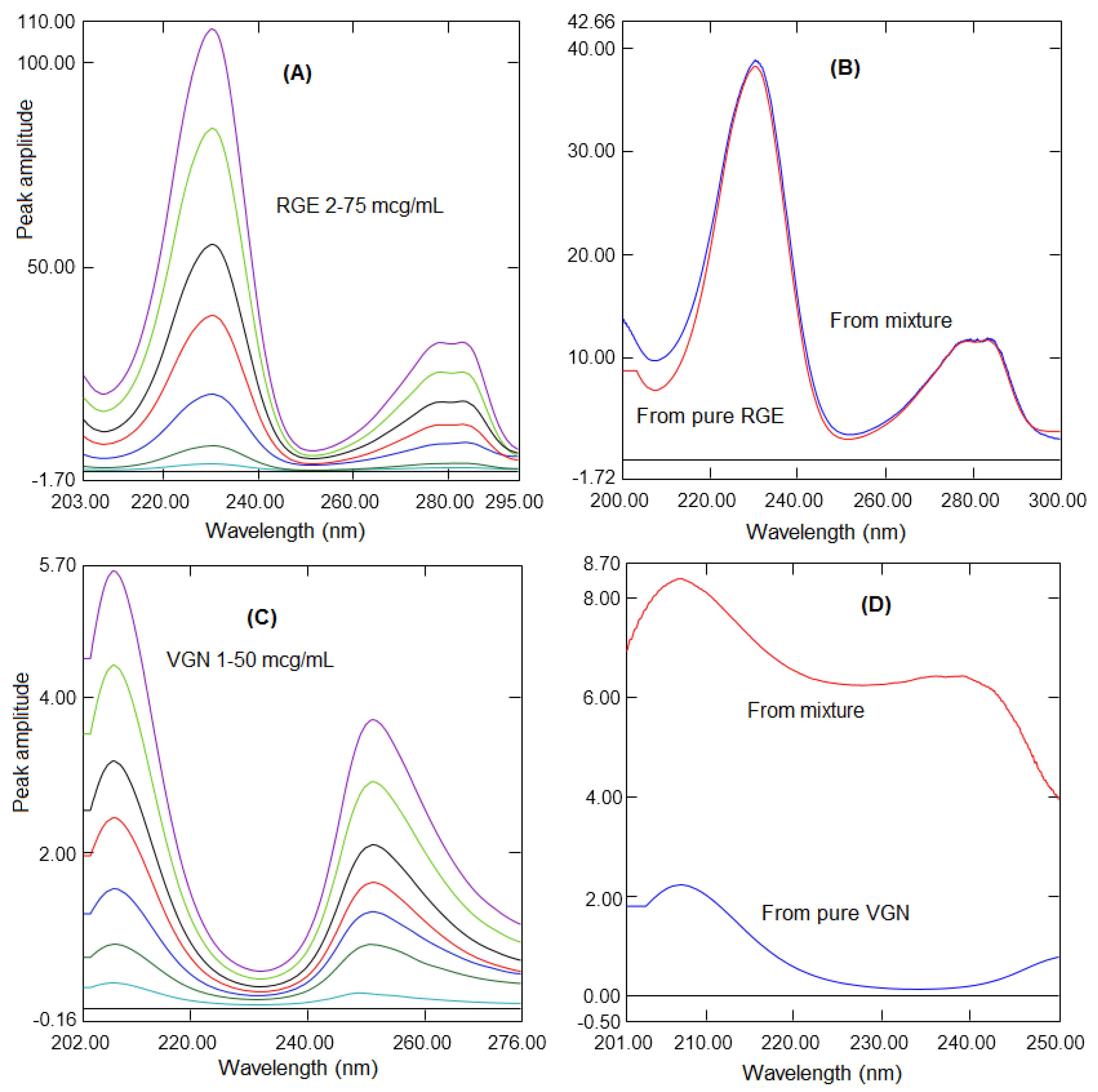
2.1. Ratio Difference Absorption Method
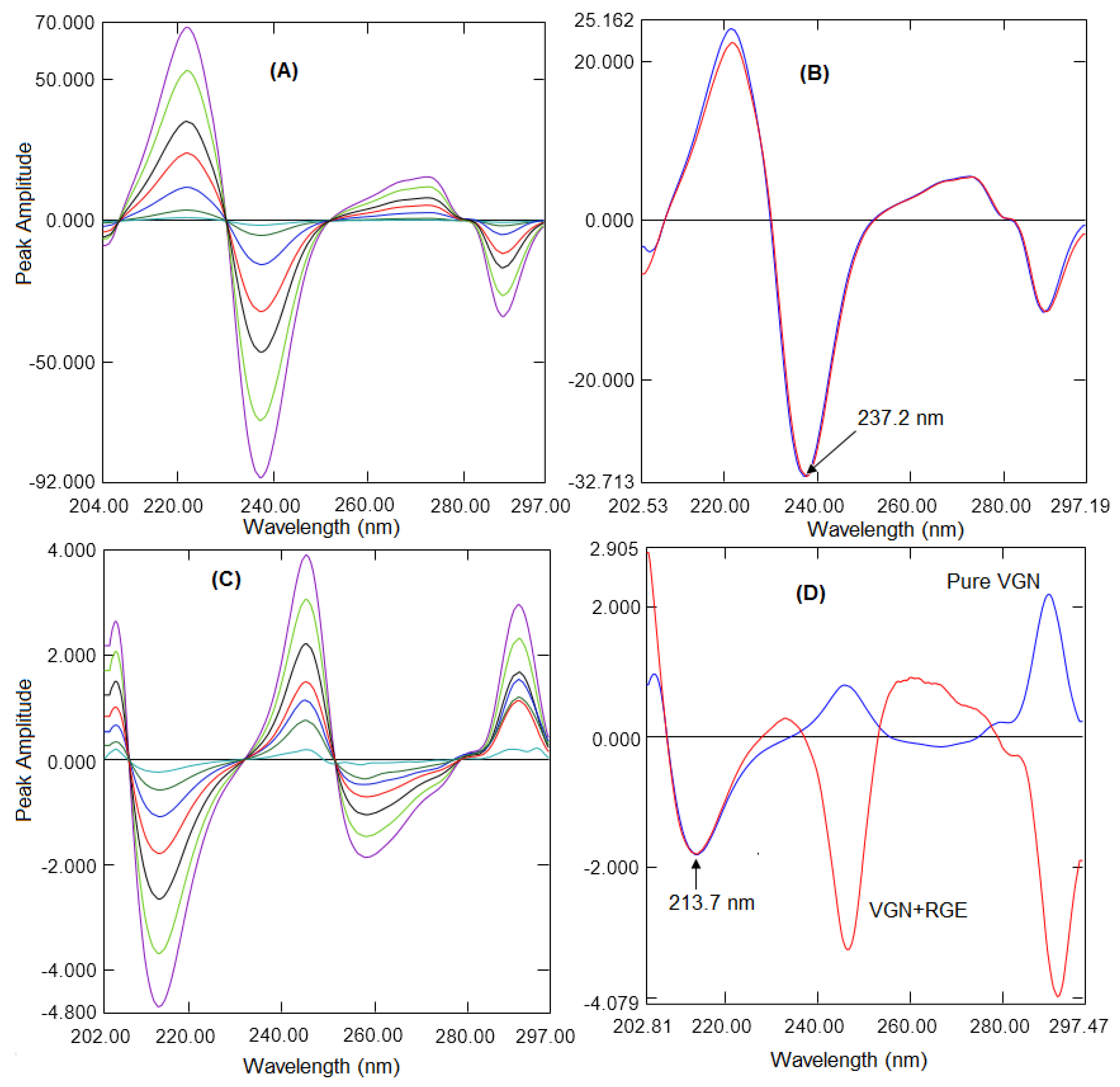
2.2. Ratio First Derivative Method
2.3. Method Validation
2.3.1. Linearity Range
2.3.2. Accuracy
2.3.3. Precision
2.3.4. Limit of Detection and Quantification
2.3.5. Specificity
2.4. Application of the Optimized Methods for the Formulation
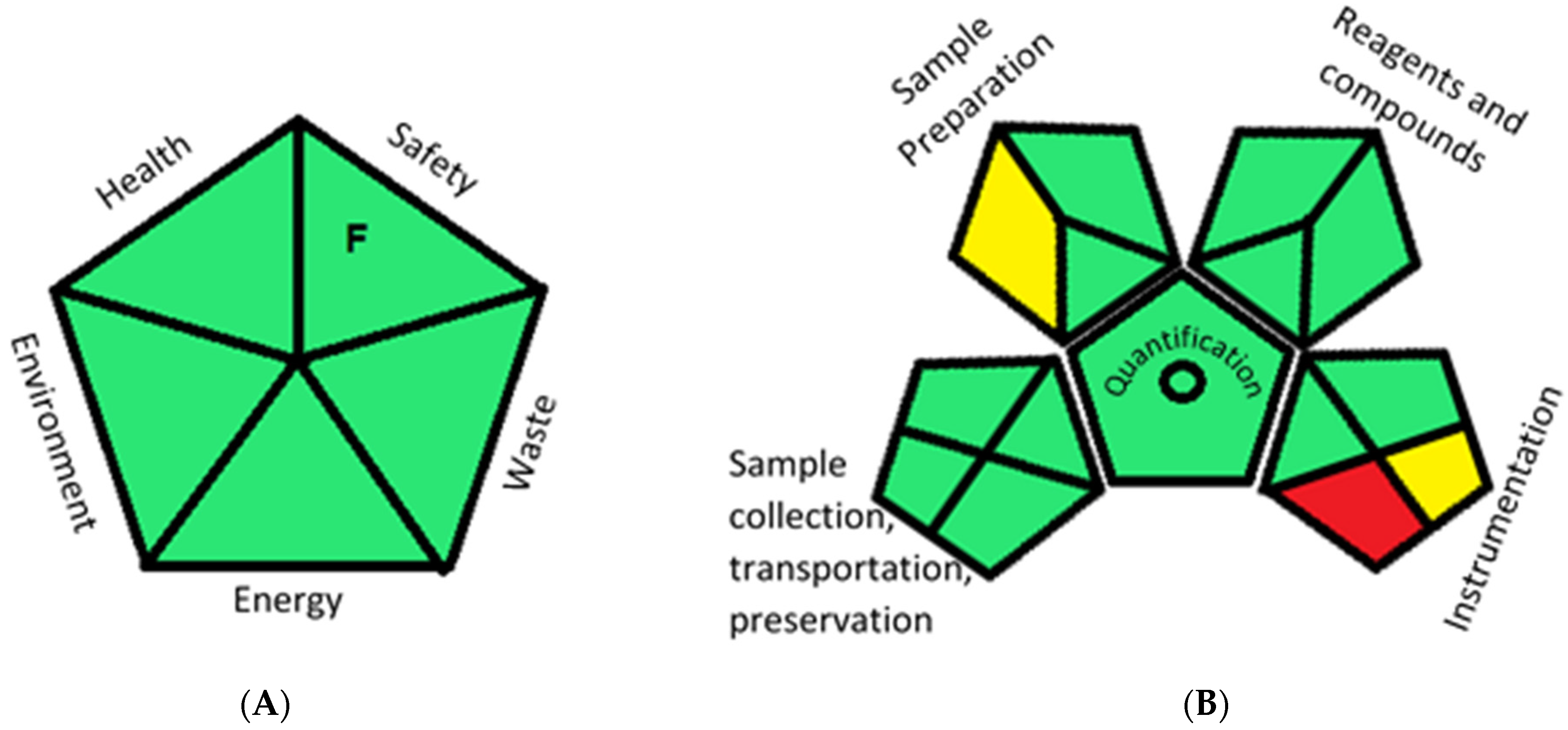
2.5. Greenness Evaluation of the Optimized Methods
3. Materials and Methods
3.1. Instruments
3.2. Chemicals
3.3. Preparation of the Standard Solutions
3.4. Preparation of the Sample Solutions
3.5. Theory of the Ratio Derivatization Technique
3.6. Procedure
3.6.1. First Derivative Spectrophotometric Method (FDS)
3.6.2. Ratio Absorption Difference Method (RAD)
3.6.3. Ratio First Derivative Absorption Method (RFA)
3.6.4. Application of the Optimized Methods to the Formulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Klein, B.E.; Klein, R.; Moss, S.E.; Cruickshanks, K.J. Parental history of diabetes in a population-based study. Diabetes Care 1996, 19, 827–830. [Google Scholar] [CrossRef]
- Ahsan, S. Effectiveness of Remogliflozin and Vildagliptin Combination in Type 2 Diabetes Mellitus Patients Uncontrolled on Triple Oral Drug Therapy. Endocr. Pract. 2021, 27, 6. [Google Scholar] [CrossRef]
- Markham, A. Remogliflozin Etabonate: First global approval. Drugs 2019, 79, 1157–1161. [Google Scholar] [CrossRef]
- Joshi, S.S.; Singh, T.; Newby, D.E.; Singh, J. Sodium-glucose co-transporter 2 inhibitor therapy: Mechanisms of action in heart failure. Heart 2021, 107, 1032–1038. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.; Bonaca, M.P. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Kawanami, D.; Takashi, Y.; Takahashi, H.; Motonaga, R.; Tanabe, M. Renoprotective Effects of DPP-4 Inhibitors. Antioxidants 2021, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Schiapaccassa, A.; Maranhão, P.A.; de Souza, M.G.C. 30-days effects of vildagliptin on vascular function, plasma viscosity, inflammation, oxidative stress, and intestinal peptides on drug-naïve women with diabetes and obesity: A randomized head-to-head metformin-controlled study. Diabetol. Metab. Syndr. 2019, 11, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dave, V.; Paresh, P. Method development and validation of UV spectrophotometric estimation of remogliflozin etabonate in bulk and its tablet dosage form. Res. J. Pharm. Technol. 2021, 14, 2042–2044. [Google Scholar] [CrossRef]
- Tayade, A.B.; Patil, A.S.; Shirkhedkar, A.A. Development and Validation of Zero Order UV-Spectrophotometric Method by Area under Curve Technique and High Performance Thin Layer Chromatography for the Estimation of Remogliflozin Etabonate in Bulk and In-House Tablets. Invent. Rapid Pharm. Anal. Qual. Assur. 2019, 3, 1–5. [Google Scholar]
- Bhatkar, T.; Badkhal, A.V.; Bhajipale, N.S. Stability Indicating RP-HPLC Method Development and Validation for the Estimation of Remogliflozin Etabonate in Bulk and Pharmaceutical Dosage Form. Int. J. Pharm. Res. 2020, 12, 4197–4207. [Google Scholar]
- Shah, D.A.; Gondalia, I.I.; Patel, V.B.; Mahajan, A.; Chhalotiya, U.K. Stability indicating liquid chromatographic method for the estimation of remogliflozin etabonate. J. Chem. Metrol. 2020, 14, 125–132. [Google Scholar] [CrossRef]
- Sigafoos, J.F.; Bowers, G.D.; Castellino, S.; Culp, A.G.; Wagner, D.S.; Reese, M.J.; Humphreys, J.E.; Hussey, E.K.; Semmes, R.L.C.; Kapur, A.; et al. Assessment of the drug interaction risk for remogliflozin etabonate, a sodium-dependent glucose cotransporter-2 inhibitor: Evidence from in vitro, human mass balance, and ketoconazole interaction studies. Drug Metab. Dispos. 2012, 40, 2090–2101. [Google Scholar] [CrossRef] [Green Version]
- Attimarad, M.; Nair, A.B.; Sreeharsha, N.; Al-Dhubiab, B.E.; Venugopala, K.N.; Shinu, P. Development and Validation of Green UV Derivative Spectrophotometric Methods for Simultaneous Determination Metformin and Remogliflozin from Formulation: Evaluation of Greenness. Int. J. Environ. Res. Public Health 2021, 18, 448. [Google Scholar] [CrossRef] [PubMed]
- Attimarad, M.; Elgorashe, R.E.E.; Subramaniam, R.; Islam, M.M.; Venugopala, K.N.; Nagaraja, S.; Balgoname, A.A. Development and Validation of Rapid RP-HPLC and Green Second-Derivative UV Spectroscopic Methods for Simultaneous Quantification of Metformin and Remogliflozin in Formulation using Experimental Design. Separations 2020, 7, 59. [Google Scholar] [CrossRef]
- Tammisetty, M.; Challa, B.R.; Puttagunta, S.B. A novel analytical method for the simultaneous estimation of remogliflozin and metformin hydrochloride by UPLC/PDA in bulk and formulation. Application to the estimation of product traces. Turk. J. Pharm. Sci. 2020, 39699, 296–305. [Google Scholar] [CrossRef]
- Dayoub, L.A.; Amali, F. Development of a new visible Spectrophotometric analytical method for determination of Vildagliptin in bulk and Pharmaceutical dosage forms. Res. J. Pharm. Technol. 2020, 13, 2807–2810. [Google Scholar] [CrossRef]
- Kumari, B.; Khansili, A. Analytical Method Development and Validation of UV-visible Spectrophotometric Method for the Estimation of Vildagliptin in Gastric Medium. Drug Res. 2020, 70, 417–423. [Google Scholar] [CrossRef]
- Waghulde, M.; Naik, J. Development and validation of analytical method for Vildagliptin encapsulated poly-ε-caprolactone microparticles. Mater. Today Proc. 2018, 5, 958–964. [Google Scholar] [CrossRef]
- Patil, K.R.; Deshmukh, T.A.; Patil, V.R. A stability indicating HPTLC method development and validation for analysis of Vildagliptin as bulk drug and from its pharmaceutical dosage form. Int. J. Pharm. Sci. Res. 2020, 11, 2310–2316. [Google Scholar] [CrossRef]
- Giordani, C.F.A.; Campanharo, S.; Wingert, N.R.; Bueno, L.M.; Manoel, J.W.; Garcia, C.V.; Volpato, N.M.; Steppe, M. UPLC-ESI/Q-TOF MS/MS Method for Determination of Vildagliptin and its Organic Impurities. J. Chromatogr. Sci. 2020, 58, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghany, M.; Abdel-Aziz, O.; Ayad, M.F.; Tadros, M.M. Validation of Different Spectrophotometric Methods for Determination of Vildagliptin and Metformin in Binary Mixture. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 125, 175–182. [Google Scholar] [CrossRef]
- Moneeb, M.S. Spectrophotometric and spectrofluorimetric methods for the determination of saxagliptin and vildagliptin in bulk and pharmaceutical preparations. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, N.K.; Jat, R.; Bhangale, J.O. Analysis of vildagliptin and nateglinide for simultaneous estimation using spectro-chromatographic methods. Eur. J. Mol. Clin. Med. 2020, 7, 741–755. [Google Scholar]
- Shakoor, A.; Ahmed, M.; Ikram, R.; Hussain, S.; Tahir, A.; Jan, B.M.; Adnan, A. Stability-indicating RP-HPLC method for simultaneous determination of metformin hydrochloride and vildagliptin in tablet and biological samples. Acta Chromatogr. 2020, 32, 39–43. [Google Scholar] [CrossRef]
- Khalil, S.; Bushara, A.; Farag, H. New Analytical Methods for the Determination of Two Gliptin Drugs in Pharmaceutical Formulations and Urine Samples. Am. J. Pharm. Technol. Res. 2020, 10, 31–43. [Google Scholar]
- Abdel Hady, K.K.; Abdel Salam, R.A.; Hadad, G.M.; Abdel Hameed, E.A. Simultaneous HPLC determination of vildagliptin, ampicillin, sulbactam and metronidazole in pharmaceutical dosage forms and human urine. J. Iran. Chem. Soc. 2021, 18, 729–738. [Google Scholar] [CrossRef]
- Kamal, A.H.; El-Malla, S.F.; Hammad, S.F. A Review on UV spectrophotometric methods for simultaneous multicomponent analysis. Eur. J. Pharm. Med. Res. 2016, 3, 348–360. [Google Scholar]
- Salinas, F.; Nevado, J.J.B.; Mansilla, A.E. A new spectrophotometric method for quantitative multicomponent analysis resolution of mixtures of salicylic and salicyluric acids. Talanta 1990, 37, 347–351. [Google Scholar] [CrossRef]
- Chohan, M.S.; Elgorashe, R.E.E.; Balgoname, A.A.; Attimarad, M.; SreeHarsha, N.; Venugopala, K.N.; Nair, A.B.; Pottathil, S. Eco-friendly Derivative UV Spectrophotometric Methods for Simultaneous Determination of Diclofenac Sodium and Moxifloxacin in Laboratory Mixed Ophthalmic Preparation. Indian J. Pharm. Edu. Res. 2019, 53, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, N.M.; Chavada, V.D.; Sanyal, M.; Shrivastav, P.S. Manipulating ratio spectra for the spectrophotometric analysis of diclofenac sodium and pantoprazole sodium in laboratory mixtures and tablet formulation. Sci. World J. 2014, 2014, 495739. [Google Scholar] [CrossRef] [PubMed]
- Attimarad, M.; Chohan, M.S.; Balgoname, A.A. Simultaneous Determination of Moxifloxacin and Flavoxate by RP-HPLC and Ecofriendly Derivative Spectrophotometry Methods in Formulations. Int. J. Environ. Res. Public Health 2019, 16, 1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raynie, D.; Driver, J. Green Assessment of Chemical Methods. In Proceedings of the 13th Annual Green Chemistry and Engineering Conference, College Park, MD, USA; 2009. [Google Scholar]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Abdalah, N.A.; Fathy, M.E.; Tolba, M.M.; El-Brashy, A.M.; Ibrahim, F.A. Green spectrofluorimetric assay of dantrolene sodium via reduction method: Application to content uniformity testing. R. Soc. Open Sci. 2021, 14, 210562. [Google Scholar] [CrossRef]
- Lotfy, H.M.; Hegazy, M.A. Simultaneous determination of some cholesterol-lowering drugs in their binary mixture by novel spectrophotometric methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 113, 107–114. [Google Scholar] [CrossRef] [PubMed]
| Validation Parameters | Remogliflozin | Vildagliptin | ||||
|---|---|---|---|---|---|---|
| FDS | RDS | RFD | FDS | RDS | RFD | |
| Wavelength (nm) | 287.7 | 230.3–251.4 | 237.2 | 213.7 | 207.2–230.6 | 213.7 |
| Linearity range (µg/mL) | 5–90 | 2–75 | 2–75 | 2–60 | 1–50 | 1–50 |
| Slope | 0.0031 | 1.328 | 1.226 | 0.012 | 0.0983 | 0.0920 |
| Intercept | −0.0051 | −1.221 | −0.884 | 0.008 | 0.2489 | 0.0841 |
| Regression coefficient (r2) | 0.9997 | 0.9998 | 0.9997 | 0.9996 | 0.9990 | 0.9994 |
| LOD (µg/mL) | 1.361 | 0.583 | 0.408 | 0.484 | 0.272 | 0.176 |
| LOQ (µg/mL) | 4.126 | 1.768 | 1.236 | 1.469 | 0.827 | 0.535 |
| Accuracy (Mean % ± SD) | 99.41 ± 1.170 | 99.13 ± 0.665 | 100.82 ± 0.910 | 99.52 ± 0.834 | 99.88 ± 1.655 | 101.07 ± 0.729 |
| Precision (%RSD) | ||||||
| Intraday | 0.730 | 1.026 | 1.843 | 0.610 | 0.871 | 0.728 |
| Interday | 0.937 | 1.383 | 0.611 | 1.748 | 0.725 | 1.287 |
| Laboratory Prepared Mixture (µg/mL) | Remogliflozin (% Recovery) | Vildagliptin (% Recovery) | |||||
|---|---|---|---|---|---|---|---|
| RGE | VGN | FDS | RDS | RFD | FDS | RDS | RFD |
| 10 | 50.00 | 99.20 | 101.60 | 98.50 | 98.30 | 98.22 | 101.04 |
| 40 | 50.00 | 97.93 | 100.70 | 99.43 | 98.58 | 99.06 | 100.78 |
| 40 | 5.00 | 100.30 | 96.48 | 100.20 | 98.60 | 99.40 | 99.80 |
| 70 | 5.00 | 99.24 | 98.06 | 99.03 | 101.20 | 100.40 | 98.60 |
| 70 | 25.00 | 100.67 | 98.73 | 98.21 | 99.24 | 99.04 | 101.36 |
| Across Mean | 99.47 | 99.11 | 99.07 | 99.18 | 99.27 | 100.32 | |
| SD | 0.964 | 1.840 | 0.702 | 1.054 | 0.705 | 1.004 | |
| Formulation Concentration | Remogliflozin (Mean %± SD) | Vildagliptin (Mean % ± SD) | |||||
|---|---|---|---|---|---|---|---|
| RGE | VGN | FDS | RDS | RFD | FDS | RDS | RFD |
| 100 mg | 50 mg | 99.42 ± 0.985 | 99.62 ± 0.788 | 99.10 ± 0.76 | 99.77 ± 1.563 | 100.04 ± 1.384 | 100.19 ± 0.797 |
| Standard Addition Method | |||||||
| Amount Added (µg/mL) | Remogliflozin (% Recovery) | Vildagliptin (% Recovery) | |||||
| 10 µg/mL | 5 | 98.70 | 100.70 | 98.30 | 99.20 | 100.40 | 99.80 |
| 20 µg/mL | 10 | 100.95 | 98.85 | 99.05 | 101.90 | 98.20 | 101.30 |
| 30 µg/mL | 15 | 96.47 | 99.30 | 100.23 | 98.20 | 101.53 | 99.47 |
| Across Mean | 98.71 | 99.62 | 99.19 | 99.77 | 100.04 | 100.19 | |
| %RSD | 1.830 | 0.788 | 0.796 | 1.563 | 1.384 | 0.797 | |
| Category | UV Spectrophotometric Methods |
|---|---|
| Sample Preparation | |
| Collection (1) | In-line |
| Preservation (2) | Nil |
| Transport (3) | Nil |
| Storage (4) | Nil |
| Type of method: direct or indirect (5) | Direct (no sample preparation) |
| Scale of extraction (6) | Nil |
| Solvents/reagents used (7) | Green Solvents |
| Additional treatments (8) | Nil |
| Reagent and solvents | |
| Amount (9) | <10 mL |
| Health hazard (10) | Ethanol, slightly toxic and irritant NFPA score 1 |
| Safety hazard (11) | Ethanol, instability score 0, flammability score 1, no special hazard |
| Instrumentation | |
| Energy (12) | ≤0.1 kWh/sample |
| Occupational hazard (13) | Hermetic sealing of the analytical procedure |
| Waste (14) | 1–10 mL |
| Waste treatment (15) | No treatment |
| Quantification | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attimarad, M.; Venugopala, K.N.; Al-Dhubiab, B.E.; Elgorashe, R.E.E.; Shafi, S. Development of Ecofriendly Derivative Spectrophotometric Methods for the Simultaneous Quantitative Analysis of Remogliflozin and Vildagliptin from Formulation. Molecules 2021, 26, 6160. https://doi.org/10.3390/molecules26206160
Attimarad M, Venugopala KN, Al-Dhubiab BE, Elgorashe REE, Shafi S. Development of Ecofriendly Derivative Spectrophotometric Methods for the Simultaneous Quantitative Analysis of Remogliflozin and Vildagliptin from Formulation. Molecules. 2021; 26(20):6160. https://doi.org/10.3390/molecules26206160
Chicago/Turabian StyleAttimarad, Mahesh, Katharigatta N. Venugopala, Bandar E. Al-Dhubiab, Rafea Elamin Elgack Elgorashe, and Sheeba Shafi. 2021. "Development of Ecofriendly Derivative Spectrophotometric Methods for the Simultaneous Quantitative Analysis of Remogliflozin and Vildagliptin from Formulation" Molecules 26, no. 20: 6160. https://doi.org/10.3390/molecules26206160







