In Vitro Phenotypic Activity and In Silico Analysis of Natural Products from Brazilian Biodiversity on Trypanosoma cruzi
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crude Extracts and Fractions
2.2. Purified Substances
3. Materials and Methods
3.1. Botanical Material and Extracts and Purified Fractions
3.2. Mammalian Cell Cultures
3.3. Mammalian Cell Toxicity
3.4. Trypanocidal Activity
3.5. In Silico ADMET Properties
3.6. Statistical Analyses
3.7. Ethics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Chagas Disease (American Trypanosomiasis). Available online: https://www.who.int/westernpacific/health-topics/chagas-disease (accessed on 20 November 2020).
- Pérez-Molina, J.A.; Crespillo-Andújar, C.; Bosch-Nicolau, P.; Molina, I. Trypanocidal Treatment of Chagas Disease. Enferm. Infecc. Microbiol. Clin. 2020. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Molina, I. Chagas Disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Bahia, M.T.; de Figueiredo Diniz, L.; Mosqueira, V.C.F. Therapeutical Approaches under Investigation for Treatment of Chagas Disease. Expert Opin. Investig. Drugs 2014, 23, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Keenan, M.; Chaplin, J.H. A New Era for Chagas Disease Drug Discovery? Prog. Med. Chem. 2015, 54, 185–230. [Google Scholar] [CrossRef] [PubMed]
- Salomao, K.; Figueiredo Sadok Menna-Barreto, R.; Lisboa de Castro, S. Stairway to Heaven or Hell? Perspectives and Limitations of Chagas Disease Chemotherapy. Curr. Top. Med. Chem. 2016, 16, 2266–2289. [Google Scholar] [CrossRef]
- Ribeiro, V.; Dias, N.; Paiva, T.; Hagström-Bex, L.; Nitz, N.; Pratesi, R.; Hecht, M. Current Trends in the Pharmacological Management of Chagas Disease. Int. J. Parasitol. Drugs Drug Resist. 2020, 12, 7–17. [Google Scholar] [CrossRef]
- Tempone, A.G.; Sartorelli, P.; Mady, C.; Fernandes, F. Natural Products to Anti-Trypanosomal Drugs: An Overview of New Drug Prototypes for American Trypanosomiasis. Cardiovasc. Hematol. Agents Med. Chem. 2007, 5, 222–235. [Google Scholar] [CrossRef]
- Varela, J.; Cerecetto, H.; González, M. Slowed Development of Natural Products for Chagas Disease, how to Move Forward? In Chagas Disease—Basic Investigations and Challenges; Nissapatorn, V., Oz, H.S., Eds.; InTech United Kingdom: London, UK, 2018; ISBN 978-1-78923-658-3. [Google Scholar]
- Álvarez-Bardón, M.; Pérez-Pertejo, Y.; Ordóñez, C.; Sepúlveda-Crespo, D.; Carballeira, N.M.; Tekwani, B.L.; Murugesan, S.; Martinez-Valladares, M.; García-Estrada, C.; Reguera, R.M.; et al. Screening Marine Natural Products for New Drug Leads against Trypanosomatids and Malaria. Mar. Drugs 2020, 18, 187. [Google Scholar] [CrossRef]
- de M. Lima, S.C.; da Silva Pacheco, J.; Marques, A.M.; Veltri, E.R.P.; Almeida-Lafetá, R.D.C.; Figueiredo, M.R.; Kaplan, M.A.C.; Torres-Santos, E.C. Leishmanicidal Activity of Withanolides from Aureliana Fasciculata Var. Fasciculata. Molecules 2018, 23, 3160. [Google Scholar] [CrossRef] [Green Version]
- Parvin, M.S.; Das, N.; Jahan, N.; Akhter, M.A.; Nahar, L.; Islam, M.E. Evaluation of in Vitro Anti-Inflammatory and Antibacterial Potential of Crescentia Cujete Leaves and Stem Bark. BMC Res. Notes 2015, 8, 412. [Google Scholar] [CrossRef] [Green Version]
- Silva, K.M.M.D.; Nóbrega, A.B.D.; Lessa, B.; Anholeti, M.C.; Lobão, A.Q.; Valverde, A.L.; Paiva, S.R.D.; Joffily, A. Clusia Criuva Cambess. (Clusiaceae): Anatomical Characterization, Chemical Prospecting and Antioxidant Activity. An. Acad. Bras. Ciênc. 2017, 89, 1565–1578. [Google Scholar] [CrossRef] [Green Version]
- da Silva, M.C.A.; Paiva, S.R. Antioxidant Activity and Flavonoid Content of Clusia Fluminensis Planch. & Triana. An. Acad. Bras. Ciênc. 2012, 84, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.F.; Camara, C.A.; de Fátima Agra, M.; Silva, T.M.S. Biflavonoids from the Unripe Fruits of Clusia Paralicola and Their Antioxidant Activity. Nat. Prod. Commun. 2012, 7, 1934578X1200701. [Google Scholar] [CrossRef] [Green Version]
- da Silva Frutuoso, V.; Monteiro, M.M.; Amendoeira, F.C.; Almeida, A.L.F.; do Nascimento, D.D.; Bérenger, A.L.R.; Kaplan, M.A.C.; Figueiredo, M.R.; Bozza, P.T.; Castro-Faria-Neto, H.C. Analgesic and Anti-Inflammatory Activity of the Aqueous Extract of Rheedia Longifolia Planch & Triana. Mem. Inst. Oswaldo Cruz 2007, 102, 91–96. [Google Scholar] [CrossRef]
- Quijia, C.R.; Araujo, V.H.; Chorilli, M. Piperine: Chemical, Biological and Nanotechnological Applications. Acta Pharm. Zagreb Croat. 2021, 71, 185–213. [Google Scholar] [CrossRef]
- Zadorozhna, M.; Tataranni, T.; Mangieri, D. Piperine: Role in Prevention and Progression of Cancer. Mol. Biol. Rep. 2019, 46, 5617–5629. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef] [Green Version]
- da Silva, J.K.; da Trindade, R.; Alves, N.S.; Figueiredo, P.L.; Maia, J.G.S.; Setzer, W.N. Essential Oils from Neotropical Piper Species and Their Biological Activities. Int. J. Mol. Sci. 2017, 18, 2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, C.-P.; Shi, Y.-N.; Liu, F.-F.; Li, H.-Z.; Zhang, Y.-J.; Yang, C.-R.; Xu, M. A Survey of the Chemical Compounds of Piper spp. (Piperaceae) and Their Biological Activities. Nat. Prod. Commun. 2016, 11, 1403–1408. [Google Scholar]
- Vásquez-Ocmín, P.G.; Gadea, A.; Cojean, S.; Marti, G.; Pomel, S.; Van Baelen, A.-C.; Ruiz-Vásquez, L.; Ruiz Mesia, W.; Figadère, B.; Ruiz Mesia, L.; et al. Metabolomic Approach of the Antiprotozoal Activity of Medicinal Piper Species Used in Peruvian Amazon. J. Ethnopharmacol. 2021, 264, 113262. [Google Scholar] [CrossRef]
- Chan-Bacab, M.J.; Peña-Rodríguez, L.M. Plant Natural Products with Leishmanicidal Activity. Nat. Prod. Rep. 2001, 18, 674–688. [Google Scholar] [CrossRef]
- Macêdo, C.G.; Fonseca, M.Y.N.; Caldeira, A.D.; Castro, S.P.; Pacienza-Lima, W.; Borsodi, M.P.G.; Sartoratto, A.; da Silva, M.N.; Salgado, C.G.; Rossi-Bergmann, B.; et al. Leishmanicidal Activity of Piper Marginatum Jacq. from Santarém-PA against Leishmania Amazonensis. Exp. Parasitol. 2020, 210, 107847. [Google Scholar] [CrossRef]
- Peres, R.B.; Ullah, A.I.; de Almeida Fiuza, L.F.; Silva, P.B.; Batista, M.M.; Corcoran, O.; Reddy, T.R.K.; de Nazaré Correia Soeiro, M. Identification and Preliminary Structure-Activity Relationship Studies of Novel Pyridyl Sulfonamides as Potential Chagas Disease Therapeutic Agents. Bioorg. Med. Chem. Lett. 2018, 28, 2018–2022. [Google Scholar] [CrossRef]
- Katsuno, K.; Burrows, J.N.; Duncan, K.; Hooft van Huijsduijnen, R.; Kaneko, T.; Kita, K.; Mowbray, C.E.; Schmatz, D.; Warner, P.; Slingsby, B.T. Hit and Lead Criteria in Drug Discovery for Infectious Diseases of the Developing World. Nat. Rev. Drug Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Filardi, L.S.; Brener, Z. Susceptibility and Natural Resistance of Trypanosoma Cruzi Strains to Drugs Used Clinically in Chagas Disease. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 755–759. [Google Scholar] [CrossRef]
- Nagafuji, S.; Okabe, H.; Akahane, H.; Abe, F. Trypanocidal Constituents in Plants 4. Withanolides from the Aerial Parts of Physalis Angulata. Biol. Pharm. Bull. 2004, 27, 193–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peraza-Sánchez, S.R.; Cen-Pacheco, F.; Noh-Chimal, A.; May-Pat, F.; Simá-Polanco, P.; Dumonteil, E.; García-Miss, M.R.; Mut-Martín, M. Leishmanicidal Evaluation of Extracts from Native Plants of the Yucatan Peninsula. Fitoterapia 2007, 78, 315–318. [Google Scholar] [CrossRef]
- Moran, M.; Guzman, J.; Ropars, A.-L.; McDonald, A.; Jameson, N.; Omune, B.; Ryan, S.; Wu, L. Neglected Disease Research and Development: How Much Are We Really Spending? PLoS Med. 2009, 6, e1000030. [Google Scholar] [CrossRef] [Green Version]
- Vieira, G.A.L.; da Silva, M.T.A.; Regasini, L.O.; Cotinguiba, F.; Laure, H.J.; Rosa, J.C.; Furlan, M.; Cicarelli, R.M.B. Trypanosoma Cruzi: Analysis of Two Different Strains after Piplartine Treatment. Braz. J. Infect. Dis. 2018, 22, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Scull, R.; Cos, P.; Setzer, W. Essential Oil from Piper Aduncum: Chemical Analysis, Antimicrobial Assessment, and Literature Review. Medicines 2017, 4, 49. [Google Scholar] [CrossRef] [Green Version]
- Villamizar, L.H.; das Graças Cardoso, M.; de Andrade, J.; Teixeira, M.L.; Soares, M.J.; Fundação Oswaldo Cruz-Fiocruz, Brasil; Universidade Federal de Lavras, Brasil. Linalool, a Piper Aduncum Essential Oil Component, Has Selective Activity against Trypanosoma Cruzi Trypomastigote Forms at 4 °C. Mem. Inst. Oswaldo Cruz 2017, 112, 131–139. [Google Scholar] [CrossRef] [Green Version]
- García-Huertas, P.; Olmo, F.; Sánchez-Moreno, M.; Dominguez, J.; Chahboun, R.; Triana-Chávez, O. Activity in vitro and in Vivo against Trypanosoma Cruzi of a Furofuran Lignan Isolated from Piper Jericoense. Exp. Parasitol. 2018, 189, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Lafetá, R.C.; Ferreira, M.J.P.; Emerenciano, V.P.; Kaplan, M.A.C. Withanolides from Aureliana Fasciculata Var. Fasciculata. Helv. Chim. Acta 2010, 93, 2478–2487. [Google Scholar] [CrossRef]
- McKerrow, J.H.; Lipinski, C.A. The Rule of Five Should Not Impede Anti-Parasitic Drug Development. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 248–249. [Google Scholar] [CrossRef]
- Zhang, H.; Samadi, A.K.; Cohen, M.S.; Timmermann, B.N. Anti-Proliferative Withanolides from the Solanaceae: A Structure-Activity Study. Pure Appl. Chem. Chim. Pure Appl. 2012, 84, 1353–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões-Silva, M.R.; De Araújo, J.S.; Oliveira, G.M.; Demarque, K.C.; Peres, R.B.; D’Almeida-Melo, I.; Batista, D.G.J.; Da Silva, C.F.; Cardoso-Santos, C.; Da Silva, P.B.; et al. Drug Repurposing Strategy against Trypanosoma Cruzi Infection: In Vitro and in Vivo Assessment of the Activity of Metronidazole in Mono- and Combined Therapy. Biochem. Pharmacol. 2017, 145, 46–53. [Google Scholar] [CrossRef]
- Meirelles, M.N.; de Araujo-Jorge, T.C.; Miranda, C.F.; de Souza, W.; Barbosa, H.S. Interaction of Trypanosoma Cruzi with Heart Muscle Cells: Ultrastructural and Cytochemical Analysis of Endocytic Vacuole Formation and Effect upon Myogenesis in Vitro. Eur. J. Cell Biol. 1986, 41, 198–206. [Google Scholar]
- Romanha, A.J.; de Castro, S.L.; de Nazaré Correia Soeiro, M.; Lannes-Vieira, J.; Ribeiro, I.; Talvani, A.; Bourdin, B.; Blum, B.; Olivieri, B.; Zani, C.; et al. In Vitro and in Vivo Experimental Models for Drug Screening and Development for Chagas Disease. Mem. Inst. Oswaldo Cruz 2010, 105, 233–238. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.F.; da Gama Jaen Batista, D.; Oliveira, G.M.; de Souza, E.M.; Hammer, E.R.; da Silva, P.B.; Daliry, A.; Araujo, J.S.; Britto, C.; Rodrigues, A.C.M.; et al. In Vitro and in Vivo Investigation of the Efficacy of Arylimidamide DB1831 and Its Mesylated Salt Form-DB1965-against Trypanosoma Cruzi Infection. PLoS ONE 2012, 7, e30356. [Google Scholar] [CrossRef] [Green Version]
- Simões-Silva, M.R.; Nefertiti, A.S.G.; De Araújo, J.S.; Batista, M.M.; Da Silva, P.B.; Bahia, M.T.; Menna-Barreto, R.S.; Pavão, B.P.; Green, J.; Farahat, A.A.; et al. Phenotypic Screening In Vitro of Novel Aromatic Amidines against Trypanosoma Cruzi. Antimicrob. Agents Chemother. 2016, 60, 4701–4707. [Google Scholar] [CrossRef] [Green Version]
- Ferreira de Almeida Fiuza, L.; Peres, R.B.; Simões-Silva, M.R.; da Silva, P.B.; da Gama Jaen Batista, D.; da Silva, C.F.; da Gama, N.S.A.; Krishna Reddy, T.R.; de Nazaré Correia Soeiro, M. Identification of Pyrazolo[3,4-e][1,4]Thiazepin Based CYP51 Inhibitors as Potential Chagas Disease Therapeutic Alternative: In Vitro and in Vivo Evaluation, Binding Mode Prediction and SAR Exploration. Eur. J. Med. Chem. 2018, 149, 257–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nefertiti, A.S.D.G.; Batista, M.M.; da Silva, P.B.; Torres-Santos, E.C.; Cunha-Júnior, E.F.; Green, J.; Kumar, A.; Farahat, A.A.; Boykin, D.W.; Soeiro, M.D.N.C. Anti-Parasitic Effect of Novel Amidines against Trypanosoma Cruzi: Phenotypic and in Silico Absorption, Distribution, Metabolism, Excretion and Toxicity Analysis. Parasitol. Open 2017, 3, e5. [Google Scholar] [CrossRef] [Green Version]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. PkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]

| Family/Species | Part of the Plant | Compound/Extract | Solvent | % of Infection Reduction after 96 h of Incubation |
|---|---|---|---|---|
| - | - | Benznidazole | - | 90.5 ± 5.7 |
| Solanaceae Aureliana fasciculata var. fasciculata | Leaves | AFFMeOH 1 | Methanol | 6.5 ± 9.0 |
| AFfPD | Dichloromethane | 100.0 | ||
| AFFHex | Hexane | 12.3 ± 5.0 | ||
| AFFAcoEt | Ethyl acetate | 11.9 ± 6.1 | ||
| AFFBuOH | Butanol | 11.1 ± 6.3 | ||
| AFFAquo | Aqueous partition | 10.3 ± 5.6 | ||
| Clusiaceae Clusia studartiana | Leaves | CSH-F 1 | Hexane | 6.8 ± 6.3 |
| Aerial parts | CSH 1 | Hexane | 65.5 ± 23.3 | |
| CSE 1 | Ethanol | 0.0 | ||
| CSTE-C | Chloroform | 5.7 ± 8 | ||
| CSTE-Ac | Ethyl acetate | 3.9 ± 5.8 | ||
| CETE-Bu | Butanol | 2.8 ± 5.8 | ||
| CSTE-Aq | Aqueous partition | 10.0 ± 6.0 | ||
| Bignoniaceae Crescentia cujete | Fruit pulp | CCPEH | Hexane | 2.9 ± 0.8 |
| CCPEC | Chloroform | 10.9 ± 3.5 | ||
| CCPEAC | Ethyl acetate | 0.0 | ||
| CCPEBU | Butanol | 0.0 | ||
| CCPEA | Aqueous partition | 1.3 ± 1.8 | ||
| CCPEACN + MEOH | Acetonitrile/methanol | 0.2 ± 0.5 | ||
| Malpighiaceae Malpighia glabra | Leaves | MGE 1 | Ethanol | 9.4 ± 3.5 |
| MGEH | Hexane | 8.8 ± 5.3 | ||
| MGED | Dichloromethane | 2.5 ± 3.6 | ||
| MGEAc | Ethyl acetate | 4.6 ± 7.3 | ||
| MGEB | Butanol | 10.4 ± 4.3 | ||
| MGEAq | Aqueous partition | 1.0 ± 1.0 | ||
| Piperaceae Piper tectoniifolium | Inflorescence | PTFrE 1 | Ethanol | 83.7 ± 15.0 |
| PTFrEPH | Hexane | 18.9 ± 5 | ||
| PTFrEPD | Dichloromethane | 19.4 ± 7.2 | ||
| PTFrEPAc | Ethyl acetate | 22.1 ± 10.3 | ||
| PTFrEPB | Butanol | 21.3 ± 6.0 | ||
| Clusiaceae Rheedia longifolia | Leaves | RLFM 1 | Methanol | 16.0 ± 2.8 |
| RLFMH | Hexane | 12.5 ± 10.2 | ||
| RLFMD | Dichloromethane | 14.2 ± 7.0 | ||
| RLFMAc | Ethyl acetate | 8.5 ± 9.7 | ||
| RLFMBu | Butanol | 12.5 ± 15.8 | ||
| RLFMAq | Aqueous partition | 12.0 ± 15.8 |
| Cp | Intracellular Forms (Tulahuen Strain) | Bloodstream Trypomastigotes (Y Strain) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 96 h | 2h | 24 h | |||||||
| EC50 | LC50 a | SI | EC50 | EC90 | EC50 | EC90 | LC50 b | SI | |
| Bz | 3.8 ± 1.8 | > 200 | > 52 | >50 | >50 | 10.2 ± 0.3 | 23.3 ± 0.6 | >750 | >83 |
| AFfPD | 9.3 ± 1.9 | 12.5 ± 1.0 | 1.3 | 38.7 ± 1.2 * | >50 | 2.2 ± 1.0 * | 7.9 ± 2.2 * | 15.2 ± 2.7 | 7.1 |
| PTFrE | 12.6 ± 1.7 | 15 ± 3.0 | 1.2 | >50 | >50 | 38.8 ± 2.1 | 47.4 ± 1.2 | 124.2 ± 6.8 | 3.2 |
| Cp | Botanical Species | Chemical Compounds | % of Infection Reduction |
|---|---|---|---|
| Bz | - | Nitro-heterocyclic | 92 ± 7 |
| 1 | Aureliana fasciculata | Aurelianolide A | 95.2 ± 0 |
| 2 | Aurelianolide B | 91 ± 16 | |
| 3 | Clusia studartiana var. fasciculata | 3 beta-friedelanol | 21 ± 10 |
| 4 | Oleanolic acid | 20 ± 18 | |
| 5 | Oleanolic methyl ester | 36 ± 24 | |
| 6 | Friedelin | 0 | |
| 7 | Piper tectoniifolium | (-)-grandisin | 25 ± 36 |
| 8 | Piper tuberculatum | Piplartine | 54.1 ± 29 |
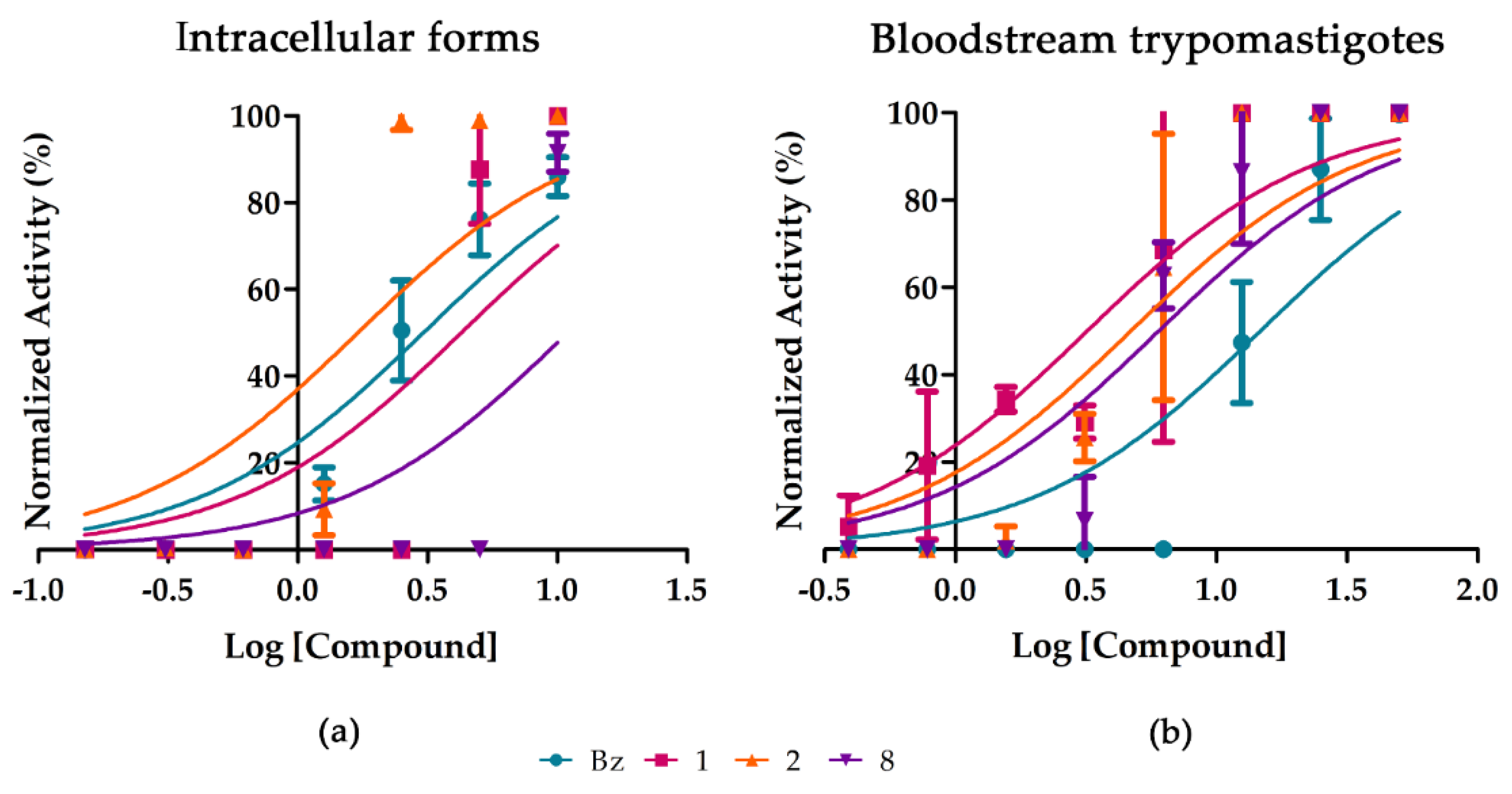
| Cp | Intracellular Forms | Bloodstream Trypomastigotes | ||||||
|---|---|---|---|---|---|---|---|---|
| 96 h | 2 h | 24 h | ||||||
| EC50 | LC50 1 | SI | EC50 | EC50 | EC90 | LC50 2 | SI | |
| Bz | 3 ± 0.6 | >200 | >66.6 | >50 | 13.13 ± 3.9 | 31.29 ± 8.8 | >750 | >57.1 |
| 1 | 4.6 ± 1.3 | 42.1 ± 28.3 | 9.15 | >50 | 5.68 ± 2.3 | 8.33 ± 3.9 | 16.36 ± 1.3 | 2.9 |
| 2 | 1.6 ± 0.4 | 27.36 ± 1 | 17.1 | >50 | 5.72 ± 1.3 | 9.25 ± 3.2 | 30.89 ± 3.8 | 5.4 |
| 8 | 8.1 ± 0.9 | 73.1 ± 75.5 | 9 | >50 | 5.38 ± 0.3 | 14.44 ± 5.8 | 33.15 ± 5.9 | 6.2 |
| Property | Reference | Bz | 1 | 2 | 8 |
|---|---|---|---|---|---|
| Molecular Weight | ≤500 | 260.253 | 528.642 | 512.643 | 317.341 |
| LogP | ≤5 | 1.1077 | 3.037 | 3.8258 | 2.0407 |
| Acceptors | ≤5 | 5 | 8 | 7 | 5 |
| Donors | ≤10 | 1 | 2 | 2 | 0 |
| Property | Reference | Bz | 1 | 2 | 8 |
|---|---|---|---|---|---|
| ABSORPTION | |||||
| Water solubility (log mol/L) | - | −2.782 | −4.994 | −5.155 | −3.7 |
| Caco2 permeability (log cm/s) | >0.9 | 0.542 | 0.85 | 0.894 | 1.419 |
| Intestinal absorption (human, %) | <30% is poorly | 75.834 | 92.85 | 87.412 | 97.785 |
| Skin Permeability (log Kp) | >−2.5 is low | −2.768 | −3.143 | −3.564 | −3.229 |
| DISTRIBUTION | |||||
| P-glycoprotein substrate | No | Yes | Yes | Yes | No |
| P-glycoprotein I inhibitor | No | No | Yes | Yes | No |
| P-glycoprotein II inhibitor | No | No | Yes | Yes | No |
| VDss (human) (log L/kg) | Low is <−0.15, High is >0.45 | −0.364 | 0.174 | −0.011 | −0.067 |
| Fraction unbound (human) | - | 0.299 | 0.061 | 0 | 0.255 |
| BBB permeability (log BB) | Poorly is <−1, High is >0.3 | −0.49 | −0.406 | −0.214 | −0.47 |
| CNS permeability(log PS) | Penetrate is >−2, Unable is <−3 | −2.731 | −2.757 | −2.501 | −2.947 |
| METABOLISM | |||||
| CYP2D6 substrate | No | No | No | No | No |
| CYP3A4 substrate | - | No | Yes | Yes | No |
| CYP1A2 inhibitior | No | No | No | No | Yes |
| CYP2C19 inhibitior | No | No | No | No | No |
| CYP2C9 inhibitior | No | No | No | No | No |
| CYP2D6 inhibitior | No | No | No | No | No |
| CYP3A4 inhibitior | No | No | No | No | No |
| EXCRETION | |||||
| Total Clearance (log ml/min/kg) | - | 0.539 | 0.335 | 0.416 | 0.266 |
| TOXICITY | |||||
| AMES toxicity | No | Yes | No | No | No |
| Max. tolerated dose (human-log) | Low is ≤0.477, High is >0.477 | 0.733 | −0.878 | −0.875 | 0.573 |
| hERG I inhibitor | No | No | No | No | No |
| hERG II inhibitor | No | No | No | No | No |
| Oral Rat Acute Toxicity (LD50) | - | 2.251 | 3.672 | 3.066 | 2.528 |
| Oral Rat Chronic Toxicity | - | 1.594 | 2.12 | 1.957 | 2.657 |
| Hepatotoxicity | No | Yes | No | No | No |
| Skin Sensitisation | No | No | No | No | No |
| T. pyriformis toxicity (log ug/L) | >−0.5 is toxic | 0.285 | 0.286 | 0.294 | 0.597 |
| Minnow toxicity (log mM) | <−0.3 is toxic | 0.803 | 2.529 | 1.526 | 1.433 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peres, R.B.; Fiuza, L.F.d.A.; da Silva, P.B.; Batista, M.M.; Camillo, F.d.C.; Marques, A.M.; de C. Brito, L.; Figueiredo, M.R.; Soeiro, M.d.N.C. In Vitro Phenotypic Activity and In Silico Analysis of Natural Products from Brazilian Biodiversity on Trypanosoma cruzi. Molecules 2021, 26, 5676. https://doi.org/10.3390/molecules26185676
Peres RB, Fiuza LFdA, da Silva PB, Batista MM, Camillo FdC, Marques AM, de C. Brito L, Figueiredo MR, Soeiro MdNC. In Vitro Phenotypic Activity and In Silico Analysis of Natural Products from Brazilian Biodiversity on Trypanosoma cruzi. Molecules. 2021; 26(18):5676. https://doi.org/10.3390/molecules26185676
Chicago/Turabian StylePeres, Raiza B., Ludmila F. de A. Fiuza, Patrícia B. da Silva, Marcos M. Batista, Flávia da C. Camillo, André M. Marques, Lavínia de C. Brito, Maria R. Figueiredo, and Maria de N. C. Soeiro. 2021. "In Vitro Phenotypic Activity and In Silico Analysis of Natural Products from Brazilian Biodiversity on Trypanosoma cruzi" Molecules 26, no. 18: 5676. https://doi.org/10.3390/molecules26185676
APA StylePeres, R. B., Fiuza, L. F. d. A., da Silva, P. B., Batista, M. M., Camillo, F. d. C., Marques, A. M., de C. Brito, L., Figueiredo, M. R., & Soeiro, M. d. N. C. (2021). In Vitro Phenotypic Activity and In Silico Analysis of Natural Products from Brazilian Biodiversity on Trypanosoma cruzi. Molecules, 26(18), 5676. https://doi.org/10.3390/molecules26185676





