Actions of Camptothecin Derivatives on Larvae and Adults of the Arboviral Vector Aedes aegypti
Abstract
:1. Introduction
1.1. Vector-Borne Diseases and Pandemics
1.2. Control of Disease Vectors
1.3. Open Science and the MMV Pandemic Response Box
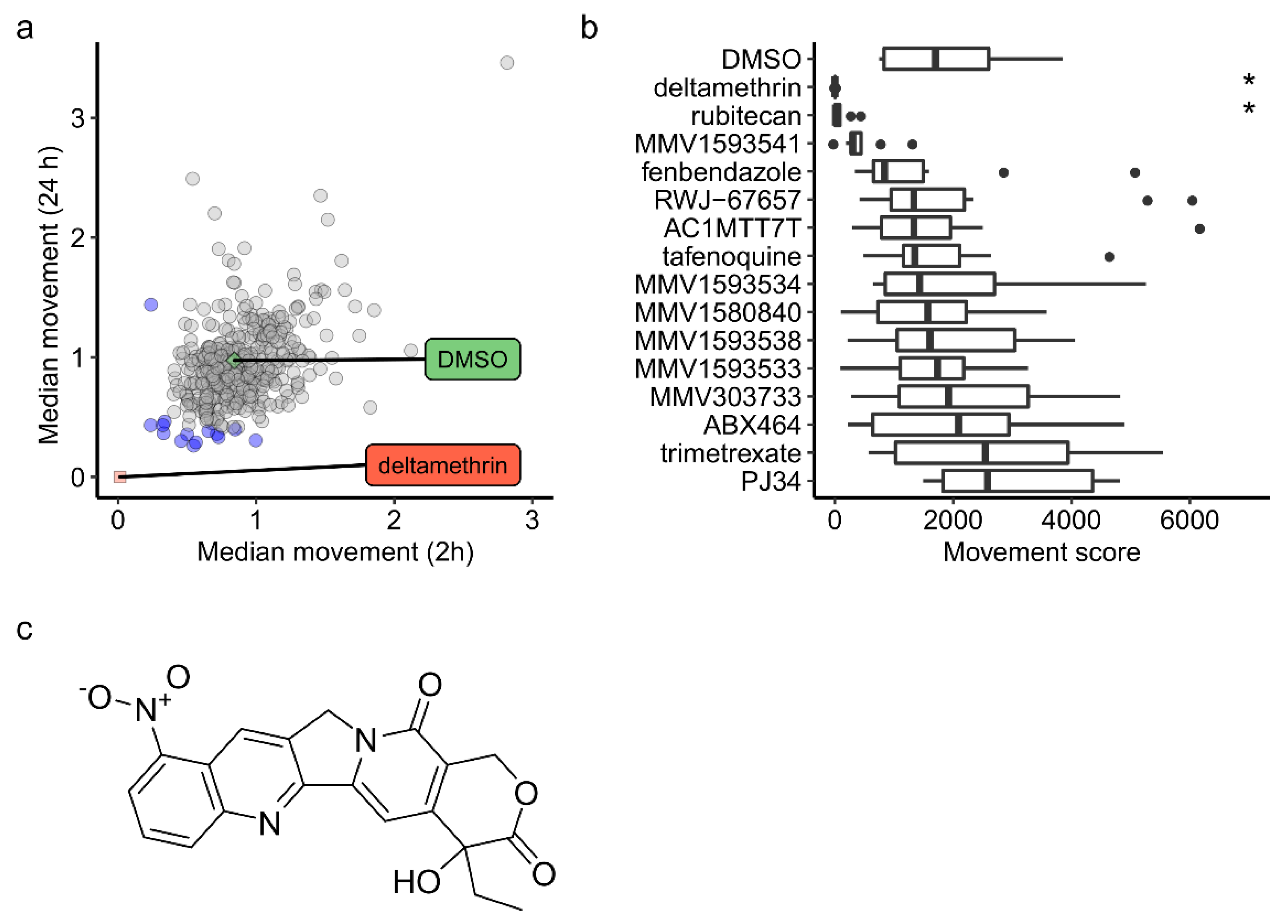
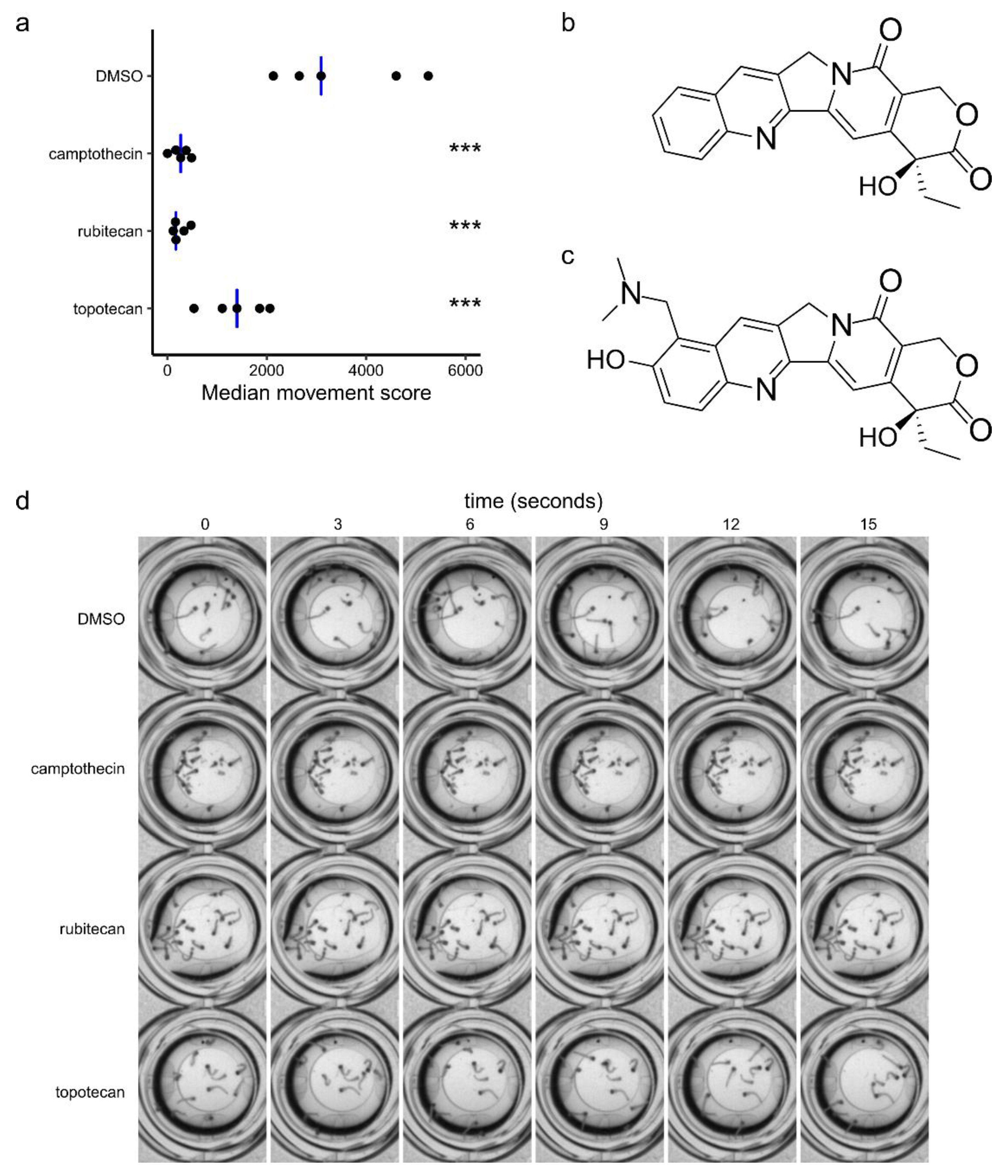
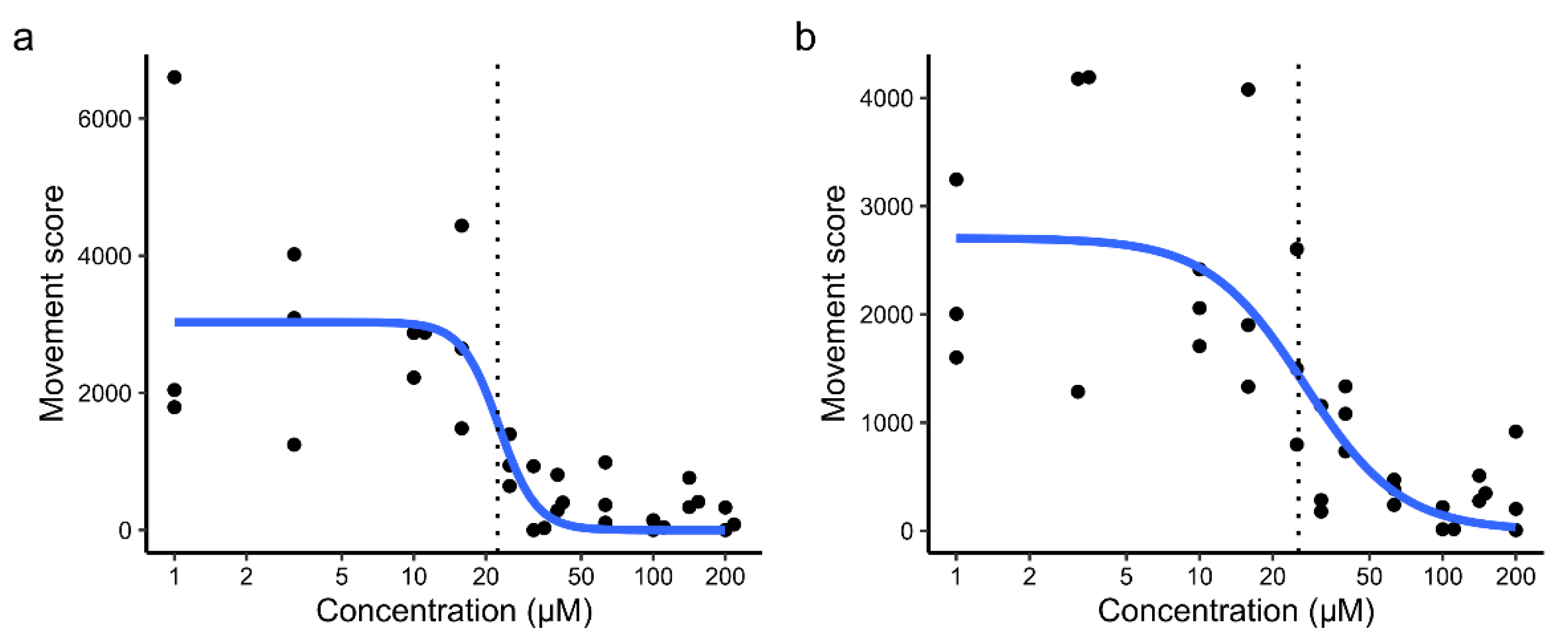
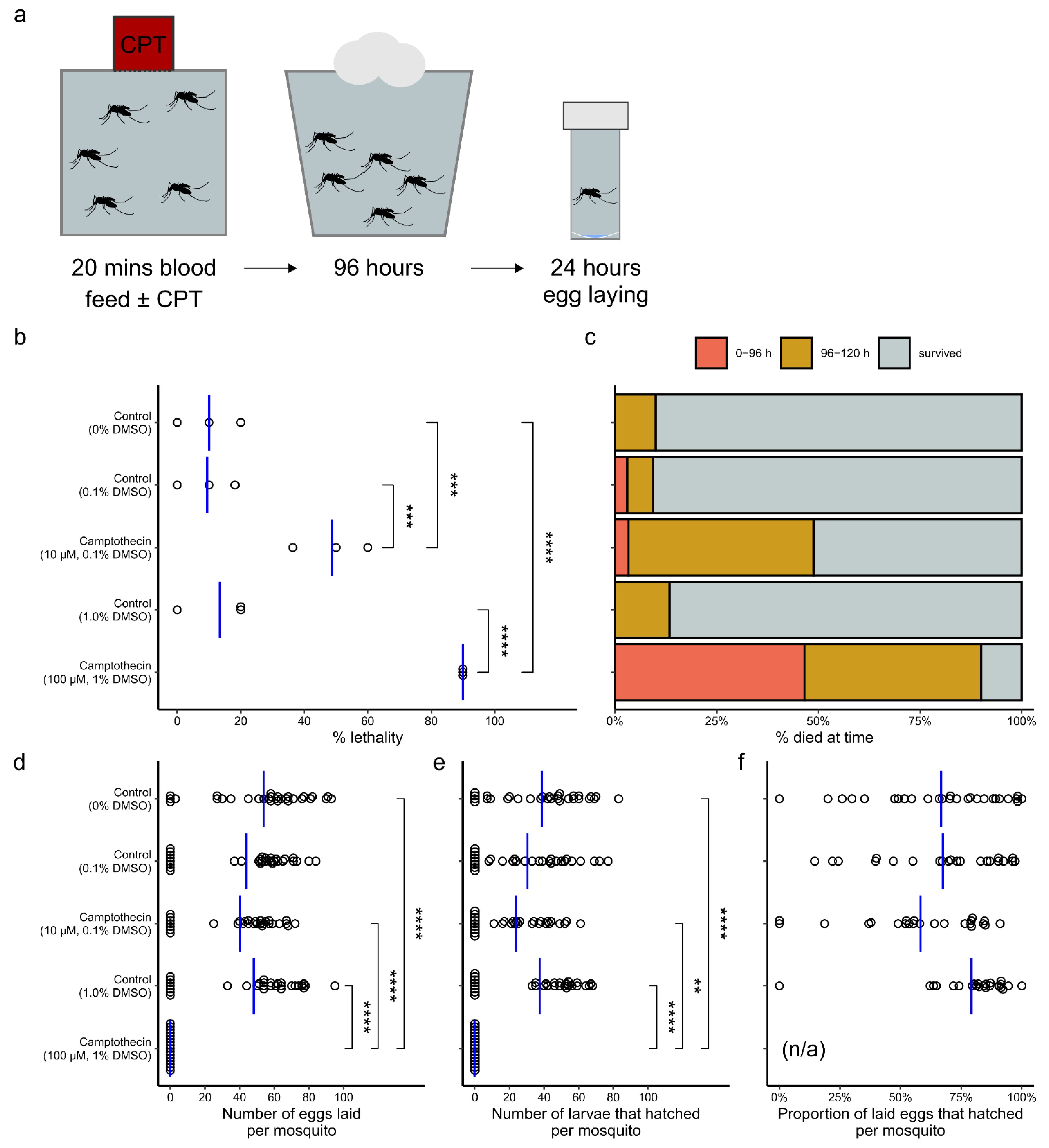
2. Results
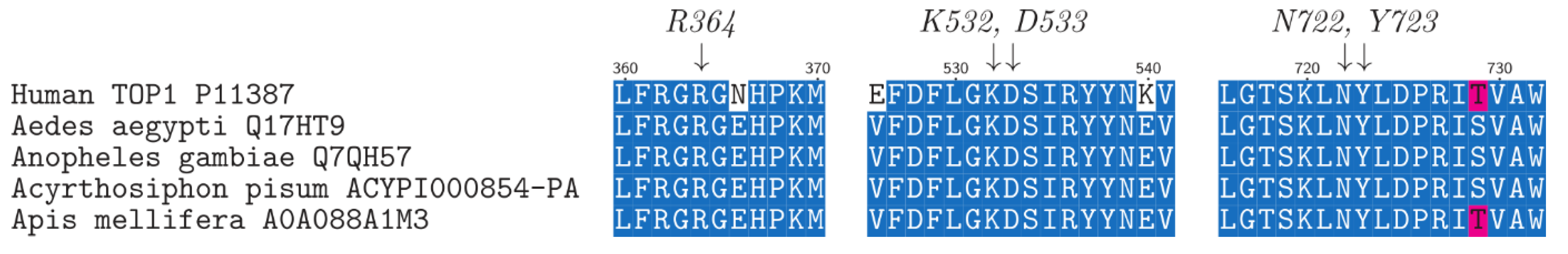
3. Discussion
3.1. Camptothecin Derivatives as Insecticides
3.2. Camptothecin Derivatives as Antivirals
3.3. Safety for Larvicidal Use
3.4. Use as an Adulticide
4. Materials and Methods
4.1. Larval Motility Assay
4.2. Adult Treatment Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bedford, J.; Farrar, J.; Ihekweazu, C.; Kang, G.; Koopmans, M.; Nkengasong, J. A new twenty-first century science for effective epidemic response. Nature 2019, 575, 130–136. [Google Scholar] [CrossRef]
- Shaw, W.R.; Catteruccia, F. Vector biology meets disease control: Using basic research to fight vector-borne diseases. Nat. Microbiol. 2019, 4, 20–34. [Google Scholar] [CrossRef]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- Musso, D.; Ko, A.I.; Baud, D. Zika virus infection—After the pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Hemingway, J.; Ranson, H.; Magill, A.; Kolaczinski, J.; Fornadel, C.; Gimnig, J.; Coetzee, M.; Simard, F.; Roch, D.K.; Hinzoumbe, C.K.; et al. Averting a malaria disaster: Will insecticide resistance derail malaria control? Lancet 2016, 387, 1785–1788. [Google Scholar] [CrossRef]
- Valle, D.; Bellinato, D.F.; Viana-Medeiros, P.F.; Lima, J.B.P.; Martins Junior, A. de J. Resistance to temephos and deltamethrin in Aedes aegypti from Brazil between 1985 and 2017. Mem. Inst. Oswaldo Cruz 2019, 114, e180544. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Alagawany, M.; Bilal, R.M.; Gewida, A.G.A.; Dhama, K.; Abdel-Latif, H.M.R.; Amer, M.S.; Rivero-Perez, N.; Zaragoza-Bastida, A.; Binnaser, Y.S.; et al. An overview on the potential hazards of pyrethroid insecticides in fish, with special emphasis on cypermethrin toxicity. Animals 2021, 11, 1880. [Google Scholar] [CrossRef] [PubMed]
- Churcher, T.S.; Lissenden, N.; Griffin, J.T.; Worrall, E.; Ranson, H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. eLife 2016, 5, e16090. [Google Scholar] [CrossRef]
- Diabate, A.; Baldet, T.; Chandre, F.; Akoobeto, M.; Guiguemde, T.R.; Darriet, F.; Brengues, C.; Guillet, P.; Hemingway, J.; Small, G.J.; et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am. J. Trop. Med. Hyg. 2002, 67, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Hauser, G.; Koella, J.C. Larval exposure to a pyrethroid insecticide and competition for food modulate the melanisation and antibacterial responses of adult Anopheles gambiae. Sci. Rep. 2020, 10, 1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partridge, F.A.; Forman, R.; Bataille, C.J.R.; Wynne, G.M.; Nick, M.; Russell, A.J.; Else, K.J.; Sattelle, D.B. Anthelmintic drug discovery: Target identification, screening methods and the role of open science. Beilstein J. Org. Chem. 2020, 16, 1203–1224. [Google Scholar] [CrossRef] [PubMed]
- Todd, M.H. Six Laws of open source drug discovery. ChemMedChem 2019, 14, 1804–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Voorhis, W.C.; Adams, J.H.; Adelfio, R.; Ahyong, V.; Akabas, M.H.; Alano, P.; Alday, A.; Resto, Y.A.; Alsibaee, A.; Alzualde, A.; et al. Open source drug discovery with the malaria box compound collection for neglected diseases and beyond. PLoS Pathog. 2016, 12, e1005763. [Google Scholar] [CrossRef] [PubMed]
- Veale, C.G.L. Unpacking the pathogen box-An open source tool for fighting neglected tropical disease. ChemMedChem 2019, 14, 386–453. [Google Scholar] [CrossRef] [PubMed]
- Partridge, F.A.; Brown, A.E.; Buckingham, S.D.; Willis, N.J.; Wynne, G.M.; Forman, R.; Else, K.J.; Morrison, A.A.; Matthews, J.B.; Russell, A.J.; et al. An automated high-throughput system for phenotypic screening of chemical libraries on C. elegans and parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 8–21. [Google Scholar] [CrossRef]
- Hurst, R.J.; Hopwood, T.; Gallagher, A.L.; Partridge, F.A.; Burgis, T.; Sattelle, D.B.; Else, K.J. An antagonist of the retinoid X receptor reduces the viability of Trichuris muris in vitro. BMC Infect. Dis. 2014, 14, 520. [Google Scholar] [CrossRef] [Green Version]
- Partridge, F.A.; Murphy, E.A.; Willis, N.J.; Bataille, C.J.R.; Forman, R.; Heyer-Chauhan, N.; Marinič, B.; Sowood, D.J.C.; Wynne, G.M.; Else, K.J.; et al. Dihydrobenz[e][1,4]oxazepin-2(3H)-ones, a new anthelmintic chemotype immobilising whipworm and reducing infectivity in vivo. PLoS Negl. Trop. Dis. 2017, 11, e0005359. [Google Scholar] [CrossRef]
- Partridge, F.A.; Forman, R.; Willis, N.J.; Bataille, C.J.R.; Murphy, E.A.; Brown, A.E.; Heyer-Chauhan, N.; Marinič, B.; Sowood, D.J.C.; Wynne, G.M.; et al. 2,4-Diaminothieno [3,2-d]pyrimidines, a new class of anthelmintic with activity against adult and egg stages of whipworm. PLoS Negl. Trop. Dis. 2018, 12, e0006487. [Google Scholar] [CrossRef] [Green Version]
- Partridge, F.A.; Bataille, C.J.R.; Forman, R.; Marriott, A.E.; Forde-Thomas, J.; Häberli, C.; Dinsdale, R.L.; O’Sullivan, J.D.B.; Willis, N.J.; Wynne, G.M.; et al. Structural Requirements for Dihydrobenzoxazepinone Anthelmintics: Actions against Medically Important and Model Parasites: Trichuris muris, Brugia malayi, Heligmosomoides polygyrus, and Schistosoma mansoni. ACS Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Buckingham, S.D.; Partridge, F.A.; Poulton, B.C.; Miller, B.S.; McKendry, R.A.; Lycett, G.J.; Sattelle, D.B. Automated phenotyping of mosquito larvae enables high-throughput screening for novel larvicides and offers potential for smartphone-based detection of larval insecticide resistance. PLoS Negl. Trop. Dis. 2021, 15, e0008639. [Google Scholar] [CrossRef]
- Clark, J.W. Rubitecan. Expert Opin. Investig. Drugs 2006, 15, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; Li, W.-Q.; Morris-Natschke, S.L.; Qian, K.; Yang, L.; Zhu, G.-X.; Wu, X.-B.; Chen, A.-L.; Zhang, S.-Y.; Song, Z.-L.; et al. Perspectives on biologically active camptothecin derivatives. Med. Res. Rev. 2015, 35, 753–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, Y.; He, W.; Ma, D.; Jiang, H. Effects of camptothecin and hydroxycamptothecin on insect cell lines Sf21 and IOZCAS-Spex-II. Pest. Manag. Sci. 2012, 68, 652–657. [Google Scholar] [CrossRef] [PubMed]
- DeMilo, A.B.; Borkovec, A.B. Camptothecin, a potent chemosterilant against the house fly. J. Econ. Entomol. 1974, 67, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; Yang, L.; Zhao, Y.-L.; Li, H.-Y. Synthesis of novel derivatives of camptothecin as potential insecticides. Pestic. Biochem. Physiol. 2010, 98, 219–223. [Google Scholar] [CrossRef]
- Ma, J.; Tong, S.; Wang, P.; Liao, W.; Liu, H.; Zhang, L. Insecticidal activity of camptothecin against Nilaparvata lugens, Brevicoryne brassicae, and Chilo suppressalis. J. Econ. Entomol. 2010, 103, 492–496. [Google Scholar] [CrossRef]
- Ong, S.-Q.; Jaal, Z. Larval age and nutrition affect the susceptibility of Culex quinquefasciatus (Diptera: Culicidae) to temephos. J. Insect Sci. 2018, 18. [Google Scholar] [CrossRef]
- Pantazis, P.; Han, Z.; Chatterjee, D.; Wyche, J. Water-insoluble camptothecin analogues as potential antiviral drugs. JBS 1999, 6, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Liu, Z.-L.; Tian, X.; Yang, L. Anti-HSV activity of camptothecin analogues. Nat. Prod. Res. 2010, 24, 509–514. [Google Scholar] [CrossRef]
- Wu, K.X.; Chu, J.J.-H. Antiviral screen identifies EV71 inhibitors and reveals camptothecin-target, DNA topoisomerase 1 as a novel EV71 host factor. Antivir. Res. 2017, 143, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Rialdi, A.; Campisi, L.; Zhao, N.; Lagda, A.C.; Pietzsch, C.; Ho, J.S.Y.; Martinez-Gil, L.; Fenouil, R.; Chen, X.; Edwards, M.; et al. Topoisomerase 1 inhibition suppresses inflammatory genes and protects from death by inflammation. Science 2016, 352, aad7993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa, C.F.; da Silva, A.V.; do Nascimento, V.A.; de Souza, V.C.; da Silva Monteiro, D.C.; Terrazaso, W.C.M.; dos Passos, R.A.; Nascimento, S.; Lima, J.B.P.; Naveca, F.G. Evidence of vertical transmission of Zika virus in field-collected eggs of Aedes aegypti in the Brazilian Amazon. PLoS Negl. Trop. Dis. 2018, 12, e0006594. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, S.; Huang, J.; Hart, C.E.; Guzman, H.; Tesh, R.B. Vertical Transmission of Zika Virus in Aedes aegypti Mosquitoes. Am. J. Trop Med. Hyg. 2016, 95, 1169–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, S.; Liu, Y.; Liu, J.; Zhao, J.; Champagne, C.; Tong, L.; Zhang, R.; Zhang, F.; Qin, C.-F.; Ma, P.; et al. Aedes mosquitoes acquire and transmit Zika virus by breeding in contaminated aquatic environments. Nat. Commun. 2019, 10, 1324. [Google Scholar] [CrossRef]
- Song, G.; Lee, E.M.; Pan, J.; Xu, M.; Rho, H.-S.; Cheng, Y.; Whitt, N.; Yang, S.; Kouznetsova, J.; Klumpp-Thomas, C.; et al. An integrated systems biology approach identifies the proteasome as a critical host machinery for ZIKV and DENV replication. Genom. Proteom. Bioinform. 2021. [Google Scholar] [CrossRef]
- Staker, B.L.; Hjerrild, K.; Feese, M.D.; Behnke, C.A.; Burgin, A.B.; Stewart, L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc. Natl. Acad. Sci. USA 2002, 99, 15387–15392. [Google Scholar] [CrossRef] [Green Version]
- Gupta, E.; Vyas, V.; Ahmed, F.; Sinko, P.; Cook, T.; Rubin, E. Pharmacokinetics of orally administered camptothecins. Ann. N. Y. Acad. Sci. 2000, 922, 195–204. [Google Scholar] [CrossRef]
- Herben, V.M.; Ten Bokkel Huinink, W.W.; Schellens, J.H.; Beijnen, J.H. Clinical pharmacokinetics of camptothecin topoisomerase I inhibitors. Pharm. World Sci. 1998, 20, 161–172. [Google Scholar] [CrossRef]
- Herben, V.M.M.; ten Bokkel Huinink, W.W.; Beijnen, J.H. Clinical pharmacokinetics of topotecan. Clin.-Pharm. 1996, 31, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.G.; Bom, D. Campthotecin design and delivery approaches for elevating anti-topoisomerase i activities in vivo. Ann. N. Y. Acad. Sci. 2000, 922, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Chowdhury, F.R. Pharmacological treatment of organophosphorus insecticide poisoning: The old and the (possible) new. Br. J. Clin. Pharmacol. 2016, 81, 462–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Jiang, T.; Li, Q.; Ling, X. Camptothecin (CPT) and its derivatives are known to target topoisomerase I (Top1) as their mechanism of action: Did we miss something in CPT analogue molecular targets for treating human disease such as cancer? Am. J. Cancer Res. 2017, 7, 2350–2394. [Google Scholar] [PubMed]
- Morham, S.G.; Kluckman, K.D.; Voulomanos, N.; Smithies, O. Targeted disruption of the mouse topoisomerase I gene by camptothecin selection. Mol. Cell Biol. 1996, 16, 6804–6809. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.X.; Chen, A.D.; Gettel, N.J.; Hsieh, T.S. Essential functions of DNA topoisomerase I in Drosophila melanogaster. Dev. Biol. 2000, 222, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Smit, M.R.; Ochomo, E.O.; Aljayyoussi, G.; Kwambai, T.K.; Abong’o, B.O.; Bousema, T.; Waterhouse, D.; Bayoh, N.M.; Gimnig, J.E.; Samuels, A.M.; et al. Human direct skin feeding versus membrane feeding to assess the mosquitocidal efficacy of high-dose ivermectin (IVERMAL Trial). Clin. Infect. Dis 2019, 69, 1112–1119. [Google Scholar] [CrossRef]
- Dreyer, S.M.; Morin, K.J.; Vaughan, J.A. Differential susceptibilities of Anopheles albimanus and Anopheles stephensi mosquitoes to ivermectin. Malar. J. 2018, 17, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, F.O. Upon entering an age of global ivermectin-based integrated mass drug administration for neglected tropical diseases and malaria. Malar. J. 2017, 16, 168. [Google Scholar] [CrossRef] [Green Version]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Partridge, F.A.; Poulton, B.C.; Lake, M.A.I.; Lees, R.A.; Mann, H.-J.; Lycett, G.J.; Sattelle, D.B. Actions of Camptothecin Derivatives on Larvae and Adults of the Arboviral Vector Aedes aegypti. Molecules 2021, 26, 6226. https://doi.org/10.3390/molecules26206226
Partridge FA, Poulton BC, Lake MAI, Lees RA, Mann H-J, Lycett GJ, Sattelle DB. Actions of Camptothecin Derivatives on Larvae and Adults of the Arboviral Vector Aedes aegypti. Molecules. 2021; 26(20):6226. https://doi.org/10.3390/molecules26206226
Chicago/Turabian StylePartridge, Frederick A., Beth C. Poulton, Milly A. I. Lake, Rebecca A. Lees, Harry-Jack Mann, Gareth J. Lycett, and David B. Sattelle. 2021. "Actions of Camptothecin Derivatives on Larvae and Adults of the Arboviral Vector Aedes aegypti" Molecules 26, no. 20: 6226. https://doi.org/10.3390/molecules26206226
APA StylePartridge, F. A., Poulton, B. C., Lake, M. A. I., Lees, R. A., Mann, H.-J., Lycett, G. J., & Sattelle, D. B. (2021). Actions of Camptothecin Derivatives on Larvae and Adults of the Arboviral Vector Aedes aegypti. Molecules, 26(20), 6226. https://doi.org/10.3390/molecules26206226





