Molecularly Imprinted Polymers (MIPs) in Sensors for Environmental and Biomedical Applications: A Review
Abstract
1. Introduction
2. Synthesis
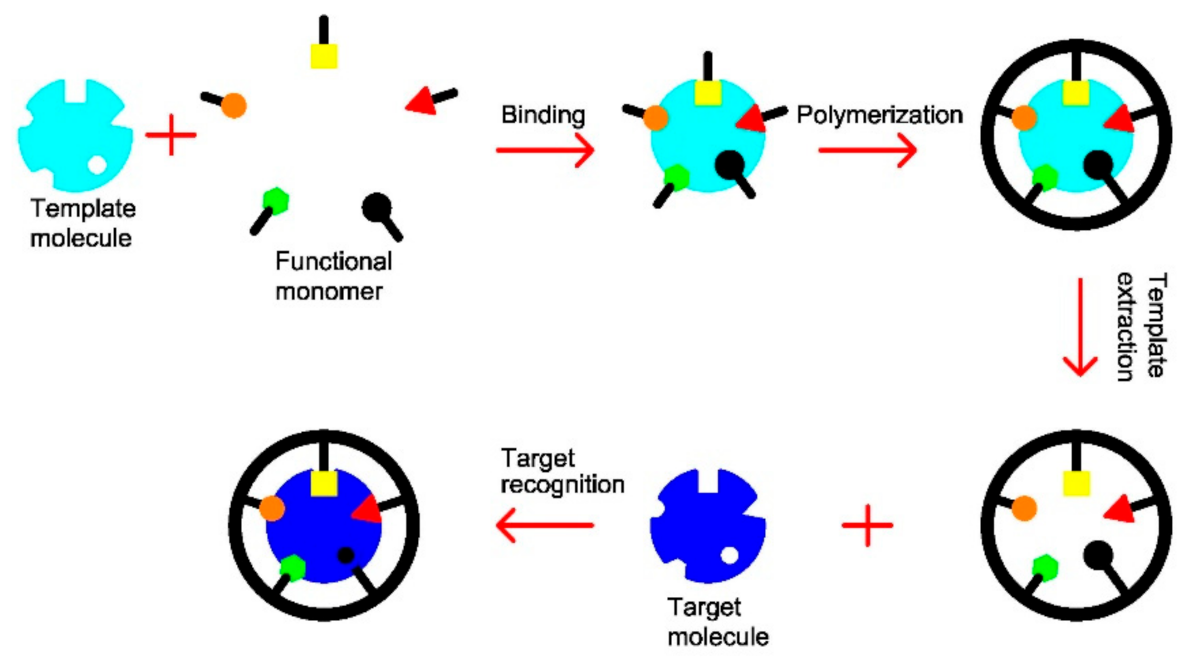
2.1. Imprinting Techniques for Sensors
2.2. Materials for MIP Fabrication
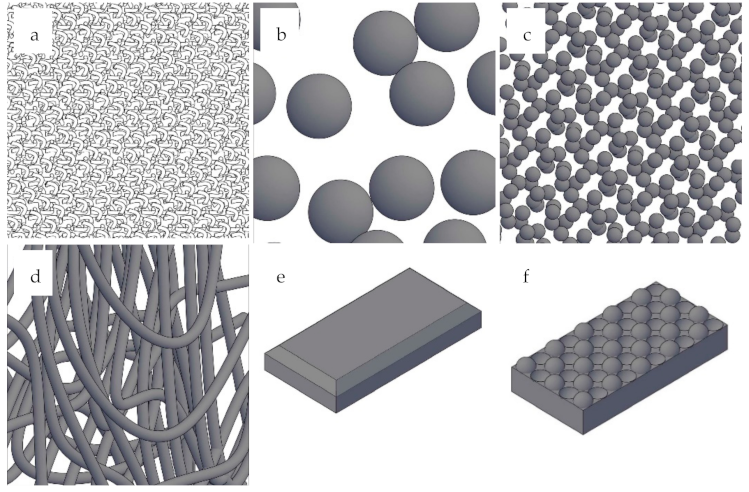
2.3. Physical Form
3. Environmental and Biomedical Applications of MIPs
3.1. Electrochemical Sensors in Environmental and Biomedical Applications
3.1.1. MIP-Electrochemical Sensors in Environmental Applications
3.1.2. MIP-Electrochemical Sensors in Biomedical Applications
3.2. Optical Sensors
3.2.1. MIP-Based Optical Sensors in Environmental Applications
3.2.2. MIP-Based Optical Sensors in Biomedical Applications
4. Technical Barriers to Commercialization of MIP Sensors and Devices
5. Summary and Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haupt, K.; Linares, A.V.; Bompart, M.; Bui, B.T.S. Molecularly Imprinted Polymers. Top. Curr. Chem. 2011, 325, 1–28. [Google Scholar] [CrossRef]
- Beltran, A.; Borrull, F.; Marcé-Recasens, R.M.; Cormack, P. Molecularly-imprinted polymers: Useful sorbents for selective extractions. TrAC Trends Anal. Chem. 2010, 29, 1363–1375. [Google Scholar] [CrossRef]
- Wulff, G.; Grobe-Einsler, R.; Vesper, W.; Sarhan, A. Enzyme-analogue built polymers, 5. On the specificity distribution of chiral cavities prepared in synthetic polymers. Makromol. Chem. 1977, 178, 2817–2825. [Google Scholar] [CrossRef]
- Whitcombe, M.J.; Rodriguez, M.E.; Villar, P.; Vulfson, E.N. A New Method for the Introduction of Recognition Site Functionality into Polymers Prepared by Molecular Imprinting: Synthesis and Characterization of Polymeric Receptors for Cholesterol. J. Am. Chem. Soc. 1995, 117, 7105–7111. [Google Scholar] [CrossRef]
- Vlatakis, G.; Andersson, L.I.; Muller, R.S.; Mosbach, K. Drug assay using antibody mimics made by molecular imprinting. Nature 1993, 361, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Arshady, R.; Mosbach, K. Synthesis of substrate-selective polymers by host-guest polymerization. Makromol. Chem. 1981, 182, 687–692. [Google Scholar] [CrossRef]
- Alexander, C.; Andersson, H.; Andersson, L.I.; Ansell, R.J.; Kirsch, N.; Nicholls, I.A.; O’Mahony, J.; Whitcombe, M.J. Molecular imprinting science and technology: A survey of the literature for the years up to and including 2003. J. Mol. Recognit. 2006, 19, 106–180. [Google Scholar] [CrossRef]
- Kupai, J.; Razali, M.; Buyuktiryaki, S.; Kecili, R.; Szekely, G. Long-term stability and reusability of molecularly imprinted polymers. Polym. Chem. 2016, 8, 666–673. [Google Scholar] [CrossRef]
- Wulff, G.; Sarhan, A. Über die Anwendung von enzymanalog gebauten Polymeren zur Racemattrennung. Angew. Chem. 1972, 84, 364. [Google Scholar] [CrossRef]
- Vasapollo, G.; del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G.; Vasapollo, G.; del Sole, R.; Mergola, L.; et al. Molecularly Imprinted Polymers: Present and Future Prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [PubMed]
- Asman, S.; Mohamad, S.; Sarih, N.M. Exploiting β-Cyclodextrin in Molecular Imprinting for Achieving Recognition of Benzylparaben in Aqueous Media. Int. J. Mol. Sci. 2015, 16, 3656–3676. [Google Scholar] [CrossRef]
- Zaidi, S.A. Molecular imprinted polymers as drug delivery vehicles. Drug Deliv. 2014, 23, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Mespouille, L.; Dubois, P.; Blankert, B.; Duez, P. Molecularly Imprinted Polymers: Compromise between Flexibility and Rigidity for Improving Capture of Template Analogues. Chem. A Eur. J. 2014, 20, 3500–3509. [Google Scholar] [CrossRef] [PubMed]
- Caro, E.; Marce, R.; Borrull, F.; Cormack, P.; Sherrington, D. Application of molecularly imprinted polymers to solid-phase extraction of compounds from environmental and biological samples. TrAC Trends Anal. Chem. 2006, 25, 143–154. [Google Scholar] [CrossRef]
- Sanagi, M.M.; Salleh, S.; Ibrahim, W.A.W.; Abu Naim, A.; Hermawan, D.; Miskam, M.; Hussain, I.; Aboul-Enein, H.Y. Molecularly imprinted polymer solid-phase extraction for the analysis of organophosphorus pesticides in fruit samples. J. Food Compos. Anal. 2013, 32, 155–161. [Google Scholar] [CrossRef]
- Bitar, M.; Cayot, P.; Bou-Maroun, E. Molecularly imprinted polymer solid phase extraction of fungicides from wine samples. Anal. Methods 2014, 6, 6467–6472. [Google Scholar] [CrossRef]
- Wang, G.N.; Yang, K.; Liu, H.Z.; Feng, M.X.; Wang, J.P. Molecularly imprinted polymer-based solid phase extraction combined high performance liquid chromatography for determination of fluoroquinolones in milk. Anal. Methods 2016, 8, 5511–5518. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, J.; Yin, J.; Shao, B.; Zhang, J. Molecularly Imprinted Solid-Phase Extraction for Selective Extraction of Bisphenol Analogues in Beverages and Canned Food. J. Agric. Food Chem. 2014, 62, 11130–11137. [Google Scholar] [CrossRef]
- Baltrons, O.; López-Mesas, M.; Palet, C.; Le Derf, F.; Portet-Koltalo, F. Molecularly imprinted polymer-liquid chromatography/fluorescence for the selective clean-up of hydroxylated polycyclic aromatic hydrocarbons in soils. Anal. Methods 2013, 5, 6297–6305. [Google Scholar] [CrossRef]
- Panahi, H.A.; Mahabadi, S.A. Application of Molecularly Imprinted Polymer for Extraction and Determination of Nalidixic Acid by High-Performance Liquid Chromatography. Sep. Sci. Technol. 2014, 50, 683–689. [Google Scholar] [CrossRef]
- Liu, Q.; Wan, J.; Cao, X. Synthesis of core-shell molecularly imprinted polymers (MIP) for spiramycin I and their application in MIP chromatography. Process. Biochem. 2018, 70, 168–178. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Jiang, Y.; Li, S.; Liu, W. Molecularly Imprinted Polymers for the Identification and Separation of Chiral Drugs and Biomolecules. Polymers 2016, 8, 216. [Google Scholar] [CrossRef]
- Li, Z.W.; Jia, X.; Xu, C.M.; Liu, L.; Fu, D.C. Chiral Separation of Amlodipine and its Enantiomer on a Molecularly Imprinted Polymer-Based Stationary Phase. Adv. Mater. Res. 2013, 706-708, 36–39. [Google Scholar] [CrossRef]
- Orozco, J.; Cortés, A.; Cheng, G.; Sattayasamitsathit, S.; Gao, W.; Feng, X.; Shen, Y.; Wang, J. Molecularly Imprinted Polymer-Based Catalytic Micromotors for Selective Protein Transport. J. Am. Chem. Soc. 2013, 135, 5336–5339. [Google Scholar] [CrossRef]
- Li, S.; Zhu, M.; Whitcombe, M.J.; Piletsky, S.A.; Turner, A.P.F. Molecularly Imprinted Polymers for Enzyme-like Catalysis: Principle, Design, and Applications. In Molecularly Imprinted Catalysts; Li, S., Cao, S., Piletsky, S.A., Turner, A.P.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–17. [Google Scholar]
- Czulak, J.; Jakubiak-Marcinkowska, A.; Trochimczuk, A. Polymer Catalysts Imprinted with Metal Ions as Biomimics of Metalloenzymes. Adv. Mater. Sci. Eng. 2013, 2013, 464265. [Google Scholar] [CrossRef]
- Wei, W.; Zhou, T.; Wu, S.; Shen, X.; Zhu, M.; Li, S. An enzyme-like imprinted-polymer reactor with segregated quantum confinements for a tandem catalyst. RSC Adv. 2018, 8, 1610–1620. [Google Scholar] [CrossRef]
- Li, S.; Ge, Y.; Turner, A.P.F. A Catalytic and Positively Thermosensitive Molecularly Imprinted Polymer. Adv. Funct. Mater. 2011, 21, 1194–1200. [Google Scholar] [CrossRef]
- Kurczewska, J.; Cegłowski, M.; Pecyna, P.; Ratajczak, M.; Gajecka, M.; Schroeder, G. Molecularly imprinted polymer as drug delivery carrier in alginate dressing. Mater. Lett. 2017, 201, 46–49. [Google Scholar] [CrossRef]
- Fareghi, A.R.; Moghadam, P.N.; Khalafy, J.; Bahram, M.; Moghtader, M. Preparation of a new molecularly imprinted polymer based on self-crosslinkable cellulose acrylate in aqueous solution: A drug delivery system for furosemide. J. Appl. Polym. Sci. 2017, 134, 45581. [Google Scholar] [CrossRef]
- Anirudhan, T.; Divya, P.; Nima, J. Silylated montmorillonite based molecularly imprinted polymer for the selective binding and controlled release of thiamine hydrochloride. React. Funct. Polym. 2013, 73, 1144–1155. [Google Scholar] [CrossRef]
- Mohajeri, S.A.; Tabassi, S.A.S.; Moghadam, M.H. Preparation of a pH-sensitive pantoprazole-imprinted polymer and evaluation of its drug-binding and -releasing properties. Sci. China Ser. B Chem. 2014, 57, 857–865. [Google Scholar] [CrossRef]
- Kempe, H.; Pujolràs, A.P.; Kempe, M. Molecularly Imprinted Polymer Nanocarriers for Sustained Release of Erythromycin. Pharm. Res. 2014, 32, 375–388. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Bulko, T.V.; Baychorov, I.H.; Archakov, A.I. Molecularly imprinted polymers (MIP) in electroanalysis of proteins. Biochem. Suppl. Ser. B Biomed. Chem. 2016, 10, 145–151. [Google Scholar] [CrossRef]
- Liu, J.; Deng, Q.; Tao, D.; Yang, K.; Zhang, L.; Liang, Z.; Zhang, Y. Preparation of protein imprinted materials by hierarchical imprinting techniques and application in selective depletion of albumin from human serum. Sci. Rep. 2014, 4, 5487. [Google Scholar] [CrossRef]
- Kuwata, T.; Uchida, A.; Takano, E.; Kitayama, Y.; Takeuchi, T. Molecularly Imprinted Polymer Arrays as Synthetic Protein Chips Prepared by Transcription-type Molecular Imprinting by Use of Protein-Immobilized Dots as Stamps. Anal. Chem. 2015, 87, 11784–11791. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, C.; Świtnicka-Plak, M.A.; Halvorsen, T.G.; Cormack, P.A.; Sellergren, B.; Reubsaet, L. Automated Protein Biomarker Analysis: Online extraction of clinical samples by Molecularly Imprinted Polymers. Sci. Rep. 2017, 7, srep44298. [Google Scholar] [CrossRef]
- Demir, E.F.; Özçalışkan, E.; Karakaş, H.; Uygun, M.; Uygun, D.A.; Akgöl, S.; Denizli, A. Synthesis and characterization of albumin imprinted polymeric hydrogel membranes for proteomic studies. J. Biomater. Sci. Polym. Ed. 2018, 29, 2218–2236. [Google Scholar] [CrossRef]
- Ji, J.; Zhou, Z.; Zhao, X.; Sun, J.; Sun, X. Electrochemical sensor based on molecularly imprinted film at Au nanoparticles-carbon nanotubes modified electrode for determination of cholesterol. Biosens. Bioelectron. 2014, 66, 590–595. [Google Scholar] [CrossRef]
- Wang, F.-R.; Lee, G.-J.; Haridharan, N.; Wu, J.J. Electrochemical Sensor Using Molecular Imprinting Polymerization Modified Electrodes to Detect Methyl Parathion in Environmental Media. Electrocatalysis 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, L.; Huang, F.; Lin, Z. [Ru(bpy)3] 2+-mediated photoelectrochemical detection of bisphenol A on a molecularly imprinted polypyrrole modified SnO2 electrode. Anal. Chim. Acta 2015, 887, 59–66. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, Y.; Li, S.; Luo, R.; Wang, C. A molecularly imprinted electrochemical sensor based on sol–gel technology and multiwalled carbon nanotubes–Nafion functional layer for determination of 2-nonylphenol in environmental samples. Sens. Actuators B Chem. 2014, 193, 844–850. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, H.; Jiang, Y.; Xu, J.; Li, X.; Zhang, W.; Qiu, F. A molecularly-imprinted-electrochemical-sensor modified with nano-carbon-dots with high sensitivity and selectivity for rapid determination of glucose. Anal. Biochem. 2018, 555, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, T.; Weber, A.; Niedergall, K.; Riegler, J.; Bryniok, D.; Hirth, T.; Tovar, G.E.M. Water treatment by molecularly imprinted polymer nanoparticles. MRS Proc. 2009, 1169. [Google Scholar] [CrossRef]
- Shen, X.; Xu, C.; Ye, L. Molecularly Imprinted Polymers for Clean Water: Analysis and Purification. Indian Eng. Chem. Res. 2012, 52, 13890–13899. [Google Scholar] [CrossRef]
- Martínez, L.D.D.L.; Rodríguez-Aguilar, M.; Perez, R.O.; Gutiérrez-Hernández, J.M.; Díaz-Barriga, F.; Batres-Esquivel, L.; Flores-Ramírez, R. Synthesis and Evaluation of a Molecularly Imprinted Polymer for the Determination of Metronidazole in Water Samples. Bull. Environ. Contam. Toxicol. 2018, 100, 395–401. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, J.; Zhang, Y.; Zhou, X.; Liu, S. Application of Molecularly Imprinted Polymers to Selective Removal of Clofibric Acid from Water. PLoS ONE 2013, 8, e78167. [Google Scholar] [CrossRef] [PubMed]
- Okutucu, B.; Sanlıer, S.H. Decolorization of textile wastewater by dye-imprinted polymer. Desalin. Water Treat. 2015, 57, 21577–21584. [Google Scholar] [CrossRef]
- Yusof, N.A.; Zakaria, N.D.; Maamor, N.A.M.; Abdullah, A.H.; Haron, J. Synthesis and Characterization of Molecularly Imprinted Polymer Membrane for the Removal of 2,4-Dinitrophenol. Int. J. Mol. Sci. 2013, 14, 3993–4004. [Google Scholar] [CrossRef]
- Kashani, T.; Jahanshahi, M.; Rahimpour, A.; Peyravi, M. Nanopore Molecularly Imprinted Polymer Membranes for Environmental Usage: Selective Separation of 2,4-Dichlorophenoxyacetic Acid as a Toxic Herbicide from Water. Polym. Technol. Eng. 2016, 55, 1700–1712. [Google Scholar] [CrossRef]
- Ghasemi, S.; Nematollahzadeh, A. Molecularly imprinted ultrafiltration polysulfone membrane with specific nano-cavities for selective separation and enrichment of paclitaxel from plant extract. React. Funct. Polym. 2018, 126, 9–19. [Google Scholar] [CrossRef]
- Gao, B.; Li, Y.; Cui, K. Molecularly imprinted membrane with innovative structure and high performance for chiral separation of amino acids. Int. J. Polym. Mater. 2017, 67, 517–527. [Google Scholar] [CrossRef]
- Ng, M.H.K.; Leo, C.P.; Abdullah, A.Z. Selective removal of dyes by molecular imprinted TiO2 nanoparticles in polysulfone ultrafiltration membrane. J. Environ. Chem. Eng. 2017, 5, 3991–3998. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2018, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Wulff, G. The role of binding-site interactions in the molecular imprinting of polymers. Trends Biotechnol. 1993, 11, 85–87. [Google Scholar] [CrossRef]
- Cormack, P.A.; Elorza, A.Z. Molecularly imprinted polymers: Synthesis and characterisation. J. Chromatogr. B 2004, 804, 173–182. [Google Scholar] [CrossRef]
- Yemiş, F.; Alkan, P.; Yenigül, B.; Yenigül, M. Molecularly Imprinted Polymers and Their Synthesis by Different Methods. Polym. Polym. Compos. 2013, 21, 145–150. [Google Scholar] [CrossRef]
- Lin, Z.; DeMarr, V.; Bao, J.; Wu, T. Molecularly Imprinted Polymer-Based Biosensors: For the Early, Rapid Detection of Pathogens, Biomarkers, and Toxins in Clinical, Environmental, or Food Samples. IEEE Nanotechnol. Mag. 2018, 12, 6–13. [Google Scholar] [CrossRef]
- Yan, H.; Row, K.H. Characteristic and Synthetic Approach of Molecularly Imprinted Polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Lee, S.H.; Doong, R.A. Design of Size-Tunable Molecularly Imprinted Polymer for Selective Adsorption of Pharmaceuticals and Biomolecules. J. Biosens. Bioelectron. 2016, 7, 4. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Zaidi, S.A. Molecular imprinting polymers and their composites: A promising material for diverse applications. Biomater. Sci. 2017, 5, 388–402. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2020, 13, 49. [Google Scholar] [CrossRef]
- Blanco-López, M.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.; Tuñón-Blanco, P. Electrochemical sensors based on molecularly imprinted polymers. Trends Anal. Chem. 2004, 23, 36–48. [Google Scholar] [CrossRef]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers—Persistent Models and New Concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef]
- Ertürk, G.; Mattiasson, B. Molecular Imprinting Techniques Used for the Preparation of Biosensors. Sensors 2017, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Rachkov, A.; Minoura, N. Towards molecularly imprinted polymers selective to peptides and proteins. The epitope approach. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 2001, 1544, 255–266. [Google Scholar] [CrossRef]
- Justino, C.I.; Freitas, A.; Pereira, R.; Duarte, A.C.; Santos, T.A.R. Recent developments in recognition elements for chemical sensors and biosensors. Trends Anal. Chem. 2015, 68, 2–17. [Google Scholar] [CrossRef]
- Algieri, C.; Drioli, E.; Guzzo, L.; Donato, L. Bio-Mimetic Sensors Based on Molecularly Imprinted Membranes. Sensors 2014, 14, 13863–13912. [Google Scholar] [CrossRef]
- Sanjuán, A.M.; Ruiz, J.A.R.; García, F.C.; García, J.M. Recent developments in sensing devices based on polymeric systems. React. Funct. Polym. 2018, 133, 103–125. [Google Scholar] [CrossRef]
- Dhanjai; Sinha, A.; Wu, L.; Lu, X.; Chen, J.; Jain, R. Advances in sensing and biosensing of bisphenols: A review. Anal. Chim. Acta 2018, 998, 1–27. [Google Scholar] [CrossRef]
- Malinauskas, A. Electrochemical response of ascorbic acid at conducting and electrogenerated polymer modified electrodes for electroanalytical applications: A review. Talanta 2004, 64, 121–129. [Google Scholar] [CrossRef]
- Rahmadhani, S.; Setiyanto, H.; Zulfikar, M.A. Electropolymerized of aniline as a new molecularly imprinted polymer for determination of phenol: A study for phenol sensor. In Proceedings of the 2017 International Seminar on Sensors, Instrumentation, Measurement and Metrology (ISSIMM), Surabaya, Indonesia, 25–26 August 2017; pp. 124–128. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Ansell, R.J. N-isopropylacrylamide as a functional monomer for noncovalent molecular imprinting. J. Mol. Recognit. 2011, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shumyantseva, V.V.; Bulko, T.V.; Suprun, E.V.; Kuzikov, A.V.; Agafonova, L.E.; Archakov, A.I. Electrochemical methods in biomedical studies. Biochem. Suppl. Ser. B Biomed. Chem. 2015, 9, 228–243. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Zhao, F.; Zhang, W.; Ye, Z. Molecularly imprinted polymer-based sensors for atrazine detection by electropolymerization of o-phenylenediamine. RSC Adv. 2015, 5, 56534–56540. [Google Scholar] [CrossRef]
- Mazzotta, E.; Turco, A.; Chianella, I.; Guerreiro, A.; Piletsky, S.A.; Malitesta, C. Solid-phase synthesis of electroactive nanoparticles of molecularly imprinted polymers. A novel platform for indirect electrochemical sensing applications. Sens. Actuators B Chem. 2016, 229, 174–180. [Google Scholar] [CrossRef]
- Udomsap, D.; Brisset, H.; Culioli, G.; Dollet, P.; Laatikainen, K.; Siren, H.; Branger, C. Electrochemical molecularly imprinted polymers as material for pollutant detection. Mater. Today Commun. 2018, 17, 458–465. [Google Scholar] [CrossRef]
- Rebocho, S.; Cordas, C.; Viveiros, R.; Casimiro, T. Development of a ferrocenyl-based MIP in supercritical carbon dioxide: Towards an electrochemical sensor for bisphenol A. J. Supercrit. Fluids 2018, 135, 98–104. [Google Scholar] [CrossRef]
- Ekomo, V.M.; Branger, C.; Gavrila, A.-M.; Sarbu, A.; Koutsouras, D.A.; Stolz, C.; Malliaras, G.G.; Brisset, H. Electrochemical molecularly imprinted polymers in microelectrode devices. MRS Commun. 2020, 10, 324–331. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; He, X.; Chen, L.; Zhang, Y. m-Aminophenylboronic acid as a functional monomer for fabricating molecularly imprinted polymer for the recognition of bovine serum albumin. React. Funct. Polym. 2008, 68, 1291–1296. [Google Scholar] [CrossRef]
- Haupt, K. Peer Reviewed: Molecularly Imprinted Polymers: The Next Generation. Anal. Chem. 2003, 75, 376A–383A. [Google Scholar] [CrossRef]
- Yuan, W.; Cai, Y.; Chen, Y.; Hong, X.; Liu, Z. Porous microsphere and its applications. Int. J. Nanomed. 2013, 8, 1111–1120. [Google Scholar] [CrossRef]
- Wackerlig, J.; Lieberzeit, P.A. Molecularly imprinted polymer nanoparticles in chemical sensing—Synthesis, characterisation and application. Sen. Actuators B Chem. 2015, 207, 144–157. [Google Scholar] [CrossRef]
- He, J.; Tang, H.; You, L.; Yuan, L.; Liu, Z.; Zhu, J.; Lu, K.; Chen, X. Synthesis of Fragment-Imprinted Microspheres of 2,6-Dichloropyrimidine as Templates and Determination of Sulfonamides in Milk Samples. Chromatographia 2013, 76, 959–965. [Google Scholar] [CrossRef]
- Lv, Y.-K.; Zhao, C.-X.; Li, P.; He, Y.-D.; Yang, Z.-R.; Sun, H.-W. Preparation of doxycycline-imprinted magnetic microspheres by inverse-emulsion suspension polymerization for magnetic dispersion extraction of tetracyclines from milk samples. J. Sep. Sci. 2013, 36, 2656–2663. [Google Scholar] [CrossRef]
- Su, X.; Li, X.; Li, J.; Liu, M.; Lei, F.; Tan, X.; Li, P.; Luo, W. Synthesis and characterization of core–shell magnetic molecularly imprinted polymers for solid-phase extraction and determination of Rhodamine B in food. Food Chem. 2014, 171, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Mayes, A.G.; Mosbach, K. Molecularly Imprinted Polymer Beads: Suspension Polymerization Using a Liquid Perfluorocarbon as the Dispersing Phase. Anal. Chem. 1996, 68, 3769–3774. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Yun, Y.; Li, Q.; Huang, Z.; Liang, Z. Preparation and characterization of monodisperse molecularly imprinted polymer microspheres by precipitation polymerization for kaempferol. Des. Monomers Polym. 2016, 20, 201–209. [Google Scholar] [CrossRef]
- Kitabatake, T.; Tabo, H.; Matsunaga, H.; Haginaka, J. Preparation of monodisperse curcumin-imprinted polymer by precipitation polymerization and its application for the extraction of curcuminoids from Curcuma longa L. Anal. Bioanal. Chem. 2013, 405, 6555–6561. [Google Scholar] [CrossRef]
- Yu, Z.; Su, Q.; Tang, Y.; Xu, Z. Preparation and evaluation of aconitine imprinted microspheres and its application to body fluid samples. J. Appl. Polym. Sci. 2012, 128, 3425–3431. [Google Scholar] [CrossRef]
- Zhao, C.; Dai, J.; Zhou, Z.; Dai, X.; Zou, Y.; Yu, P.; Zou, T.; Li, C.; Yan, Y. One-pot method for obtaining hydrophilic tetracycline-imprinted particles via precipitation polymerization in ethanol. J. Appl. Polym. Sci. 2013, 131, 1–11. [Google Scholar] [CrossRef]
- Puoci, F.; Hampel, S.; Parisi, O.I.; Hassan, A.; Cirillo, G.; Picci, N. Imprinted microspheres doped with carbon nanotubes as novel electroresponsive drug-delivery systems. J. Appl. Polym. Sci. 2013, 130, 829–834. [Google Scholar] [CrossRef]
- Zhou, T.; Shen, X.; Chaudhary, S.; Ye, L. Molecularly imprinted polymer beads prepared by pickering emulsion polymerization for steroid recognition. J. Appl. Polym. Sci. 2013, 131, 1–7. [Google Scholar] [CrossRef]
- Hang, H.; Li, C.; Pan, J.; Li, L.; Dai, J.; Dai, X.; Yu, P.; Feng, Y. Selective separation of lambdacyhalothrin by porous/magnetic molecularly imprinted polymers prepared by Pickering emulsion polymerization. J. Sep. Sci. 2013, 36, 3285–3294. [Google Scholar] [CrossRef]
- Zhou, T.; Kamra, T.; Ye, L. Preparation of diclofenac-imprinted polymer beads for selective molecular separation in water. J. Mol. Recognit. 2017, 31, e2608. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, L.; Hang, H.; Wu, R.; Dai, X.; Shi, W.; Yan, Y. Fabrication and Evaluation of Magnetic/Hollow Double-Shelled Imprinted Sorbents Formed by Pickering Emulsion Polymerization. Langmuir 2013, 29, 8170–8178. [Google Scholar] [CrossRef]
- Li, X.-J.; Zhong, S.-A.; Li, C.-E. Synthesis of novel photoresponsive molecularly imprinted polymer microspheres with special binding properties. J. Appl. Polym. Sci. 2013, 130, 869–876. [Google Scholar] [CrossRef]
- Zheng, X.-F.; Lian, Q.; Yang, H.; Wang, X. Surface Molecularly Imprinted Polymer of Chitosan Grafted Poly(methyl methacrylate) for 5-Fluorouracil and Controlled Release. Sci. Rep. 2016, 6, 21409. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Feng, R.; Qi, N.; Zhang, Y.-P.; Bai, L.-Y.; Huang, M.-X. Synthesis and Evaluation of Cyromazine Molecularly Imprinted Polymeric Microspheres by Two-Step Seed Swelling Polymerization. Asian J. Chem. 2013, 25, 8329–8332. [Google Scholar] [CrossRef]
- Chen, J.; Bai, L.; Zhang, Y.; Chen, N.; Zhang, Y. Fabrication of Atrazine Molecularly Imprinted Polymer Microsphere by Two Step Seed Swelling Polymerization Method. J. Chin. Chem. Soc. 2012, 59, 1493–1499. [Google Scholar] [CrossRef]
- Qi, N.; Chen, J.; Zhang, Y.P.; Zhang, Y.J.; Bai, L.Y. Preparation of Melamine Molecularly Imprinted Polymeric Microspheres by Two-Step Seed Swelling Polymerization. Adv. Mater. Res. 2013, 668, 128–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, X. Preparation of molecularly imprinted polymer for vanillin via seed swelling and suspension polymerization. Polym. Sci. Ser. B 2014, 56, 538–545. [Google Scholar] [CrossRef]
- Wolska, J.; Bryjak, M. Removal of Bisphenol A from Aqueous Solution by Molecularly Imprinted Polymers. Sep. Sci. Technol. 2014, 49, 1643–1653. [Google Scholar] [CrossRef]
- Shi, X.; Wu, A.; Zheng, S.; Li, R.; Zhang, D. Molecularly imprinted polymer microspheres for solid-phase extraction of chloramphenicol residues in foods. J. Chromatogr. B 2007, 850, 24–30. [Google Scholar] [CrossRef]
- Brooks, B. Suspension Polymerization Processes. Chem. Eng. Technol. 2010, 33, 1737–1744. [Google Scholar] [CrossRef]
- Nabavi, S.A.; Vladisavljević, G.T.; Eguagie, E.M.; Li, B.; Georgiadou, S.; Manović, V. Production of spherical mesoporous molecularly imprinted polymer particles containing tunable amine decorated nanocavities with CO2 molecule recognition properties. Chem. Eng. J. 2016, 306, 214–225. [Google Scholar] [CrossRef]
- Qian, L.-W.; Hu, X.-L.; Guan, P.; Gao, B.; Wang, D.; Wang, C.-L.; Li, J.; Du, C.-B.; Song, W.-Q. Thermal preparation of lysozyme-imprinted microspheres by using ionic liquid as a stabilizer. Anal. Bioanal. Chem. 2014, 406, 7221–7231. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Gong, S. Preparation of molecularly imprinted polymers for vanillin via reversible addition-fragmentation chain transfer suspension polymerization. J. Appl. Polym. Sci. 2012, 128, 2927–2932. [Google Scholar] [CrossRef]
- Suwanwong, Y.; Kulkeratiyut, S.; Prachayasittikul, V.; Boonpangrak, S. Effects of Polymerization Methods and Functional Monomers on Curcumin Imprinted Polymer Properties. Sep. Sci. Technol. 2014, 49, 1086–1095. [Google Scholar] [CrossRef]
- Sun, H.; Lai, J.-P.; Chen, F.; Zhu, D.-R. Molecularly imprinted microspheres synthesized by a simple, fast, and universal suspension polymerization for selective extraction of the topical anesthetic benzocaine in human serum and fish tissues. Anal. Bioanal. Chem. 2015, 407, 1745–1752. [Google Scholar] [CrossRef]
- Moreno Bondi, M.C.; Urraca, J.L.; Carrasco, S.; Navarro-Villoslada, F. Preparation of Molecularly Imprinted Polymers. In Handbook of Molecularly Imprinted Polymers; Álvarez-Lorenzo, C., Concheiro, Á., Eds.; Smithers Rapra Technology Ltd.: Billingham, UK, 2013. [Google Scholar]
- Li, G.L.; Möhwald, H.; Shchukin, D.G. Precipitation polymerization for fabrication of complex core–shell hybrid particles and hollow structures. Chem. Soc. Rev. 2013, 42, 3628–3646. [Google Scholar] [CrossRef]
- Cacho, C.; Turiel, E.; Pérez-Conde, C. Molecularly imprinted polymers: An analytical tool for the determination of benzimidazole compounds in water samples. Talanta 2009, 78, 1029–1035. [Google Scholar] [CrossRef]
- Pardeshi, S.; Dhodapkar, R.; Kumar, A. Molecularly imprinted microspheres and nanoparticles prepared using precipitation polymerisation method for selective extraction of gallic acid from Emblica officinalis. Food Chem. 2013, 146, 385–393. [Google Scholar] [CrossRef]
- Ye, L.; Cormack, P.A.G.; Mosbach, K. Molecularly imprinted monodisperse microspheres for competitive radioassay. Anal. Commun. 1999, 36, 35–38. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Han, J.; Dong, X. Synthesis of tetracycline-imprinted polymer microspheres by reversible addition-fragmentation chain-transfer precipitation polymerization using polyethylene glycol as a coporogen. J. Sep. Sci. 2014, 37, 1118–1125. [Google Scholar] [CrossRef]
- Gao, F.-X.; Ma, X.-T.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Smart surface imprinting polymer nanospheres for selective recognition and separation of glycoprotein. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 191–199. [Google Scholar] [CrossRef]
- Azodi-Deilami, S.; Abdouss, M.; Kordestani, D.; Shariatinia, Z. Preparation of N,N-p-phenylene bismethacryl amide as a novel cross-link agent for synthesis and characterization of the core–shell magnetic molecularly imprinted polymer nanoparticles. J. Mater. Sci. Mater. Electron. 2013, 25, 645–656. [Google Scholar] [CrossRef]
- Yao, T.; Gu, X.; Li, T.; Li, J.; Li, J.; Zhao, Z.; Wang, J.; Qin, Y.; She, Y. Enhancement of surface plasmon resonance signals using a MIP/GNPs/rGO nano-hybrid film for the rapid detection of ractopamine. Biosens. Bioelectron. 2015, 75, 96–100. [Google Scholar] [CrossRef]
- Tan, F.; Zhao, Q.; Teng, F.; Sun, D.; Gao, J.; Quan, X.; Chen, J. Molecularly imprinted polymer/mesoporous carbon nanoparticles as electrode sensing material for selective detection of ofloxacin. Mater. Lett. 2014, 129, 95–97. [Google Scholar] [CrossRef]
- Sellergren, B.; Rückert, B.; Hall, A. Layer-by-Layer Grafting of Molecularly Imprinted Polymers via Iniferter Modified Supports. Adv. Mater. 2002, 14, 1204–1208. [Google Scholar] [CrossRef]
- Rückert, B.; Hall, A.J.; Sellergren, B. Molecularly imprinted composite materials via iniferter-modified supports. J. Mater. Chem. 2002, 12, 2275–2280. [Google Scholar] [CrossRef]
- Barahona, F.; Turiel, E.; Cormack, P.A.G.; Martín-Esteban, A. Chromatographic performance of molecularly imprinted polymers: Core-shell microspheres by precipitation polymerization and grafted MIP films via iniferter-modified silica beads. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1058–1066. [Google Scholar] [CrossRef]
- Minko, S. Grafting on Solid Surfaces: “Grafting to”and “Grafting from” Methods. In Polymer Surfaces and Interfaces: Characterization, Modification and Applications; Stamm, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 215–234. [Google Scholar]
- Chern, C. Emulsion polymerization mechanisms and kinetics. Prog. Polym. Sci. 2006, 31, 443–486. [Google Scholar] [CrossRef]
- Yamak, H.B. Emulsion Polymerization: Effects of Polymerization Variables on the Properties of Vinyl Acetate Based Emulsion Polymers. In Polymer Science; Yilmaz, F., Ed.; InTech: London, UK, 2013. [Google Scholar]
- Matsui, J.; Kato, T.; Takeuchi, T.; Suzuki, M.; Yokoyama, K.; Tamiya, E.; Karube, I. Molecular recognition in continuous polymer rods prepared by a molecular imprinting technique. Anal. Chem. 1993, 65, 2223–2224. [Google Scholar] [CrossRef]
- Matsui, J.; Miyoshi, Y.; Matsui, R.; Takeuchi, T. Rod-Type Affinity Media for Liquid Chromatography Prepared by in-situ-Molecular Imprinting. Anal. Sci. 1995, 11, 1017–1019. [Google Scholar] [CrossRef]
- MacDougall, D.; Francis, J.A.; Cox, G.V.; Grosby, D.G.; Estes, F.L.; Freeman, D.H.; Gibbs, W.E.; Gordon, G.E.; Keith, L.H.; Lal, J.; et al. Guidelines for data acquisition and data quality evaluation in environmental chemistry. Anal. Chem. 1980, 52, 2242–2249. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C. Handbook of Molecularly Imprinted Polymers; Smithers Information Ltd.: Akron, OH, USA, 2013. [Google Scholar]
- Liu, C.-C. Electrochemical Sensors. In The Biomedical Engineering Handbook Medical Devices and Systems, 3rd ed.; Bronzino, J.D., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- Jolly, P.; Tamboli, V.; Harniman, R.L.; Estrela, P.; Allender, C.J.; Bowen, J.L. Aptamer–MIP hybrid receptor for highly sensitive electrochemical detection of prostate specific antigen. Biosens. Bioelectron. 2016, 75, 188–195. [Google Scholar] [CrossRef]
- Bakas, I.; Hayat, A.; Piletsky, S.; Piletska, E.; Chehimi, M.M.; Noguer, T.; Rouillon, R. Electrochemical impedimetric sensor based on molecularly imprinted polymers/sol–gel chemistry for methidathion organophosphorous insecticide recognition. Talanta 2014, 130, 294–298. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, H.; Chen, M.; Zhang, X.; Wang, S. Surface-enhanced molecularly imprinted electrochemiluminescence sensor based on Ru@SiO 2 for ultrasensitive detection of fumonisin B1. Biosens. Bioelectron. 2017, 96, 55–61. [Google Scholar] [CrossRef]
- Graniczkowska, K.; Pütz, M.; Hauser, F.M.; De Saeger, S.; Beloglazova, N.V. Capacitive sensing of N-formylamphetamine based on immobilized molecular imprinted polymers. Biosens. Bioelectron. 2017, 92, 741–747. [Google Scholar] [CrossRef]
- Lenain, P.; de Saeger, S.; Mattiasson, B.; Hedström, M. Affinity sensor based on immobilized molecular imprinted synthetic recognition elements. Biosens. Bioelectron. 2015, 69, 34–39. [Google Scholar] [CrossRef]
- Warwick, C.; Guerreiro, A.; Gomez-Caballero, A.; Wood, E.; Kitson, J.; Robinson, J.; Soares, A. Conductance based sensing and analysis of soluble phosphates in wastewater. Biosens. Bioelectron. 2013, 52, 173–179. [Google Scholar] [CrossRef]
- Rosy; Chasta, H.; Goyal, R.N. Molecularly imprinted sensor based on o-aminophenol for the selective determination of norepinephrine in pharmaceutical and biological samples. Talanta 2014, 125, 167–173. [Google Scholar] [CrossRef]
- Uygun, Z.O.; Dilgin, Y. A novel impedimetric sensor based on molecularly imprinted polypyrrole modified pencil graphite electrode for trace level determination of chlorpyrifos. Sens. Actuators B Chem. 2013, 188, 78–84. [Google Scholar] [CrossRef]
- Ratautaite, V.; Janssens, S.; Haenen, K.; Nesládek, M.; Ramanaviciene, A.; Baleviciute, I.; Ramanavicius, A. Molecularly Imprinted Polypyrrole Based Impedimentric Sensor for Theophylline Determination. Electrochim. Acta 2014, 130, 361–367. [Google Scholar] [CrossRef]
- Gurtova, O.; Ye, L.; Chmilenko, F. Potentiometric propranolol-selective sensor based on molecularly imprinted polymer. Anal. Bioanal. Chem. 2012, 405, 287–295. [Google Scholar] [CrossRef]
- Bagheri, H.; Shirzadmehr, A.; Rezaei, M. Designing and fabrication of new molecularly imprinted polymer-based potentiometric nano-graphene/ionic liquid/carbon paste electrode for the determination of losartan. J. Mol. Liq. 2015, 212, 96–102. [Google Scholar] [CrossRef]
- Basozabal, I.; Guerreiro, A.; Gomez-Caballero, A.; Goicolea, M.A.; Barrio, R.J. Direct potentiometric quantification of histamine using solid-phase imprinted nanoparticles as recognition elements. Biosens. Bioelectron. 2014, 58, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Alexander, S. Design and fabrication of molecularly imprinted polymer-based potentiometric sensor from the surface modified multiwalled carbon nanotube for the determination of lindane (γ-hexachlorocyclohexane), an organochlorine pesticide. Biosens. Bioelectron. 2015, 64, 586–593. [Google Scholar] [CrossRef]
- Rizk, M.; Toubar, S.S.; Sayour, H.E.E.-D.; Mohamed, D.; Touny, R.M. A new potentiometric sensor based on molecularly imprinted polymer for analysis of a veterinary drug imidocarb dipropionate. Eur. J. Chem. 2014, 5, 18–23. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Liang, Y.; Liu, Y. Designing of molecularly imprinted polymer-based potentiometric sensor for the determination of heparin. Anal. Biochem. 2013, 434, 242–246. [Google Scholar] [CrossRef]
- Kou, L.-J.; Liang, R.-N.; Wang, X.-W.; Chen, Y.; Qin, W. Potentiometric sensor for determination of neutral bisphenol A using a molecularly imprinted polymer as a receptor. Anal. Bioanal. Chem. 2013, 405, 4931–4936. [Google Scholar] [CrossRef]
- Zarezadeh, A.; Rajabi, H.R.; Sheydaei, O.; Khajehsharifi, H. Application of a nano-structured molecularly imprinted polymer as an efficient modifier for the design of captopril drug selective sensor: Mechanism study and quantitative determination. Mater. Sci. Eng. C 2018, 94, 879–885. [Google Scholar] [CrossRef]
- Mamo, S.K.; Gonzalez-Rodriguez, J. Development of a Molecularly Imprinted Polymer-Based Sensor for the Electrochemical Determination of Triacetone Triperoxide (TATP). Sensors 2014, 14, 23269–23282. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, B.; Cui, L.; Wang, M. Molecularly imprinted electrochemical sensor for the highly selective and sensitive determination of melamine. Mater. Sci. Eng. C 2015, 55, 457–461. [Google Scholar] [CrossRef]
- Tan, X.; Hu, Q.; Wu, J.; Li, X.; Li, P.; Yu, H.; Li, X.; Lei, F. Electrochemical sensor based on molecularly imprinted polymer reduced graphene oxide and gold nanoparticles modified electrode for detection of carbofuran. Sens. Actuators B Chem. 2015, 220, 216–221. [Google Scholar] [CrossRef]
- Zhao, L.; Zeng, B.; Zhao, F. Electrochemical determination of tartrazine using a molecularly imprinted polymer—Multiwalled carbon nanotubes—Ionic liquid supported Pt nanoparticles composite film coated electrode. Electrochim. Acta 2014, 146, 611–617. [Google Scholar] [CrossRef]
- Pacheco, J.; Castro, M.; Machado, S.; Barroso, M.F.; Nouws, H.; Delerue-Matos, C. Molecularly imprinted electrochemical sensor for ochratoxin A detection in food samples. Sens. Actuators B Chem. 2015, 215, 107–112. [Google Scholar] [CrossRef]
- Nezhadali, A.; Mojarrab, M. Fabrication of an electrochemical molecularly imprinted polymer triamterene sensor based on multivariate optimization using multi-walled carbon nanotubes. J. Electroanal. Chem. 2015, 744, 85–94. [Google Scholar] [CrossRef]
- Lei, R.; Guo, C.; Xiong, H.; Dong, C.; Zhang, X.; Wang, S. A Novel Electrochemical Sensor for β2-Agonists with High Sensitivity and Selectivity Based on Surface Molecularly Imprinted Sol-gel Doped with Antimony-Doped Tin Oxide. Electroanalysis 2014, 26, 1004–1012. [Google Scholar] [CrossRef]
- Gholivand, M.; Torkashvand, M. The fabrication of a new electrochemical sensor based on electropolymerization of nanocomposite gold nanoparticle-molecularly imprinted polymer for determination of valganciclovir. Mater. Sci. Eng. C 2016, 59, 594–603. [Google Scholar] [CrossRef]
- Li, Y.; Song, H.; Zhang, L.; Zuo, P.; Ye, B.-C.; Yao, J.; Chen, W. Supportless electrochemical sensor based on molecularly imprinted polymer modified nanoporous microrod for determination of dopamine at trace level. Biosens. Bioelectron. 2016, 78, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhao, F. Electrochemical sensor for dimetridazole based on novel gold nanoparticles@molecularly imprinted polymer. Sens. Actuators B Chem. 2015, 220, 1017–1022. [Google Scholar] [CrossRef]
- Dadkhah, S.; Ziaei, E.; Mehdinia, A.; Kayyal, T.B.; Jabbari, A. A glassy carbon electrode modified with amino-functionalized graphene oxide and molecularly imprinted polymer for electrochemical sensing of bisphenol A. Microchim. Acta 2016, 183, 1933–1941. [Google Scholar] [CrossRef]
- Silva, H.; Pacheco, J.; Silva, J.; Viswanathan, S.; Delerue-Matos, C. Molecularly imprinted sensor for voltammetric detection of norfloxacin. Sens. Actuators B Chem. 2015, 219, 301–307. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, F.; Quan, X.; Yu, H.; Chen, S.; Zhao, H.; Liu, Z.; Hilal, N. An electrochemical sensor for selective determination of sulfamethoxazole in surface water using a molecularly imprinted polymer modified BDD electrode. Anal. Methods 2015, 7, 2693–2698. [Google Scholar] [CrossRef]
- Kumar, N.; Rosy; Goyal, R.N. A melamine based molecularly imprinted sensor for the determination of 8-hydroxydeoxyguanosine in human urine. Talanta 2017, 166, 215–222. [Google Scholar] [CrossRef]
- Toro, M.J.U.; Marestoni, L.D.; Sotomayor, M.D.P.T. A new biomimetic sensor based on molecularly imprinted polymers for highly sensitive and selective determination of hexazinone herbicide. Sens. Actuators B Chem. 2015, 208, 299–306. [Google Scholar] [CrossRef]
- Rao, H.; Chen, M.; Ge, H.; Lu, Z.; Liu, X.; Zou, P.; Wang, X.; He, H.; Zeng, X.; Wang, Y. A novel electrochemical sensor based on Au@PANI composites film modified glassy carbon electrode binding molecular imprinting technique for the determination of melamine. Biosens. Bioelectron. 2016, 87, 1029–1035. [Google Scholar] [CrossRef]
- Cai, R.; Rao, W.; Zhang, Z.; Long, F.; Yin, Y. An imprinted electrochemical sensor for bisphenol A determination based on electrodeposition of a graphene and Ag nanoparticle modified carbon electrode. Anal. Methods 2014, 6, 1590–1597. [Google Scholar] [CrossRef]
- Akhoundian, M.; Rüter, A.; Shinde, S. Ultratrace Detection of Histamine Using a Molecularly-Imprinted Polymer-Based Voltammetric Sensor. Sensors 2017, 17, 645. [Google Scholar] [CrossRef]
- Prasad, B.B.; Singh, K. An electroconducting copper (II) imprinted sensor using algae as cheap substitute of multiwalled carbon nanotubes. Electrochim. Acta 2016, 187, 193–203. [Google Scholar] [CrossRef]
- Tan, Y.; Jin, J.; Zhang, S.; Shi, Z.; Wang, J.; Zhang, J.; Pu, W.; Yang, C. Electrochemical Determination of Bisphenol A Using a Molecularly Imprinted Chitosan-acetylene Black Composite Film Modified Glassy Carbon Electrode. Electroanalysis 2015, 28, 189–196. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Z.; Kuang, Y. Electrochemical determination of bisphenol A in plastic bottled drinking water and canned beverages using a molecularly imprinted chitosan–graphene composite film modified electrode. Food Chem. 2014, 157, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Motia, S.; Tudor, I.A.; Popescu, L.M.C.; Piticescu, R.M.; Bouchikhi, B.; El Bari, N. Development of a novel electrochemical sensor based on electropolymerized molecularly imprinted polymer for selective detection of sodium lauryl sulfate in environmental waters and cosmetic products. J. Electroanal. Chem. 2018, 823, 553–562. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhang, Y.; Gu, M.; Wang, D.; Dang, Y.-Y.; Ye, B.-C.; Li, Y. A robust electrochemical sensing platform using carbon paste electrode modified with molecularly imprinted microsphere and its application on methyl parathion detection. Biosens. Bioelectron. 2018, 106, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yan, X.; Li, C.; Zheng, B.; Li, Y.; Liu, W.; Zhang, Z.; Yang, M. Biomimetic sensor based on molecularly imprinted polymer with nitroreductase-like activity for metronidazole detection. Biosens. Bioelectron. 2015, 77, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-J.; Zhang, Z.-H.; Cai, R.; Kong, X.-Q.; Chen, X.; Liu, Y.-N.; Yao, S.-Z. Molecularly imprinted electrochemical sensor based on a reduced graphene modified carbon electrode for tetrabromobisphenol A detection. Analyst 2013, 138, 2769–2776. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Cai, R.; Rao, W.; Long, F. Molecularly imprinted electrochemical sensor based on nickel nanoparticles-graphene nanocomposites modified electrode for determination of tetrabromobisphenol A. Electrochim. Acta 2014, 117, 385–392. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, R.; Long, F.; Wang, J. Development and application of tetrabromobisphenol A imprinted electrochemical sensor based on graphene/carbon nanotubes three-dimensional nanocomposites modified carbon electrode. Talanta 2015, 134, 435–442. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; Liu, M.; Deng, P.; Tang, S.; Jiang, J.; Feng, H.; Qian, D.; He, L. Ag/N-doped reduced graphene oxide incorporated with molecularly imprinted polymer: An advanced electrochemical sensing platform for salbutamol determination. Biosens. Bioelectron. 2017, 90, 210–216. [Google Scholar] [CrossRef]
- Hassan, A.H.; Moura, S.L.; Ali, F.; Moselhy, W.A.; Sotomayor, M.; Pividori, M.I. Electrochemical sensing of methyl parathion on magnetic molecularly imprinted polymer. Biosens. Bioelectron. 2018, 118, 181–187. [Google Scholar] [CrossRef]
- Anirudhan, T.; Athira, V.; Sekhar, V.C. Electrochemical sensing and nano molar level detection of Bisphenol-A with molecularly imprinted polymer tailored on multiwalled carbon nanotubes. Polymer 2018, 146, 312–320. [Google Scholar] [CrossRef]
- Nezhadali, A.; Bonakdar, G.A. Multivariate optimization of mebeverine analysis using molecularly imprinted polymer electrochemical sensor based on silver nanoparticles. J. Food Drug Anal. 2018, 27, 305–314. [Google Scholar] [CrossRef]
- Tan, F.; Cong, L.; Li, X.; Zhao, Q.; Zhao, H.; Quan, X.; Chen, J. An electrochemical sensor based on molecularly imprinted polypyrrole/graphene quantum dots composite for detection of bisphenol A in water samples. Sens. Actuators B Chem. 2016, 233, 599–606. [Google Scholar] [CrossRef]
- Karimian, N.; Zavar, M.H.A.; Chamsaz, M.; Turner, A.; Tiwari, A. On/off-switchable electrochemical folic acid sensor based on molecularly imprinted polymer electrode. Electrochem. Commun. 2013, 36, 92–95. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, C.; Zeng, Y.; Liu, H.; Yin, Z.; Li, L. Determination of Bisphenol a Using an Electrochemical Sensor Based on a Molecularly Imprinted Polymer-Modified Multiwalled Carbon Nanotube Paste Electrode. Anal. Lett. 2014, 47, 996–1014. [Google Scholar] [CrossRef]
- Saksena, K.; Shrivastava, A.; Kant, R. Chiral analysis of ascorbic acid in bovine serum using ultrathin molecular imprinted polyaniline/graphite electrode. J. Electroanal. Chem. 2017, 795, 103–109. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Z.; Li, J.; Kuang, Y. Acetylene black paste electrode modified with a molecularly imprinted chitosan film for the detection of bisphenol A. Microchim. Acta 2013, 180, 861–869. [Google Scholar] [CrossRef]
- Nie, D.; Han, Z.; Yu, Y.; Shi, G. Composites of multiwalled carbon nanotubes/polyethyleneimine (MWCNTs/PEI) and molecularly imprinted polymers for dinitrotoluene recognition. Sens. Actuators B Chem. 2016, 224, 584–591. [Google Scholar] [CrossRef]
- Huang, X.; Wei, S.; Yao, S.; Zhang, H.; He, C.; Cao, J. Development of molecularly imprinted electrochemical sensor with reduced graphene oxide and titanium dioxide enhanced performance for the detection of toltrazuril in chicken muscle and egg. J. Pharm. Biomed. Anal. 2018, 164, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Singh, R.; Singh, K.; Fatma, S.; Prasad, B.B. Enantioselective analysis of D- and l- Serine on a layer-by-layer imprinted electrochemical sensor. Biosens. Bioelectron. 2018, 124–125, 176–183. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Development of cardiac troponin-I biosensor based on boron nitride quantum dots including molecularly imprinted polymer. Biosens. Bioelectron. 2018, 126, 418–424. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, L.; Waterhouse, G.I.; Wang, M.; Qiao, X.; Xu, Z. Novel three-dimensional electrochemical sensor with dual signal amplification based on MoS2 nanosheets and high-conductive NH2-MWCNT@COF for sulfamerazine determination. Sens. Actuators B Chem. 2018, 281, 107–114. [Google Scholar] [CrossRef]
- Alizadeh, T.; Atashi, F.; Ganjali, M.R. Molecularly imprinted polymer nano-sphere/multi-walled carbon nanotube coated glassy carbon electrode as an ultra-sensitive voltammetric sensor for picomolar level determination of RDX. Talanta 2018, 194, 415–421. [Google Scholar] [CrossRef]
- Yu, R.; Zhou, H.; Li, M.; Song, Q. Rational selection of the monomer for molecularly imprinted polymer preparation for selective and sensitive detection of 3-methylindole in water. J. Electroanal. Chem. 2018, 832, 129–136. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yang, R.; Li, J.; Qu, L. A highly sensitive and selective electrochemical sensor based on polydopamine functionalized graphene and molecularly imprinted polymer for the 2,4-dichlorophenol recognition and detection. Talanta 2018, 195, 691–698. [Google Scholar] [CrossRef]
- Li, H.-H.; Wang, H.-H.; Li, W.-T.; Fang, X.-X.; Guo, X.-C.; Zhou, W.-H.; Cao, X.; Kou, D.-X.; Zhou, Z.-J.; Wu, S.-X. A novel electrochemical sensor for epinephrine based on three dimensional molecularly imprinted polymer arrays. Sens. Actuators B Chem. 2016, 222, 1127–1133. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, M. QCM sensing of melphalan via electropolymerized molecularly imprinted polythiophene films. Biosens. Bioelectron. 2015, 74, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.; Sharma, S.; Dutra, R.A.; Noronha, J.P.; Cass, A.E.; Sales, M.G.F. Protein-responsive polymers for point-of-care detection of cardiac biomarker. Sens. Actuators B Chem. 2014, 196, 123–132. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Marques, A.C.; Santos, L.; Carvalho, A.; Costa, F.M.; Martins, R.; Sales, M.G.F.; Fortunato, E. Molecularly-imprinted chloramphenicol sensor with laser-induced graphene electrodes. Biosens. Bioelectron. 2018, 124–125, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Han, Q.; Wang, Y.; Wu, J.; Wen, T.; Wang, R.; Hong, J.; Zhou, X.; Jiang, H. Amperometric detection of dopamine in human serumby electrochemical sensor based on gold nanoparticles doped molecularly imprinted polymers. Biosens. Bioelectron. 2013, 49, 199–203. [Google Scholar] [CrossRef]
- Yarman, A.; Scheller, F.W. The First Electrochemical MIP Sensor for Tamoxifen. Sensors 2014, 14, 7647–7654. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Liu, X.; Yang, J.; Lu, X. Preparation of an amperometric sensor for norfloxacin based on molecularly imprinted grafting photopolymerization. Anal. Bioanal. Chem. 2013, 405, 2525–2533. [Google Scholar] [CrossRef]
- Azevedo, S.D.; Lakshmi, D.; Chianella, I.; Whitcombe, M.; Karim, K.; Ivanova-Mitseva, P.K.; Subrahmanyam, S.; Piletsky, S. Molecularly Imprinted Polymer-Hybrid Electrochemical Sensor for the Detection of β-Estradiol. Indian Eng. Chem. Res. 2013, 52, 13917–13923. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, H.; Quan, X.; Tan, F. Amperometric Sensor for Tetracycline Determination Based on Molecularly Imprinted Technique. Proced. Environ. Sci. 2013, 18, 249–257. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Ramanavicius, A. Molecularly imprinted polypyrrole-based synthetic receptor for direct detection of bovine leukemia virus glycoproteins. Biosens. Bioelectron. 2004, 20, 1076–1082. [Google Scholar] [CrossRef]
- Ratautaite, V.; Topkaya, S.N.; Mikoliunaite, L.; Ozsoz, M.; Oztekin, Y.; Ramanaviciene, A.; Ramanavicius, A. Molecularly Imprinted Polypyrrole for DNA Determination. Electroanalysis 2013, 25, 1169–1177. [Google Scholar] [CrossRef]
- Hirsch, R.; Ternes, T.; Haberer, K.; Kratz, K.-L. Occurrence of antibiotics in the aquatic environment. Sci. Total. Environ. 1999, 225, 109–118. [Google Scholar] [CrossRef]
- Al Qarni, H.; Collier, P.; O’Keeffe, J.; Akunna, J. Investigating the removal of some pharmaceutical compounds in hospital wastewater treatment plants operating in Saudi Arabia. Environ. Sci. Pollut. Res. 2016, 23, 13003–13014. [Google Scholar] [CrossRef] [PubMed]
- A Ternes, T.; Stüber, J.; Herrmann, N.; McDowell, D.; Ried, A.; Kampmann, M.; Teiser, B. Ozonation: A tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater? Water Res. 2003, 37, 1976–1982. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Dempsey-Hibbert, N.C.; Peeters, M.; Tridente, A.; Banks, C.E. Molecularly imprinted polymer based electrochemical biosensors: Overcoming the challenges of detecting vital biomarkers and speeding up diagnosis. Talanta Open 2020, 2, 100018. [Google Scholar] [CrossRef]
- Dietl, S.; Sobek, H.; Mizaikoff, B. Epitope-imprinted polymers for biomacromolecules: Recent strategies, future challenges and selected applications. TrAC Trends Anal. Chem. 2021, 143, 116414. [Google Scholar] [CrossRef]
- Al-Kindy, S.; Badía, R.; Suárez-Rodríguez, J.L.; Díaz-García, M.E. Molecularly Imprinted Polymers and Optical Sensing Applications. Crit. Rev. Anal. Chem. 2000, 30, 291–309. [Google Scholar] [CrossRef]
- Henry, O.Y.F.; Cullen, D.; Piletsky, S.A. Optical interrogation of molecularly imprinted polymers and development of MIP sensors: A review. Anal. Bioanal. Chem. 2005, 382, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Feng, S.; Hu, Y.; Wang, S.; Lu, X. Rapid determination of atrazine in apple juice using molecularly imprinted polymers coupled with gold nanoparticles-colorimetric/SERS dual chemosensor. Food Chem. 2018, 276, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ge, L.; Li, M.; Li, W.; Li, L.; Wang, Y.; Yu, J. Photoelectrochemical Sensor Based on Molecularly Imprinted Polymer-Coated TiO2 Nanotubes for Lindane Specific Recognition and Detection. J. Inorg. Organomet. Polym. Mater. 2013, 23, 703–711. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, Y.; Zhang, L.; Ge, S.; Yu, J. A novel microfluidic paper-based colorimetric sensor based on molecularly imprinted polymer membranes for highly selective and sensitive detection of bisphenol A. Sens. Actuators B Chem. 2017, 243, 130–136. [Google Scholar] [CrossRef]
- Sergeyeva, T.; Chelyadina, D.S.; Gorbach, L.A.; Brovko, O.O.; Piletska, E.V.; Piletsky, S.; Sergeeva, L.M.; El’Skaya, A.V. Colorimetric biomimetic sensor systems based on molecularly imprinted polymer membranes for highly-selective detection of phenol in environmental samples. Biopolym. Cell 2014, 30, 209–215. [Google Scholar] [CrossRef]
- Huang, K.; Chen, Y.; Zhou, F.; Zhao, X.; Liu, J.; Mei, S.; Zhou, Y.; Jing, T. Integrated ion imprinted polymers-paper composites for selective and sensitive detection of Cd(II) ions. J. Hazard. Mater. 2017, 333, 137–143. [Google Scholar] [CrossRef]
- Foguel, M.V.; Ton, X.-A.; Zanoni, M.V.; Sotomayor, M.; Haupt, K.; Bui, B.T.S. A molecularly imprinted polymer-based evanescent wave fiber optic sensor for the detection of basic red 9 dye. Sens. Actuators B Chem. 2015, 218, 222–228. [Google Scholar] [CrossRef]
- Liu, X.; Yu, D.; Yu, Y.; Ji, S. Preparation of a magnetic molecularly imprinted polymer for selective recognition of rhodamine B. Appl. Surf. Sci. 2014, 320, 138–145. [Google Scholar] [CrossRef]
- Yan, K.; Yang, Y.; Zhang, J. A self-powered sensor based on molecularly imprinted polymer-coupled graphitic carbon nitride photoanode for selective detection of bisphenol A. Sens. Actuators B Chem. 2018, 259, 394–401. [Google Scholar] [CrossRef]
- Dai, J.; Vu, D.; Nagel, S.; Lin, C.-H.; De Cortalezzi, M.F. Colloidal crystal templated molecular imprinted polymer for the detection of 2-butoxyethanol in water contaminated by hydraulic fracturing. Microchim. Acta 2017, 185, 32. [Google Scholar] [CrossRef] [PubMed]
- Kadhem, A.J.; Xiang, S.; Nagel, S.; Lin, C.-H.; de Cortalezzi, M.F. Photonic Molecularly Imprinted Polymer Film for the Detection of Testosterone in Aqueous Samples. Polymers 2018, 10, 349. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, Y.; Yu, L.; He, H. Surface molecularly imprinted polymers fabricated by differential UV–vis spectra and reverse prediction method for the enrichment and determination of sterigmatocystin. Food Chem. 2021, 367, 130715. [Google Scholar] [CrossRef]
- Tan, L.; Chen, K.; Huang, C.; Peng, R.; Luo, X.; Yang, R.; Cheng, Y.; Tang, Y. A fluorescent turn-on detection scheme for α-fetoprotein using quantum dots placed in a boronate-modified molecularly imprinted polymer with high affinity for glycoproteins. Microchim. Acta 2015, 182, 2615–2622. [Google Scholar] [CrossRef]
- Xu, L.; Fang, G.; Pan, M.; Wang, X.; Wang, S. One-pot synthesis of carbon dots-embedded molecularly imprinted polymer for specific recognition of sterigmatocystin in grains. Biosens. Bioelectron. 2016, 77, 950–956. [Google Scholar] [CrossRef]
- Ren, X.; Chen, L. Preparation of molecularly imprinted polymer coated quantum dots to detect nicosulfuron in water samples. Anal. Bioanal. Chem. 2015, 407, 8087–8095. [Google Scholar] [CrossRef]
- Xiong, Y.; Ye, Z.; Xu, J.; Liu, Y.; Zhang, H. A microvolume molecularly imprinted polymer modified fiber-optic evanescent wave sensor for bisphenol A determination. Anal. Bioanal. Chem. 2014, 406, 2411–2420. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, R.; Wang, Y.; Sun, L.; Chen, L.; Dai, X.; Pan, J.; Pan, G.; Yan, Y. Surface-imprinted fluorescence microspheres as ultrasensitive sensor for rapid and effective detection of tetracycline in real biological samples. Sens. Actuators B Chem. 2018, 263, 533–542. [Google Scholar] [CrossRef]
- Lu, X.; Yang, Y.; Zeng, Y.; Li, L.; Wu, X. Rapid and reliable determination of p-nitroaniline in wastewater by molecularly imprinted fluorescent polymeric ionic liquid microspheres. Biosens. Bioelectron. 2018, 99, 47–55. [Google Scholar] [CrossRef]
- Wei, X.; Xu, G.; Gong, C.; Qin, F.; Gong, X.; Li, C. Fabrication and evaluation of sulfanilamide-imprinted composite sensors by developing a custom-tailored strategy. Sens. Actuators B Chem. 2018, 255, 2697–2703. [Google Scholar] [CrossRef]
- Wei, X.; Hao, T.; Xu, Y.; Lu, K.; Li, H.; Yan, Y.; Zhou, Z. Facile polymerizable surfactant inspired synthesis of fluorescent molecularly imprinted composite sensor via aqueous CdTe quantum dots for highly selective detection of λ-cyhalothrin. Sens. Actuators B Chem. 2016, 224, 315–324. [Google Scholar] [CrossRef]
- Wagner, S.; Bell, J.; Biyikal, M.; Gawlitza, K.; Rurack, K. Integrating fluorescent molecularly imprinted polymer (MIP) sensor particles with a modular microfluidic platform for nanomolar small-molecule detection directly in aqueous samples. Biosens. Bioelectron. 2018, 99, 244–250. [Google Scholar] [CrossRef]
- Xu, S.; Lu, H. Mesoporous structured MIPs@CDs fluorescence sensor for highly sensitive detection of TNT. Biosens. Bioelectron. 2016, 85, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiu, H.; Shen, H.; Pan, J.; Dai, X.; Yan, Y.; Pan, G.; Sellergren, B. Molecularly imprinted fluorescent hollow nanoparticles as sensors for rapid and efficient detection λ-cyhalothrin in environmental water. Biosens. Bioelectron. 2016, 85, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, N.; Jiang, J.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. Molecularly imprinted magnetic microparticles for the simultaneous detection and extraction of Rhodamine B. Sens. Actuators B Chem. 2017, 246, 286–292. [Google Scholar] [CrossRef]
- Amjadi, M.; Jalili, R. Molecularly imprinted mesoporous silica embedded with carbon dots and semiconductor quantum dots as a ratiometric fluorescent sensor for diniconazole. Biosens. Bioelectron. 2017, 96, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Z.; Li, J.; You, H.; Li, Y.; Chen, L. Molecularly imprinted polymers-coated gold nanoclusters for fluorescent detection of bisphenol A. Sens. Actuators B Chem. 2015, 211, 507–514. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Liu, Y.-J.; Gao, X.; Gao, K.-C.; Xia, H.; Luo, M.-F.; Wang, X.-J.; Ye, L.; Shi, Y.; Lu, B. Monitoring bisphenol A and its biodegradation in water using a fluorescent molecularly imprinted chemosensor. Chemosphere 2015, 119, 515–523. [Google Scholar] [CrossRef]
- Ren, X.; Chen, L. Quantum dots coated with molecularly imprinted polymer as fluorescence probe for detection of cyphenothrin. Biosens. Bioelectron. 2015, 64, 182–188. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Z.; Jiang, X.; Feng, D.-Q.; Zhao, J.; Fan, D.; Wang, W. In-situ hydrothermal synthesis of molecularly imprinted polymers coated carbon dots for fluorescent detection of bisphenol A. Sens. Actuators B Chem. 2016, 228, 302–307. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Zakery, M.; Rezaei, B. An optical sensor with specific binding sites for the detection of thioridazine hydrochloride based on ZnO-QDs coated with molecularly imprinted polymer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 206, 460–465. [Google Scholar] [CrossRef]
- Fang, M.; Zhou, L.; Zhang, H.; Liu, L.; Gong, Z.-Y. A molecularly imprinted polymers/carbon dots-grafted paper sensor for 3-monochloropropane-1,2-diol determination. Food Chem. 2018, 274, 156–161. [Google Scholar] [CrossRef]
- Feng, J.; Tao, Y.; Shen, X.; Jin, H.; Zhou, T.; Zhou, Y.; Hu, L.; Luo, D.; Mei, S.; Lee, Y.-I. Highly sensitive and selective fluorescent sensor for tetrabromobisphenol-A in electronic waste samples using molecularly imprinted polymer coated quantum dots. Microchem. J. 2018, 144, 93–101. [Google Scholar] [CrossRef]
- Mehrzad-Samarin, M.; Faridbod, F.; Ganjali, M.R. A luminescence nanosensor for Ornidazole detection using graphene quantum dots entrapped in silica molecular imprinted polymer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 206, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, H.; Hosseini, S.M.M. A solid-phase luminescence sensor based on molecularly imprinted polymer-CdSeS/ZnS quantum dots for selective extraction and detection of sulfasalazine in biological samples. Talanta 2018, 194, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Shirani, M.P.; Rezaei, B.; Ensafi, A.A. A novel optical sensor based on carbon dots embedded molecularly imprinted silica for selective acetamiprid detection. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2018, 210, 36–43. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, Y.; Liu, Y.; Long, S.; Du, L.; Liang, J.; Huang, C.; Swihart, M.; Tan, K. Core-shell quantum dots coated with molecularly imprinted polymer for selective photoluminescence sensing of perfluorooctanoic acid. Talanta 2018, 194, 1–6. [Google Scholar] [CrossRef]
- Dai, J.; Dong, X.; de Cortalezzi, M.F. Molecularly imprinted polymers labeled with amino-functionalized carbon dots for fluorescent determination of 2,4-dinitrotoluene. Microchim. Acta 2017, 184, 1369–1377. [Google Scholar] [CrossRef]
- Dai, J.; de Cortalezzi, M.F. Influence of pH, ionic strength and natural organic matter concentration on a MIP-Fluorescent sensor for the quantification of DNT in water. Heliyon 2019, 5, e01922. [Google Scholar] [CrossRef] [PubMed]
- Sa-Nguanprang, S.; Phuruangrat, A.; Bunkoed, O. An optosensor based on a hybrid sensing probe of mesoporous carbon and quantum dots embedded in imprinted polymer for ultrasensitive detection of thiamphenicol in milk. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 264, 120324. [Google Scholar] [CrossRef]
- Cui, Y.; Su, A.; Feng, J.; Dong, W.; Li, J.; Wang, H.; Ni, X.; Jiang, Y. Development of silica molecularly imprinted polymer on carbon dots as a fluorescence probe for selective and sensitive determination of cetirizine in saliva and urine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 264, 120293. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, Y.; Luo, J.; Kou, J.; Zheng, H.; Li, B.; Zhang, Z. A molecularly imprinted polymer based a lab-on-paper chemiluminescence device for the detection of dichlorvos. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 141, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Li, L.; Wang, X.; Wang, Y.; Li, J.; Luo, C. A sensitive and selective chemiluminescence sensor for the determination of dopamine based on silanized magnetic graphene oxide-molecularly imprinted polymer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 139, 374–379. [Google Scholar] [CrossRef]
- Qiu, H.; Fan, L.; Li, X.; Li, L.; Sun, M.; Luo, C. A microflow chemiluminescence sensor for indirect determination of dibutyl phthalate by hydrolyzing based on biological recognition materials. J. Pharm. Biomed. Anal. 2013, 75, 123–129. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, G.; Wang, X.; Zhang, F.; Liu, J.; Zheng, W.; Wang, S. Electrochemiluminescent graphene quantum dots enhanced by MoS2 as sensing platform: A novel molecularly imprinted electrochemiluminescence sensor for 2-methyl-4-chlorophenoxyacetic acid assay. Electrochim. Acta 2017, 228, 107–113. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, G.; Wang, X.; Liu, G.; Wang, S. Imprinting of molecular recognition sites combined with π-donor–acceptor interactions using bis-aniline-crosslinked Au–CdSe/ZnS nanoparticles array on electrodes: Development of electrochemiluminescence sensor for the ultrasensitive and selective detection of 2-methyl-4-chlorophenoxyacetic acid. Biosens. Bioelectron. 2015, 77, 1134–1143. [Google Scholar] [CrossRef]
- Wang, S.; Ge, L.; Li, L.; Yan, M.; Ge, S.; Yu, J. Molecularly imprinted polymer grafted paper-based multi-disk micro-disk plate for chemiluminescence detection of pesticide. Biosens. Bioelectron. 2013, 50, 262–268. [Google Scholar] [CrossRef]
- Duan, H.; Li, L.; Wang, X.; Wang, Y.; Li, J.; Luo, C. CdTe quantum dots@luminol as signal amplification system for chrysoidine with chemiluminescence-chitosan/graphene oxide-magnetite-molecularly imprinting sensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 535–541. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, D.; Cao, Y.; Gan, N. An antibody-free and signal-on type electrochemiluminescence sensor for diethylstilbestrol detection based on magnetic molecularly imprinted polymers-quantum dots labeled aptamer conjugated probes. J. Electroanal. Chem. 2017, 789, 1–8. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, T.; Feng, J.; Jin, H.; Tao, Y.; Luo, D.; Mei, S.; Lee, Y.-I. A rapid and sensitive molecularly imprinted electrochemiluminescence sensor for Azithromycin determination in biological samples. J. Electroanal. Chem. 2018, 813, 1–8. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, S.; Liang, Y.; Wang, X.; Zhuang, X.; Tian, C.; Luan, F.; Chen, L. Selective detection of enrofloxacin in biological and environmental samples using a molecularly imprinted electrochemiluminescence sensor based on functionalized copper nanoclusters. Talanta 2021, 236, 122835. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Pesavento, M.; Zeni, L. High selectivity and sensitivity sensor based on MIP and SPR in tapered plastic optical fibers for the detection of l-nicotine. Sens. Actuators B Chem. 2014, 191, 529–536. [Google Scholar] [CrossRef]
- Cennamo, N.; De Maria, L.; D’Agostino, G.; Zeni, L.; Pesavento, M. Monitoring of Low Levels of Furfural in Power Transformer Oil with a Sensor System Based on a POF-MIP Platform. Sensors 2015, 15, 8499–8511. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, A.M.; Mishra, S.K.; Gupta, B.D. Surface Plasmon Resonance-Based Fiber Optic Sensor for the Detection of Ascorbic Acid Utilizing Molecularly Imprinted Polyaniline Film. Plasmonics 2015, 10, 1853–1861. [Google Scholar] [CrossRef]
- Shrivastav, A.; Usha, S.P.; Gupta, B.D. Fiber optic profenofos sensor based on surface plasmon resonance technique and molecular imprinting. Biosens. Bioelectron. 2015, 79, 150–157. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Reut, J.; Öpik, A.; Furchner, A.; Syritski, V. Hybrid molecularly imprinted polymer for amoxicillin detection. Biosens. Bioelectron. 2018, 118, 102–107. [Google Scholar] [CrossRef]
- Jiang, S.; Peng, Y.; Ning, B.; Bai, J.; Liu, Y.; Zhang, N.; Gao, Z. Surface plasmon resonance sensor based on molecularly imprinted polymer film for detection of histamine. Sens. Actuators B Chem. 2015, 221, 15–21. [Google Scholar] [CrossRef]
- Rahtuvanoğlu, A.; Akgönüllü, S.; Karacan, S.; Denizli, A. Biomimetic Nanoparticles Based Surface Plasmon Resonance Biosensors for Histamine Detection in Foods. Chem. Select 2020, 5, 5683–5692. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.; Zhao, F.; Xia, N.; Liu, L. Self-assembled biotin-phenylalanine nanoparticles for the signal amplification of surface plasmon resonance biosensors. Microchim. Acta 2020, 187, 1–7. [Google Scholar] [CrossRef]
- Çakır, O.; Baysal, Z. Pesticide analysis with molecularly imprinted nanofilms using surface plasmon resonance sensor and LC-MS/MS: Comparative study for environmental water samples. Sens. Actuators B Chem. 2019, 297, 126764. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. SPR nanosensor based on molecularly imprinted polymer film with gold nanoparticles for sensitive detection of aflatoxin B1. Talanta 2020, 219, 121219. [Google Scholar] [CrossRef] [PubMed]
- Özgür, E.; Topçu, A.A.; Yılmaz, E.; Denizli, A. Surface plasmon resonance based biomimetic sensor for urinary tract infections. Talanta 2020, 212, 120778. [Google Scholar] [CrossRef] [PubMed]
- Kamra, T.; Zhou, T.; Montelius, L.; Schnadt, J.; Ye, L. Implementation of Molecularly Imprinted Polymer Beads for Surface Enhanced Raman Detection. Anal. Chem. 2015, 87, 5056–5061. [Google Scholar] [CrossRef] [PubMed]
- Kamra, T.; Xu, C.; Montelius, L.; Schnadt, J.; Wijesundera, S.A.; Yan, M.; Ye, L. Photoconjugation of Molecularly Imprinted Polymer Nanoparticles for Surface-Enhanced Raman Detection of Propranolol. ACS Appl. Mater. Interfaces 2015, 7, 27479–27485. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, S.; Gao, F.; Li-Chan, E.C.; Grant, E.; Lu, X. Detection of melamine in milk using molecularly imprinted polymers–surface enhanced Raman spectroscopy. Food Chem. 2015, 176, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Li, X.; Han, S.; Liu, J.H.; Zhao, Y.Y. Synthesis of surface-imprinted Ag nanoplates for detecting organic pollutants in water environments based on surface enhanced Raman scattering. RSC Adv. 2015, 5, 99914–99919. [Google Scholar] [CrossRef]
- Guo, Y.; Kang, L.; Chen, S.; Li, X. High performance surface-enhanced Raman scattering from molecular imprinting polymer capsulated silver spheres. Phys. Chem. Chem. Phys. 2015, 17, 21343–21347. [Google Scholar] [CrossRef]
- Chang, L.; Ding, Y.; Li, X. Surface molecular imprinting onto silver microspheres for surface enhanc24 June 2013ed Raman scattering applications. Biosens. Bioelectron. 2013, 50, 106–110. [Google Scholar] [CrossRef]
- Ye, J.; Chen, Y.; Liu, Z. A Boronate Affinity Sandwich Assay: An Appealing Alternative to Immunoassays for the Determination of Glycoproteins. Angew. Chem. Int. Ed. 2014, 53, 10386–10389. [Google Scholar] [CrossRef]
- Xue, J.-Q.; Li, D.-W.; Qu, L.; Long, Y. Surface-imprinted core–shell Au nanoparticles for selective detection of bisphenol A based on surface-enhanced Raman scattering. Anal. Chim. Acta 2013, 777, 57–62. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, L.; Lv, H.; Yu, Z.; Zhao, B. Magnetic imprinted surface enhanced Raman scattering (MI-SERS) based ultrasensitive detection of ciprofloxacin from a mixed sample. Anal. Methods 2013, 6, 1627–1632. [Google Scholar] [CrossRef]
- Yin, W.; Wu, L.; Ding, F.; Li, Q.; Wang, P.; Li, J.; Lu, Z.; Han, H. Surface-imprinted SiO2@Ag nanoparticles for the selective detection of BPA using surface enhanced Raman scattering. Sens. Actuators B Chem. 2017, 258, 566–573. [Google Scholar] [CrossRef]
- Ren, X.; Li, X. Flower-like Ag coated with molecularly imprinted polymers as a surface-enhanced Raman scattering substrate for the sensitive and selective detection of glibenclamide. Anal. Methods 2020, 12, 2858–2864. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Li, Y.; Qiao, Y.; Liu, L.; Wang, Q.; Che, G. High-sensitive molecularly imprinted sensor with multilayer nanocomposite for 2,6-dichlorophenol detection based on surface-enhanced Raman scattering. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 228, 117784. [Google Scholar] [CrossRef]
- Zhou, J.; Sheth, S.; Zhou, H.; Song, Q. Highly selective detection of l-Phenylalanine by molecularly imprinted polymers coated Au nanoparticles via surface-enhanced Raman scattering. Talanta 2020, 211, 120745. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Lee, M.-H.; Thomas, J.L.; Shih, C.-P.; Hung, T.-L.; Whang, T.-J.; Lin, H.-Y. Optical sensing of phenylalanine in urine via extraction with magnetic molecularly imprinted poly(ethylene-co-vinyl alcohol) nanoparticles. Nanotechnology 2015, 26, 305502. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, P.; Ge, S.; Ge, L.; Yu, J.; Yan, M. Photoelectrochemical sensor for pentachlorophenol on microfluidic paper-based analytical device based on the molecular imprinting technique. Biosens. Bioelectron. 2014, 56, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Yang, X.; Zhang, L.; Gong, J. Ultrasensitive photoelectrochemical determination of chromium(VI) in water samples by ion-imprinted/formate anion-incorporated graphitic carbon nitride nanostructured hybrid. J. Hazard. Mater. 2016, 312, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Dai, W.; Ge, L.; Yan, M.; Ge, S.; Yu, J. Visible light photoelectrochemical sensor based on Au nanoparticles and molecularly imprinted poly(o-phenylenediamine)-modified TiO2nanotubes for specific and sensitive detection chlorpyrifos. Analyst 2012, 138, 939–945. [Google Scholar] [CrossRef]
- Lu, B.; Liu, M.; Shi, H.; Huang, X.; Zhao, G. A Novel Photoelectrochemical Sensor for Bisphenol A with High Sensitivity and Selectivity Based on Surface Molecularly Imprinted Polypyrrole Modified TiO2Nanotubes. Electroanalysis 2013, 25, 771–779. [Google Scholar] [CrossRef]
- Sun, X.; Gao, C.; Zhang, L.; Yan, M.; Yu, J.; Ge, S. Photoelectrochemical sensor based on molecularly imprinted film modified hierarchical branched titanium dioxide nanorods for chlorpyrifos detection. Sens. Actuators B Chem. 2017, 251, 1–8. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Yuan, S.; Xue, J.; Cao, B.; Yu, J. Engineering anatase hierarchically cactus-like TiO 2 arrays for photoelectrochemical and visualized sensing platform. Biosens. Bioelectron. 2016, 90, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zang, D.; Ge, S.; Ge, L.; Yu, J.; Yan, M. A novel microfluidic origami photoelectrochemical sensor based on CdTe quantum dots modified molecularly imprinted polymer and its highly selective detection of S-fenvalerate. Electrochim. Acta 2013, 107, 147–154. [Google Scholar] [CrossRef]
- Liu, M.; Ding, X.; Yang, Q.; Wang, Y.; Zhao, G.; Yang, N. A pM leveled photoelectrochemical sensor for microcystin-LR based on surface molecularly imprinted TiO2 @CNTs nanostructure. J. Hazard. Mater. 2017, 331, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Li, J.; Xiong, H.; Lu, W.; Peng, H.; Chen, L. Thermosensitive molecularly imprinted polymers on porous carriers: Preparation, characterization and properties as novel adsorbents for bisphenol A. Talanta 2014, 130, 182–191. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Fang, T.; Zhang, L.; Gong, J. Disposable photoelectrochemical sensing strip for highly sensitive determination of perfluorooctane sulfonyl fluoride on functionalized screen-printed carbon electrode. Talanta 2018, 181, 147–153. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Cheng, L.; Yang, C.; Zhang, J. Photoelectrochemical Aptasensing of Kanamycin Using Visible Light-Activated Carbon Nitride and Graphene Oxide Nanocomposites. Anal. Chem. 2014, 86, 9372–9375. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Zhang, L.; Gong, J. Electrospun template directed molecularly imprinted nanofibers incorporated with BiOI nanoflake arrays as photoactive electrode for photoelectrochemical detection of triphenyl phosphate. Biosens. Bioelectron. 2017, 92, 61–67. [Google Scholar] [CrossRef]
- Tran, T.; Li, J.; Feng, H.; Cai, J.; Yuan, L.; Wang, N.; Cai, Q. Molecularly imprinted polymer modified TiO2 nanotube arrays for photoelectrochemical determination of perfluorooctane sulfonate (PFOS). Sens. Actuators B Chem. 2014, 190, 745–751. [Google Scholar] [CrossRef]
- Wang, R.; Yan, K.; Wang, F.; Zhang, J. A highly sensitive photoelectrochemical sensor for 4-aminophenol based on CdS-graphene nanocomposites and molecularly imprinted polypyrrole. Electrochim. Acta 2014, 121, 102–108. [Google Scholar] [CrossRef]
- Gong, J.; Fang, T.; Peng, D.; Li, A.; Zhang, L. A highly sensitive photoelectrochemical detection of perfluorooctanic acid with molecularly imprined polymer-functionalized nanoarchitectured hybrid of AgI–BiOI composite. Biosens. Bioelectron. 2015, 73, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Viter, R.; Kunene, K.; Genys, P.; Jevdokimovs, D.; Erts, D.; Sutka, A.; Bisetty, K.; Viksna, A.; Ramanaviciene, A.; Ramanavicius, A. Photoelectrochemical Bisphenol S Sensor Based on ZnO-Nanoroads Modified by Molecularly Imprinted Polypyrrole. Macromol. Chem. Phys. 2019, 221, 1900232. [Google Scholar] [CrossRef]
- Whitcombe, M.J.; Chianella, I.; Larcombe, L.; Piletsky, S.A.; Noble, J.; Porter, R.; Horgan, A. The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem. Soc. Rev. 2010, 40, 1547–1571. [Google Scholar] [CrossRef] [PubMed]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays—An overview of the current status quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef]
| Sensor | Functional Monomer | Electrode | Target | Sample | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| LOD | ||||||
| Capacitance | ||||||
| Aptamer-MIP | Dopamine | Au | Prostate specific antigen | TBST buffer | 0.1–100 ng/mL | [133] |
| 1 pg/mL | ||||||
| Sol-gel-MIP | N,N-methylene bis acrylamide (MBAA) | C | Methidathion | Wastewater | 40–200 μg/L | [134] |
| 5.14 μg/L | ||||||
| Silica NP-Au NP-MIP-Chitosan | MAA | GCE | Fumosin B1 | Maize, milk | 0.001–100 ng/mL | [135] |
| 0.35 pg/mL | ||||||
| MIP | MAA | Au | N-formylamphetamine | Ultrapure water | Variable | [136] |
| 10 μM | ||||||
| MIP | MAA | Au | Metergoline | PBS | 1–50 μM | [137] |
| 1 μM | ||||||
| MIP | N-allylthiourea (thiourea) | Pt | Phosphate | Wastewater | 0.66–8 mg P/L | [138] |
| 0.16 mg P/L | ||||||
| MIP | o-aminophenol | GCE | Norepinephrine | Human plasma and urine, pharmaceuticals | 5 × 10−8–10−5 M | [139] |
| 4.9 × 10−10 M | ||||||
| MIP | Pyrrole | PGE | Chlorpyrifos | Tap water, non-agricultural soil, corn leaves | 20–300 μg/L | [140] |
| 4.5 μg/L | ||||||
| MIP | Pyrrole | B-doped nanocrystalline diamond | Theophylline | PBS | NI | [141] |
| NI | ||||||
| Potentiometry | ||||||
| MIP | MAA | PVC | Propranolol | Water, pharmaceuticals | 10−4–10−1/10−5–10−1 M * | [142] |
| 10−4/10−5 M * | ||||||
| MIP-Nanographene-IL | MAA | CPE | Losartan | Urine, pharmaceuticals | 3 × 10−9–10−2 M | [143] |
| 1.82 × 10−9 M | ||||||
| MIP NP | MAA | PVC | Histamine | Wine, fish | 1.12 × 10−6–10−2 M | [144] |
| 1.12 × 10−6 M | ||||||
| MIP-MWCNT | MAA | Cu | Lindane | Ground, tap, and sea water, orange, grape, tomato, cabbage | 10−9–10−5; 10−5–10−3 M | [145] |
| 10−10 M | ||||||
| MIP | MAA | PVC | Imidocarb dipropionate | Bovine liver and kidney | 10−5–10−2 M | [146] |
| 2 × 10−6 M | ||||||
| MIP | MAA | GCE | Heparine | Heparine sodium injection | 3 × 10−9–7 × 10−7 M | [147] |
| 10−9 M | ||||||
| MIP | 4-VP | PVC | Bisphenol A | Polycarbonate drinking water bottle in water solution | 0.1–1 μM | [148] |
| 0.02 μM | ||||||
| Nanostructured MIP particles | MAA | GCE CPE | Captopril | Captopril tablet | 10−6–10−1 M (GCE); 3 × 10−9–10−1 M (CPE) | [149] |
| NI (GCE); 10−9 M (CPE) | ||||||
| Voltammetry | ||||||
| MIP | Pyrrole | GCE | Triacetone triperoxide | Acetonitrile | 82–44,300 μg/L | [150] |
| 26.9 μg/L | ||||||
| MIP-C NT/IL | Pyrrole | GCE | Melamine | Milk | 0.4–9.2 μM | [151] |
| 0.11 μM | ||||||
| MIP-RGO-Au NP | MAA | GCE | Carbofuran | Vegetables | 5 × 10−8–2 × 10−5 M | [152] |
| 2 × 10−8 M | ||||||
| MIP-MWCNT-IL-Pt | 4-vinylpyridine | GCE | Tartrazine | Orange powder and soft drinks | 0.03–5; 5–20 μM | [153] |
| 8 nM | ||||||
| MIP-MWCNT | Pyrrole | GCE | Ochratoxin A | Beer, wine | 5 × 10−8–10−6 M | [154] |
| 4.1 × 10−9 M | ||||||
| MIP-MWCNT | Pyrrole | PGE | Triamterene | Human serum, pharmaceuticals | 0.08–265 μM | [155] |
| 3.35 nM | ||||||
| Sol-gel-MIP | Antimony-doped tin oxide (ATO) | Pt | β2-agonists | Human serum | 5.5 nM–6.3 μM | [156] |
| 1.7 nM | ||||||
| MIP-MWCNT | 2,2′-dithiodianiline | GCE | Valganciclovir | Human serum, tablet | 1–500; 500–2000 nM | [157] |
| 0.3 nM | ||||||
| MIP | o-aminophenol | Au-Ag | Dopamine | Rabbit serum, rat brain tissue | 2 × 10−13–2 × 10−8 M | [158] |
| 7.63 × 10−14 M | ||||||
| Au NP-MIP | Au NPs@IL, ionic liquid (IL, i.e., 3-propyl-1-vinylimidazolium bromide) | GCE | Dimetridazole | Milk, honey | 2 × 10−9–2.5 × 10−7; 2.5 × 10−7–3 × 10−6 M | [159] |
| 5 × 10−10 M | ||||||
| MIP-GO | APTES | GCE | Bisphenol A | Milk, mineralized water | 0.006–0.1; 0.2–20 μM | [160] |
| 0.003 μM | ||||||
| MIP-MWCNT | Pyrrole | GCE | Norfloxacin | Human urine | 10−7–8 × 10−6 M | [161] |
| 4.6 × 10−8 M | ||||||
| MIP | Pyrrole | BDD | Sulfamethoxazole | Surface water | 0.1–100 μM | [162] |
| 24.1 nM | ||||||
| MIP-Melamine | Melamine | EPPG | 8-hydroxydeoxyguanosine | Human urine | 2 × 10−8–3 × 10−6 M | [163] |
| 3 × 10−9 M | ||||||
| MIP-Chitosan-C dots | 3-aminobenzeneboronic acid | GCE | Glucose | Human serum | 0.5–40; 50–600 μM | [43] |
| 0.09 μM | ||||||
| MIP | 2-vinylpyridine, methacrylic acid, and acrylamide | CPE | Hexazinone | River water | 1.19 × 10−11–1.1 × 10−9 M | [164] |
| 2.6 × 10−12 M | ||||||
| MIP-Au-Polyaniline | MAA | GCE | Melamine | Milk, food | 10−5–10 μM | [165] |
| 1.39 × 10−6 μM | ||||||
| MIP-GO-Ag NP | Pyrrole | C | Bisphenol A | PVC and soil in water solution | 10−11–10−8 M | [166] |
| 3.2 × 10−12 M | ||||||
| MIP | MAA | CPE | Histamine | Human serum | 10−10–7 × 10−9; 7 × 10−9–4 × 10−7 M | [167] |
| 7.4 × 10−11 M | ||||||
| MIP-Algae | N-methacryloyl glutamic acid | C | Cu(II) | Human serum, soil, lake water, pharmaceutical tablet | Depending on the sample | [168] |
| MIP-MWNT-Au NP | P-aminothiophenol (p-ATP) | GCE | Cholesterol | Ethanol | 10−13–10−9 M | [39] |
| 3.3 × 10−14 M | ||||||
| MIP-Chitosan-Acetylene black | Chitosan | GCE | Bisphenol A | Drinking water | 0.005–0.2; 0.5–10 mM | [169] |
| 2 nM | ||||||
| MIP-Chitosan-Graphene | Chitosan (CHI) | ABPE | Bisphenol A | Bottled water, canned beverages | 0.008–1; 1–20 μM | [170] |
| 0.006 μM | ||||||
| MIP | 2-aminothiophenol (2-ATP) | Au | Sodium lauryl sulfate | River and wastewater, personal hygiene products | 0.1–1000 pg/mL | [171] |
| 0.18 pg/mL | ||||||
| MIP | MAA | CPE | Methyl parathion | Soil, vegetables | 10−12–8 × 10−9 M | [172] |
| 3.4 × 10−13 M | ||||||
| MIP | o-phenylenediamine (o-PD) | Au | Atrazine | Deionized water | 5 × 10−9–1.4 × 10−7 M | [76] |
| 10−9 M | ||||||
| MIP-Cu-Melamine | Melamine | GCE | Metronidazole | Standard injection | 0.5–1000 μM | [173] |
| 0.12 μM | ||||||
| MIP-Graphene | Pyrrole | C | Tetrabromobisphenol A | Rain and lake water | 0.5–4.5 nM | [174] |
| 0.23 nM | ||||||
| Ni NP-MIP-Graphene | Pyrrole | C | Tetrabromobisphenol A | Rain, lake, and tap water | 0.5–104 nM | [175] |
| 0.13 nM | ||||||
| MIP-Graphene-C NT | Pyrrole | C | Tetrabromobisphenol A | Fish | 10−11–10−8 M | [176] |
| 3.7 × 10−12 M | ||||||
| Ag-N-RGO-MIP | o-phenylenediamine (o-PD) | GCE | Salbutamol | Human urine, pork | 0.03–20 μM | [177] |
| 7 nM | ||||||
| Magnetite NP-MIP | MAA | mGEC | Methyl parathion | Fish | NI | [178] |
| 1.22 × 10−6 mg/L | ||||||
| MIP-MWNT | MAA and vinylpyridine | GCE | Bisphenol A | Baby feeding bottle in PBS | 0.1 nM–400 μM | [179] |
| 0.02 nM | ||||||
| MIP-Ag NP | Pyrrole | PGE | Mebeverine | Human serum, capsule | 10−8–10−6; 10−5–10−3 M | [180] |
| 8.6 × 10−9 M | ||||||
| MIP-Graphene QD | Pyrrole | GCE | Bisphenol A | Tap and sea water | 0.1–50 μM | [181] |
| 0.04 μM | ||||||
| MIP | Amine terminated poly(N-isopropylacrylamide) (PNIPAAm) and o-phenylenediamine (o-PD) | Au | Folic acid | PBS | 1–200 μM | [182] |
| 0.9 μM | ||||||
| Au NP-MIP | Acetate buffer (pH 6.0), quercetin, resorcinol, methyl parathion, KClO4 | GCE | Methyl parathion | Water, tangerine juice, sweet potato leaves | 0.05–15 μM | [40] |
| 0.01 μM | ||||||
| MIP-MWCNT | 4-vinylpyridine | CPE | Bisphenol A | River, tap, and pure water | 0.08–100 μM | [183] |
| 0.022 μM | ||||||
| MIP | Aniline | PGE | L-ascorbic acid | Bovine serum, water | 1–100 μM | [184] |
| 1 μM | ||||||
| MIP-Chitosan | Chitosan | ABPE | Bisphenol A | Polycarbonate baby feeding and water bottle, PVC bottle, PVC food package in water solution | 80 nM–10 μM | [185] |
| 60 nM | ||||||
| MWCNT-Polyethyleneimine-MIP | Acrylamide | GCE | 2,4-dinitrotoluene | River and wastewater | 2.2 × 10−9–10−6 M | [186] |
| 10−9 M | ||||||
| MIP-RGO-TiO2 | Carboxymethyl-β-cyclodextrin (CM-β-CD) | Pt | Toltrazuril | Chicken muscles, egg | 0.43–42.54 μg/L | [187] |
| 0.21 μg/L | ||||||
| MIP-RGO | Acrylamide | PGE | D-, L-serine | Blood serum, cerebrospinal fluid, water, pharmaceutics | Depending on the sample | [188] |
| 0.24 ng/mL | ||||||
| MIP-BN QD | Pyrrole | GCE | Cardiac Troponin-I | Human plasma | 0.01–5 ng/mL | [189] |
| 0.0005 ng/mL | ||||||
| Conductive NH2-MWCNT-MoS2-MIP | p-aminobenzoic acid | GCE | Sulfamerazine | Pork, chicken | 3 × 10−7–2 × 10−4 M | [190] |
| 1.1 × 10−7 M | ||||||
| Nano MIP-MWCNT | MAA | GCE | Trinitroperhydro-1,3,5-triazine | Tap, river, and sea water | 0.1–10 nM; 0.01–1 μM | [191] |
| 20 pM | ||||||
| MIP | o-Phenylenediamine (o-PD) | GCE | 3-methylindole | Tap and lake water | 0.01–1.2 μM | [192] |
| 4 nM | ||||||
| MIP-Polydopamine-RGO | o-phenylenediamine (o-PD) | GCE | 2,4-dichlorophenol | Lake water | 2–10; 10–100 nM | [193] |
| 0.8 nM | ||||||
| MIP-ZnO | Pyrrole | ITO/PET | Epinephrine | Epinephrine hydrochloride injections | 1–10; 10–800 μM | [194] |
| 1 μM | ||||||
| MIP | 3-thiophene acetic acid (3-TAA) | Au | Melphalan | Pharmaceutical tablet | 0.01–0.07 mM | [195] |
| NI | ||||||
| MIP | Vinylferrocene, 4-vinylpyridine | CPE | Benzo(a)pyrene | Ultrapure water | 0–8; 2–16 μM | [78] |
| 0.93 μM | ||||||
| MIP | Ferrocenylmethyl methacrylate | C SPE | Bisphenol A | PBS | 4.7–8 nM | [79] |
| 3.2 nM | ||||||
| MIP microbeads | Ferrocenylmethyl methacrylate | CPE | Bisphenol A | Ultrapure water | NI | [80] |
| NI | ||||||
| Capacitance. Voltammetry | ||||||
| MIP | Aminophenol (AP) | Au SPE | Myoglobin | Synthetic human serum | 1.5–4 (EIS); 0.8–3.5 (SWV) μg/mL | [196] |
| 1.5 (EIS); 0.8 (SWV) μg/mL | ||||||
| MIP | Eriochrome black T (EBT) | Graphene SPE C SPE | Chloramphenicol | Acetonitrile buffer | 1 nM–10 mM | [197] |
| 0.62 nM | ||||||
| Amperometry | ||||||
| MIP-MWCNT-Nafion | APTES and PTMS | GCE | 2-nonylphenol | Tap and river water, soil | 0.2–360 μM | [42] |
| 0.06 μM | ||||||
| Au NP-MIP | Functionalized AuNPs (F-AuNPs) | Au | Dopamine | Human serum | 0.02–0.54 μM | [198] |
| 7.8 nM | ||||||
| MIP | O-phenylenediamine‒resorcinol | GCE | Tamoxifen | Acetate buffer | 1–100 nM | [199] |
| NI | ||||||
| MIP | Acrylamide | Au | Norfloxacin | Human urine | 10−9–10−3 M | [200] |
| 10−10 M | ||||||
| Conducting MIP | N-phenylethylene diamine methacrylamide (NPEDMA) | Au | 17β-estradiol | Ethanol/PBS solutions | 10−7–8 × 10−7 M | [201] |
| 6.89 × 10−7 M | ||||||
| MIP-Pt | MAA | Ti | Tetracycline | Ultrapure water | 0.1–10 mg/L | [202] |
| 0.026 mg/L | ||||||
| MIP NP | Vinylferrocene, ferrocenylmethyl methacrylate | GCE | Vancomycin | TRIS buffer | 83–410 μM | [77] |
| NI | ||||||
| MIP | Pyrrole | Pt wire sealed in glass | Bovine leukemia virus glycoproteins | Ultrapure water | NI | [203] |
| NI | ||||||
| MIP | Pyrrole | PGE | DNA | Acetate buffer | NI | [204] |
| NI | ||||||
| Sensor | Form or Electrode | Functional Monomer | Target | Sample | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| LOD | ||||||
| UV/Visible spectroscopy | ||||||
| ZnFe2O4/MIP | Membrane | Acrylamide (AM) | Bisphenol A | Ultrapure water | 10–1000 nM | [214] |
| 6.18 nM | ||||||
| MIP | Membrane | Itaconic acid (IA) | Phenol | Drinking, natural, and wastewater | 50 nM–10 mM | [215] |
| 50 nM | ||||||
| MIP | Paper | MAA + polyethylenimine (PEI), | Cd(II) | Lake water | 1–100 ng/mL | [216] |
| 0.4 ng/mL | ||||||
| MIP | Particles | 2-acrylamido-2-methyl-1-propanesulfonic acid (AMPSA) | Basic red 9 | Ultrapure water | NI | [217] |
| NI | ||||||
| Magnetite-MIP | Microspheres | MAA | Rhodamine B | Wine | 0.04–1.4 μg/mL | [218] |
| 1.05 μg/L | ||||||
| MIP-Graphitic C3N4 | FTO | 4-vinylpyridine (4-VP) | Bisphenol A | Bottled water | 5–200 μM | [219] |
| 1.3 μM | ||||||
| MIP | Film | Acrylic acid (AA) | 2-butoxyethanol | Hydraulic fracking wastewater | 10 ppb–100 ppm | [220] |
| 3.4 ppb | ||||||
| MIP | Film | AA | Testosterone | Ultrapure water | 5–100 ppb | [221] |
| 4.2 ppb | ||||||
| Magnetic MIP | NP | Triallyl isocyanurate | Sterigmatocystin | Wheat | 1.8–25 ng/g | [222] |
| 0.63 ng/g | ||||||
| Fluorescence | ||||||
| MIP/Mn-ZnS QD | NP | 4-vinylphenylboronic acid and methyl methacrylate | α-fetoprotein | Human serum | 0.05–10 μg/L | [223] |
| 48 ng/L | ||||||
| C dots-MIP | NP | MAA | Sterigmatocystin | Millet, rice, corn | 0.05–2 mg/L | [224] |
| 0.019 mg/L | ||||||
| MIP/Mn-ZnS QD | NP | 3-aminopropyltriethoxysilane (APTES) | Nicosulfuron | River water | 12–6000 nM | [225] |
| 1.1 nM | ||||||
| MIP/POF | Capillary tube | MAA | Bisphenol A | Mineral water bottle in ethanol and water solutions | 3 × 10−9–5 × 10−6 g/mL | [226] |
| 1.7 × 10−9 g/mL | ||||||
| Allyl fluorescein-MIP | Microspheres | 4-vinylphenylboronic acid+ MAA (VPBA/MAA) | Tetracycline | Etracycline hydrochloride, human serum, swine urine | 4.26–150 nM | [227] |
| 4.26 nM | ||||||
| MIP-IL | Microspheres | 3-(anthracen-9-ylmethyl)-1-vinyl-1H-imidazol-3-ium chloride (Fluorescent IL monomer) | p-nitroaniline | River, lake, and tap water | 10 nM–10 M | [228] |
| 9 nM | ||||||
| MIP-SiO2-CdTe QD | Composite | Acrylamide (AM) | Sulfanilamide | River water | 2–30 μM | [229] |
| 0.17 μM | ||||||
| CdTe QD-MIP | Composite | AM | λ-cyhalothrin | River water | 0.1–16 μM | [230] |
| 0.03 μM | ||||||
| MIP | Core-shell particles | methacrylamide | 2,4-diclorophenoxyacetic acid | River water | 20 nM–5 μM | [231] |
| 20 nM | ||||||
| MIP-C dots | Mesoporous structure | Amino-CDs | TNT | Tap water, soil | 0.5–20 μM | [232] |
| 17 nM | ||||||
| MIP | Hollow NP | AM | λ-cyhalothrin | Canal water | 10.26–160 nM | [233] |
| 10.26 nM | ||||||
| Fe3O4-SiO2-MIP | Core-shell magnetic NP | nitrobenzoxadiazole fluorophore (NBD-MA) | Rhodamine B | Methanol | NI | [234] |
| 10−8 M | ||||||
| CdTe/CdS QD-MIP | Mesoporous structure | 3-Aminopropyltriethoxysilane (APTES) | Diniconazole | Soil, river and wastewater | 20–160 μg/L | [235] |
| 6.4 μg/L | ||||||
| MIP | Au NC | APTES | Bisphenol A | Seawater | 0.1–13 μM | [236] |
| 0.1 μM | ||||||
| MIP | Microplate | Dansyl methacrylate | Bisphenol A | Tap, river and distilled water | 10–2000 μg/L | [237] |
| 3 μg/L | ||||||
| QD-MIP | Composite | Acrylamide (AM) | Cyphenothrin | River water | 0.1–80 μM | [238] |
| 9 nM | ||||||
| MIP-C dots | Nanocomposite | APTES | Bisphenol A | River water | 10−7–4.2 × 10−6 M | [239] |
| 3 × 10−8 M | ||||||
| MIP-ZnO QD | Nanocomposite | APTES | Thioridazine hydrochloride | Human plasma | 4–120 nM | [240] |
| 0.43 nM | ||||||
| MIP-C dots | Paper | MAA | 3-monochloropropane-1,2-diol | Soy sauce | 1–150 ng/mL | [241] |
| 0.6 ng/mL | ||||||
| MIP-QD | Composite | APTES | Tetrabromobisphenol-A | Electronic waste | 1–60 ng/mL | [242] |
| 3.6 ng/mL | ||||||
| MIP-Graphene QD | Composite | 3-aminopropyltriethoxysilane (APTS) | Omidazole | Human plasma | 0.75–30 μM | [243] |
| 0.24 μM | ||||||
| MIP-CdSeS/ZnS QD | Glass slide | MAA | Sulfasalazine | Human plasma and urine | 0.02–1.5 μM | [244] |
| 0.0071 μM | ||||||
| SiH4-C dots-MIP | Nanocomposite | APTES | Acetamiprid | Wastewater, apple | 7–107 nM | [245] |
| 2 nM | ||||||
| QD-MIP | Core-shell | APTES | Perfluorooctanoic acid | Tap and river water | 0.25–15 μM | [246] |
| 25 nM | ||||||
| MIP-C dots | Film | Poly(methyl acrylate-co-acrylic acid) | 2,4- dinitrotoluene | Ultrapure water | 1–15 ppm | [247] |
| 0.28 ppm | ||||||
| MIP-C dots | Film | Acrylic acid(AA) + methyl acrylate (MA) | 2,4- dinitrotoluene | Lake and tap water | 1–15 ppm | [248] |
| 0.28 ppm | ||||||
| MIP-QD | Nanocomposite | APTES | Thiamphenicol | Milk | 0.10–100 μM | [249] |
| 0.04 μM | ||||||
| MIP-C dots | Film | APTES | Cetricine | Urine, saliva | 0.5–500 ng/mL | [250] |
| 0.41 ng/mL | ||||||
| Chemiluminescence | ||||||
| MIP/Cromatography paper | Paper strip | MAA | Dichlorvos | Cabbage leaves, tomato skin | 0.003–10 μg/mL | [251] |
| 0.8 ng/mL | ||||||
| Silanized magnetic graphene-MIP/Capillary | Capillary tube | Acrylamide (AM) | Dopamine | Urine, dopamine hydrochloride injection | 8–200 ng/mL | [252] |
| 1.5 ng/mL | ||||||
| MIP-Magnetic NP | NP | MAA | Dibutyl phthalate | Juice | 3.84 × 10−8–2.08 × 10−5 M | [253] |
| 2.09 × 10−9 M | ||||||
| MoS2-Graphene QD-MIP | GCE | o-phenyl-enediamine (o–PD) | 2-methyl-4-chlorophenoxyacetic acid | Oat, tap, and lake water | 10 pM–0.1 μM | [254] |
| 5.5 pM | ||||||
| MIP-Au NP-CdSe/ZnS QD | Au electrode | Thioanilin | 2-methyl-4-chlorophenoxyacetic acid | Tap, lake and river water, oat, rice | 10 pM–50 μM | [255] |
| 2.2 nM | ||||||
| MIP/Chromatography paper | Paper disk | AM | 2,4-dichlorophenoxyacetic acid | Tap and lake water | 5 pM–10 μM | [256] |
| 1 pM | ||||||
| CdTe QD-Chitosan-GO-Magnetite-MIP | NP | MAA | Chrysoidine | Paper, fabric | 10−7–10−5 M | [257] |
| 3.2 × 10−9 M | ||||||
| MIP-Aptamer-CdS QD | SPE | Dopamine | Diethylstilbestrol | Fish | 0.3–100,000 pg/mL | [258] |
| 0.1 pg/mL | ||||||
| MIP | CPE | MAA | Azithromycin | Blood serum, urine | 10−10–4 × 10−7 M | [259] |
| 2.3 × 10−11 M | ||||||
| MIP-Cu nanoclusters | GCE | o-phenylenediamine | Enrofloxacin | Meat, lake water, bovine serum, human urine | 0.1 nM–1 μM | [260] |
| 27 pM | ||||||
| Surface plasmon resonance | ||||||
| MIP/POF | Optical fiber | MAA | L-nicotine | Ultrapure water | 1.86 × 10−4–10−3 M | [261] |
| 1.86 × 10−4 M | ||||||
| MIP/POF | Optical fiber | MAA | Furfural | Transformer oil | 9–30 ppb | [262] |
| 9 ppb | ||||||
| MIP/Ag-POF | Optical fiber | Polyaniline | Ascorbic acid | Ultrapure water | 10−8–10−7; 10−6–10−4 M | [263] |
| 1.28 × 10−10 M | ||||||
| MIP/Ag-POF | Optical fiber | MAA | Profenofos | PBS | 10−4 μg/L | [264] |
| 2.5 × 10−6 μg/L | ||||||
| MIP | Film | Methacrylamide | Amoxicillin | Tap water, PBS | 0.1–2.6 nM | [265] |
| 73 pM | ||||||
| MIP | Film | MAA | Histamine | Fish | 25–1000 μg/L | [266] |
| 25 μg/L | ||||||
| MIP | NP | N-methacryloyl-(L)-histidine methyl ester | Histamine | Canned tuna, cheese | 0.001–10 μg/L | [267] |
| 0.58 ng/L | ||||||
| MIP | NP | Biotinylated phenylalanine | Prostate-specific antigen | Blood | 0.001–0.2 ng/mL | [268] |
| 1 pg/mL | ||||||
| MIP | Nanofilm | N-methacryloyl-(L)-tryptophan methyl ester | Carbofuran, dimethoate | River water | 10–1000 ng/L | [269] |
| 7.11 (carbofuran); 8.37 (dimethoate) ng/L | ||||||
| MIP-Au NP | Film | N-methacryloyl-(L)-phenylalanine | Aflatoxin B1 | Corn, peanut | 0.0001–10 ng/mL | [270] |
| 1.04 pg/mL | ||||||
| MIP-Ag NP | Film | N-methacryloyl-(L)-histidine methyl ester | Escherichia coli | Mimic urine | 15–1,500,000 CFU/mL | [271] |
| 0.57 CFU/mL | ||||||
| Raman scattering | ||||||
| MIP microspheres/Au-Klarite substrate | Microsphres | MAA | Nicotine | Acetonitrile | NI | [272] |
| NI | ||||||
| MIP/Au-Disulfide-derivatized perfluorophenylazide-Klarite substrate | NP | MAA | Propranolol | Human urine | NI | [273] |
| 7.7 × 10−4 M | ||||||
| MIP/Ag dentrite nanostructure substrate | Fine particles | MAA | Melamine | Milk | 0.005–0.05 mM | [274] |
| 0.012 mM | ||||||
| Ag-MIP | Core-shell nanoplates | Methacrylamide | Rhodamine B | Ultrapure water | NI | [275] |
| 10−12 M | ||||||
| Ag-MIP | Core-shell | AM | Rhodamine 6G | Water | 10−12–10−6 M | [276] |
| 10−14 M | ||||||
| Ag-MIP | Core-shell | MAA | 4-mercaptobenzoic acid | Water | NI | [277] |
| 10−15 M | ||||||
| Boronate affinity MIP-Boronate affinity SERS | Sandwich assay | APTES | α-fetoprotein | Human serum | 0.001–10 μg/mL | [278] |
| 0.1 ng/mL | ||||||
| MIP-Au NP | Core-shell | 3-(triethoxysilyl)propyl isocyanate (TEPIC) | Bisphenol A | Surface water, plastic-bottled beverages | 2.2 × 10−6–10−4 M | [279] |
| 5.37 × 10−7 M | ||||||
| MIP-Magnetic NP | Core-shell | MAA | Ciprofloxacin | Fetal bovine serum | 10−7–10−4 M | [280] |
| 10−7 M | ||||||
| MIP-SiO2-AgNP | Core-shell | tetraethyl orthosilicate (TEOS) | Bisphenol A | Tap and lake water, milk | 1.75 × 10−11–1.75 × 10−6 M | [281] |
| 1.46 × 10−11 M | ||||||
| MIP-Ag | Core-shell | Acrylamide | Glibenclamide | Water | 1 ng/mL–100 μg/mL | [282] |
| 1 ng/mL | ||||||
| SiO2/rGO/Au-MIP | NP | Methacrylic acid, acrylamide | 2,6-dichlorophenol | Water | 1–100 nM | [283] |
| 0.02 nM | ||||||
| MIP-Au NP | Core-shell | Phenyltrimethoxysilane | L-Phenylalanine | Bovine serum | 10−8–10−4 M | [284] |
| 10−9 M | ||||||
| UV/visible spectroscopy. Raman scattering | ||||||
| MIP-Au NP | Fine particles | MAA | Atrazine | Apple juice | NA (Color); 0.005–1 mg/L (SERS) | [212] |
| 0.005 (Color); 0.0012 (SERS) mg/L | ||||||
| Fluorescence. Raman scattering | ||||||
| Magnetic MIP | NP | Poly(ethylene-co-vinyl alcohol) | Phenylalanine | Human urine | 7–100 (F); 5–800 μg/mL (RS) | [285] |
| NI (F); 0.4 μg/mL (RS) | ||||||
| Photoelectrochemical | ||||||
| MIP | ITO | Pyrrole | Bisphenol A | River and tap water | 2–500 nM | [41] |
| 1.2 nM | ||||||
| MIP-Au NP-ZnO NP | Paper | Pyrrole | Pentacholorophenol | Drinking and river water | 0.01–100 ng/mL | [286] |
| 4 pg/mL | ||||||
| F graphitic C3N4-MIP | FTO | 4-vinylpyridine (4-VP) | Cr(VI) | Tap and river water | 0.01–100 ppb | [287] |
| 0.006 ppb | ||||||
| MIP/TiO2 NT | Thin film | o-phenyl-enediamine (o–PD) | Lindane | Drinking and river water | 0.1–10 μM | [213] |
| 0.03 μM | ||||||
| MIP/Au NP-TiO2 NT | Thin film | o-PD | Chlorpyrifos | Green vegetables | 0.05–10 μM | [288] |
| 0.96 nM | ||||||
| MIP/TiO2 NT | Vertical NT | Pyrrole | Bisphenol A | Drinking, river, and tap water, domestic sewage | 4.5–108 nM | [289] |
| 2 nM | ||||||
| MIP-TiO2 | Nanorods | P-aminothiophenol (ATP) | Chlorpyrifos | Drinking and river water | 0.01–100 ng/mL | [290] |
| 7.4 pg/mL | ||||||
| MIP-TiO2 | FTO glass substrate | APTES | RNase B | Human serum | 0.5 pM–2 μM | [291] |
| 0.12 pM | ||||||
| MIP-CdTe QD-Au NP | Paper/SPE | AAM | S-fenvalerate | Apple, pear, tomato, cucumber | 10−8–10−6 M | [292] |
| 3.2 × 10−9 M | ||||||
| MIP-TiO2-MWCNT | Core-shell | TiO2 | Microcystin-LR | Tap, pond, and river water | 1 pM–3 nM | [293] |
| 0.4 pM | ||||||
| MIP | Porous carrier | 4-vinylpyridine (VP) and N-isopropylacrylamide (NIPAM) | Bisphenol A | Seawater, yogurt | NI | [294] |
| 11.14 μg/L | ||||||
| MIP | Strip/SPE | AM | Perfluorooctane sulfonyl fluoride | Tap, river, and lake water | 0.05–500 ppb | [295] |
| 0.01 ppb | ||||||
| Aptamer-MIP-GO-C3N4 | FTO | Aptamer | Kanamycin | Na2SO4 solution | 1–230 nM | [296] |
| 0.2 nM | ||||||
| MIP-BiIO nanoflake array | Nanofibers | Chitosan | Triphenyl Phosphate | Tap, river, and lake water | 0.01–500 ng/mL | [297] |
| 0.008 ng/mL | ||||||
| MIP/TiO2 NT | Vertical NT | AM | Perfluorooctane sulfonate | Tap, river, and mountain water | 0.5–10 μM | [298] |
| 86 ng/mL | ||||||
| CdS dots-Graphene-MIP | FTO | Pyrrole | 4-aminophenol | Lake water | 5 × 10−8–3.5 × 10−6 M | [299] |
| 2.3 × 10−8 M | ||||||
| MIP-AgI NP-BiIO nanoflake array | FTO | AM | Perfluorooctanic acid | Tap and river water | 0.02–1000 ppb | [300] |
| 0.01 ppb | ||||||
| Glass/ZnO/MIP | Nanorods | Pyrrole | Bisphenol S | PBS | 2.5–12.5 μM | [301] |
| 0.7 μM | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadhem, A.J.; Gentile, G.J.; Fidalgo de Cortalezzi, M.M. Molecularly Imprinted Polymers (MIPs) in Sensors for Environmental and Biomedical Applications: A Review. Molecules 2021, 26, 6233. https://doi.org/10.3390/molecules26206233
Kadhem AJ, Gentile GJ, Fidalgo de Cortalezzi MM. Molecularly Imprinted Polymers (MIPs) in Sensors for Environmental and Biomedical Applications: A Review. Molecules. 2021; 26(20):6233. https://doi.org/10.3390/molecules26206233
Chicago/Turabian StyleKadhem, Abbas J., Guillermina J. Gentile, and Maria M. Fidalgo de Cortalezzi. 2021. "Molecularly Imprinted Polymers (MIPs) in Sensors for Environmental and Biomedical Applications: A Review" Molecules 26, no. 20: 6233. https://doi.org/10.3390/molecules26206233
APA StyleKadhem, A. J., Gentile, G. J., & Fidalgo de Cortalezzi, M. M. (2021). Molecularly Imprinted Polymers (MIPs) in Sensors for Environmental and Biomedical Applications: A Review. Molecules, 26(20), 6233. https://doi.org/10.3390/molecules26206233







