Nitrogen as a Probe Molecule for the IR Studies of the Heterogeneity of OH Groups in Zeolites
Abstract
:1. Introduction
2. Results
2.1. Free OH Groups
2.2. OH Groups Interacting with Nitrogen
3. Discussion
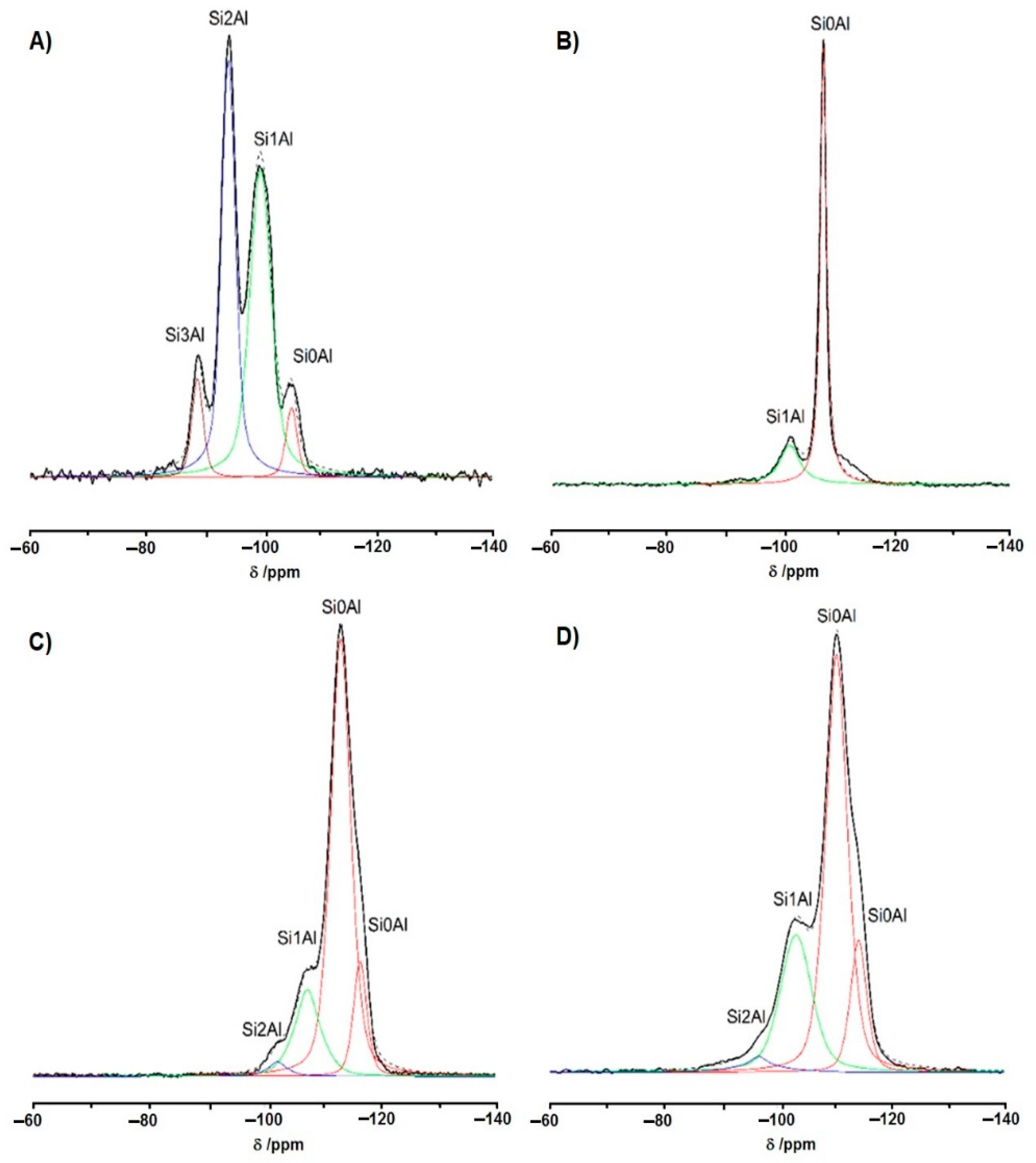
4. Materials and Methods
4.1. Materials
4.2. IR Studies
4.3. NMR Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Vermeiren, W.; Gilson, J.P. Impact of zeolites on the petroleum and petrochemical industry. Top. Catal. 2009, 52, 1131–1161. [Google Scholar] [CrossRef]
- Corma, A. Inorganic solid acids and their use in acid-catalyzed hydrocarbon reactions. Chem. Rev. 1995, 95, 559–614. [Google Scholar] [CrossRef]
- Clerici, M.G. Zeolites for fine chemicals production. Top. Catal. 2000, 13, 373–386. [Google Scholar] [CrossRef]
- Guisnet, M.; Gilson, J.P. Zeolites for Cleaner Technologies; Catalytic Science Series: Volume 3; Imperial College Press: London, UK, 2002; ISBN 1-86094-329-2. [Google Scholar]
- Soler-Illia, G.J.A.A.; Sanchez, C.; Lebeau, B.; Patarin, J. Chemical strategies to design textured materials: From microporous and mesoporous oxides to nanonetworks and hierarchical structures. Chem. Rev. 2002, 102, 4093–4138. [Google Scholar] [CrossRef] [PubMed]
- Van Donk, S.; Janssen, A.H.; Bitter, J.H.; De Jong, K.P. Generation, characterization, and impact of mesopores in zeolite catalysts. Catal. Rev. 2003, 45, 297–319. [Google Scholar] [CrossRef] [Green Version]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry and Use; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Olson, D.H.; Kokotailo, G.T.; Lawton, S.L.; Meier, W.M. Crystal structure and structure-related properties of ZSM-5. J. Phys. Chem. 1981, 85, 2238–2243. [Google Scholar] [CrossRef]
- Cejka, J.; Centi, G.; Perez-Pariente, J.; Roth, W.J. Zeolite-based materials for novel catalytic applications: Opportunities, perspectives and open problems. Catal. Today 2012, 179, 2–15. [Google Scholar] [CrossRef]
- Védrine, J.C.; Abou-Kais, A.; Massardier, J.; Dalmai-Imelik, G. ESR study of deep-bed calcined NH4Y and aluminum deficient zeolites. J. Catal. 1973, 29, 120–128. [Google Scholar] [CrossRef]
- Derouane, E.G.; Védrine, J.C.; Ramos Pinto, R.; Borges, P.M.; Costa, L.; Lemos, M.A.N.D.A.; Lemos, F.; Ramôa Ribeiro, F. The acidity of zeolites: Concepts, measurements and relation to catalysis: A review on experimental and theoretical methods for the study of zeolite acidity. Catal. Rev. Sci. Eng. 2013, 55, 454–515. [Google Scholar] [CrossRef]
- Egerton, T.A.; Hardin, A.H. The application of Raman spectroscopy to surface chemical studies. Catal. Rev. Sci. Eng. 1975, 11, 71–116. [Google Scholar] [CrossRef]
- Lercher, J.A.; Grundling, C.; Eder-Mirth, G. Infrared studies of the surface acidity of oxides and zeolites using adsorbed probe molecules. Catal. Today 1996, 27, 353–376. [Google Scholar] [CrossRef] [Green Version]
- Paukshtis, E.A.; Yurchenko, E.N. Study of the acid–base properties of heterogeneous catalysts by infrared spectroscopy. Russ. Chem. Rev. 1983, 52, 42. [Google Scholar] [CrossRef]
- Knözinger, H. Infrared spectroscopy for the characterization of surface acidity and basicity. In Handbook of Heterogeneous Catalysis; Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J., Eds.; Wiley: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Mapes, J.; Eischens, R. The infrared spectra of ammonia chemisorbed on cracking catalysts. J. Phys. Chem. 1954, 58, 1059. [Google Scholar] [CrossRef]
- Datka, J.; Góra-Marek, K. IR studies of the formation of ammonia dimers in zeolites TON. Catal. Today 2006, 114, 205–210. [Google Scholar] [CrossRef]
- Parry, E.P. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J. Catal. 1963, 2, 371. [Google Scholar] [CrossRef]
- Hughes, T.R.; White, H.M. A Study of the surface structure of decationized y zeolite by quantitative infrared spectroscopy. J. Phys. Chem. 1967, 71, 2192. [Google Scholar] [CrossRef]
- Gil, B.; Mierzyńska, K.; Szczerbińska, M.; Datka, J. In situ IR and catalytic studies of the effect of coke on acid properties of steamed zeolite Y. Microporous Mesoporous Mater. 2007, 99, 328–333. [Google Scholar] [CrossRef]
- Cerqueira, H.S.; Ayrault, P.; Datka, J.; Guisnet, M. Influence of coke on the acid properties of a USHY zeolite. Microporous Mesoporous Mater. 2000, 38, 197–205. [Google Scholar] [CrossRef]
- Datka, J.; Gil, B. In situ IR and catalytic studies of the regeneration of acid sites in coked zeolite Y. Microporous Mesoporous Mater. 2007, 103, 225–229. [Google Scholar] [CrossRef]
- Datka, J.; Gil, B.; Kubacka, A. Heterogeneity of OH groups in H-mordenite. Effect of Na/H exchange degree. Zeolites 1997, 18, 245. [Google Scholar] [CrossRef]
- Gackowski, M.; Podobiński, J.; Broclawik, E.; Datka, J. IR and NMR studies of the status of Al and of acid sites in desilicated zeolite Y. Molecules 2020, 25, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knozinger, H. Infrared spectroscopy as a probe of surface acidity. In Elementary Reaction Steps in Heterogeneous Catalysis; Joyner, R.W., van Santen, R.A., Eds.; Kluwer: Dordrecht, The Netherlands, 1993; p. 267. [Google Scholar]
- Eder, F.; Lercher, J.A. On the role of the pore size and tortuosity for sorption of alkanes in molecular sieves. J. Phys. Chem. B 1997, 101, 1273. [Google Scholar] [CrossRef]
- Datka, J. Transformations of butenes on dehydroxylated Y zeolites studied by infrared spectroscopy. J. Chem. Soc. Faraday Trans. 1981, 77, 2633. [Google Scholar] [CrossRef]
- Datka, J. The interaction of ethene molecules with OH groups in IMaHA and NaHZSM-5 zeolites studied by i.r. spectroscopy. Zeolites 1991, 11, 739. [Google Scholar] [CrossRef]
- Davydov, A.A. Donor-acceptor properties of the cations of oxide surfaces. Russ. J. Phys. Chem. 1992, 66, 1736–1739. [Google Scholar]
- Makarova, M.A.; Ojo, A.F.; Karim, K.; Hunger, M.; Dwyer, J. FTIR study of weak hydrogen bonding of bronsted hydroxyls in zeolites and aluminophosphates. J. Phys. Chem. 1994, 98, 3619. [Google Scholar] [CrossRef]
- Janin, A.; Lavalley, J.C.; Benazzi, E.; Schott-Darie, C.; Kessler, H. Acidity of cloverite. In Catalysis by Microporous Mater; Beyer, K., Karge, H.G., Kiricsi, I., Nagy, J.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 94, p. 124. [Google Scholar]
- Su, B.L.; Barthomeuf, D. Quantitative measurement by infrared spectroscopy of the protonic acidity of H-SAPO-37 and HY using benzene as a probe. J. Catal. 1993, 139, 81. [Google Scholar] [CrossRef]
- Boehm, H.P.; Knozinger, H. Nature and Estimation of Functional Groups on Solid Surfaces. In Catalysis—Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1983; Volume 4, p. 39. [Google Scholar]
- Jentys, A.; Lercher, J.A. IR study of the Adsorption of Benzene on HZSM-5. In Zeolites as Catalysts, Sorbents and Detergent Builders—Applications and Innovations; Karge, H.G., Weitkamp, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 46, p. 585. [Google Scholar]
- Kotrla, J.; Kubelkova, L. Activation of reactants by hydroxyl groups of solid acids. An FTIR study. In Catalysis by Microporous Mater; Beyer, H.K., Karge, H.G., Kiricsi, I., Nagy, J.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 94, p. 509. [Google Scholar]
- Florian, J.; Kubelkova, L. Proton transfer between H-Zeolite and adsorbed acetone or acetonitrile: Quantum chemical and FTIR Study. J. Phys. Chem. 1994, 98, 8734. [Google Scholar] [CrossRef]
- Pelmenshikov, A.G.; van Santen, R.A. Water Adsorption on Zeolites: Ab-Initio Interpretation of IR Data. J. Phys. Chem. 1993, 97, 10768. [Google Scholar] [CrossRef]
- Lavalley, J.C.; Caillod, J. CD3OCD2H: une nouvelle molécule-sonde pour l’étude infrarouge de l’acidité de Lewis des sites d’adsorption des oxydes metalliques. J. Chim. Phys. 1980, 77, 379–386. [Google Scholar] [CrossRef]
- Knozinger, H. Specific Poisoning and Characterization of Catalytically Active Oxide Surfaces. Adv. Catal. 1976, 25, 184. [Google Scholar] [CrossRef]
- Lercher, J.A.; Noller, H. Infrared spectroscopic study of hydroxyl group acid strength of silica, alumina, and magnesia mixed oxides. J. Catal. 1982, 77, 152. [Google Scholar] [CrossRef]
- Lercher, J.A.; Vinek, H.; Noller, H. Acid–base properties of silica–alumina samples derived from NaX zeolites. Part 1. Physical characterization and an infrared study of the adsorption of acetone. J. Chem. Soc. Faraday Trans. 1984, 80, 1239. [Google Scholar] [CrossRef]
- Busca, G.; Lorenzelli, V. Infrared study of methanol, formaldehyde, and formic acid adsorbed on hematite. J. Catal. 1980, 66, 155. [Google Scholar] [CrossRef]
- Lavalley, J.C.; Lamotte, J.; Busca, G.; Lorenzelli, V. Fourier transform i.r. evidence of the formation of dioxymethylene species from formaldehyde adsorption on anatase and thoria. J. Chem. Soc. Chem. Commun. 1985, 14, 1006–1007. [Google Scholar] [CrossRef]
- Gil, B.; Kałahurska, K.; Kowalczyk, A. A study of the external and internal sites of 2D and 3D zeolites through the FTIR investigation of the adsorption of ammonia and pivalonitrile. Appl. Catal. A Gen. 2019, 578, 63–69. [Google Scholar] [CrossRef]
- Inagaki, S.; Park, S.; Yamazaki, H.; Kondo, J.N.; Kubota, Y. Investigation of the acidic nature of MCM-68 zeolite based on the adsorption of CO and bulky probe molecules. Microporous Mesoporous Mater. 2018, 272, 16–23. [Google Scholar] [CrossRef]
- Armaroli, T.; Trombetta, M.; Alejandre, A.; Solis, J.R.; Busca, G. FTIR study of the interaction of some branched aliphatic molecules with the external and internal sites of H-ZSM5 zeolite. Phys. Chem. Chem. Phys. 2000, 2, 3341. [Google Scholar] [CrossRef]
- Ungureanu, A.; Hoang, T.V.; On, D.T.; Dumitriu, E.; Kaliaguine, S. An investigation of the acid properties of UL-ZSM-5 by FTIR of adsorbed 2,6-ditertbutylpyridine and aromatic transalkylation test reaction. Appl. Catal. A Gen. 2005, 294, 92–105. [Google Scholar] [CrossRef]
- Trombetta, M.; Busca, G.; Lenarda, M.; Storaro, L.; Pavan, M. An investigation of the surface acidity of mesoporous Al-containing MCM-41 and of the external surface of ferrierite through pivalonitrile adsorption. Appl. Catal. A Gen. 1999, 182, 225–235. [Google Scholar] [CrossRef]
- Holm, M.S.; Svelle, S.; Joensen, F.; Beato, P.; Christensen, C.H.; Bordiga, S.; Bjørgen, M. Assessing the acid properties of desilicated ZSM-5 by FTIR using CO and 2,4,6-trimethylpyridine (collidine) as molecular probes. Appl. Catal. A 2009, 356, 23. [Google Scholar] [CrossRef]
- Datka, J.; Boczar, M.; Gil, B. Heterogeneity of hydroxyl groups in zeolites studied by IR spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 1995, 105, 1–18. [Google Scholar] [CrossRef]
- Creyghton, E.J.; Elings, J.A.; Dewing, R.S.; Sheldon, R.A.; van Bekkum, H. New multifunctional probe for testing outer surface activity of zeolites: Application to surface-located platinum clusters and acid sites. Microporous Mater. 1996, 5, 299. [Google Scholar] [CrossRef]
- Aurault, P.; Datka, J.; Laforge, S.; Martin, D.; Guisnet, M. Characterization of internal and external acidity of H-MCM-22 Zeolites. J. Phys. Chem. B 2004, 108, 13755. [Google Scholar] [CrossRef]
- Sadowska, K.; Góra-Marek, K.; Datka, J. Accessibility of acid sites in hierarchic zeolites—Quantitative IR Studies of pivalonitrile adsorption. J. Phys. Chem. 2013, 117, 9239. [Google Scholar] [CrossRef]
- Bose, H.; Forster, H. Sorption states of isoelectronic linear molecules in zeolites. J. Mol. Struct. 1990, 218, 393. [Google Scholar] [CrossRef]
- Bordiga, B.; Palomino, G.T.; Paze, C.; Zecchina, A. Vibrational spectroscopy of H2, N2, CO and NO adsorbed on H, Li, Na, K-exchanged ferrierite. Microporous Mesoporous Mater. 2000, 34, 67–80. [Google Scholar] [CrossRef]
- Gribov, E.N.; Cocina, D.; Spoto, G.; Bordiga, S.; Ricchiardi, G.; Zecchina, A. Vibrational and thermodynamic properties of Ar, N2, O2, H2 and CO adsorbed and condensed into (H,Na)-Y zeolite cages as studied by variable temperature IR spectroscopy. Phys. Chem. Chem. Phys. 2006, 8, 1186. [Google Scholar] [CrossRef]
- Neyman, K.M.; Strodel, P.; Ruzankin, S.P.; Schlensog, N.; Knozinger, H.; Rosch, N. N2 and CO molecules as probes of zeolite acidity: An infrared spectroscopy and density functional investigation. Catal. Lett. 1995, 31, 273. [Google Scholar] [CrossRef]
- Chakarova, K.; Hadjiivanov, K. H-Bonding of zeolite hydroxyls with weak bases: FTIR study of CO and N2 adsorption on H-D-ZSM-5. J. Phys. Chem. C 2011, 115, 4806–4817. [Google Scholar] [CrossRef]
- Borovkov, V.Y.; Musyka, I.S.; Kazansky, V.B. The study of the adsorption of molecular and dissociative hydrogen on γ – and η-alumina at the temperature interval between 80–300 K by means of DRIR spectroscopy. Dokl. Akad. Nauk SSSR 1982, 265, 109. [Google Scholar]
- Gackowski, M.; Kuterasiński, Ł.; Podobiński, J.; Datka, J. Hydroxyl groups of exceptionally high acidity in desilicated zeolites Y. ChemPhysChem 2018, 19, 3372–3379. [Google Scholar] [CrossRef] [PubMed]
- Gackowski, M.; Kuterasiński, Ł.; Podobiński, J.; Korzeniowska, A.; Sulikowski, B.; Datka, J. Mazzite type hierarchical Zeolites: IR and NMR and catalytic Studies. Appl. Catal. A 2019, 578, 53. [Google Scholar] [CrossRef]
- Datka, J.; Boczar, M.; Rymarowicz, P. Heterogeneity of OH groups in NaH-ZSM 5 zeolites studied by IR spectroscopy. J. Catal. 1988, 114, 368. [Google Scholar] [CrossRef]
- Datka, J.; Gil, B. Heterogeneity of hydroxyl groups in faujasites studied by IR spectroscopy. J. Catal. 1994, 145, 372. [Google Scholar] [CrossRef]
- Kazanski, V.B. Structure and Reactivity of Modified Zeolites; Elsevier: Amsterdam, The Netherlands, 1984; p. 61. [Google Scholar]
- Zhidomirov, G.M.; Kazanski, V.B. Quantum chemical cluster models of acid-base sites of oxide catalysts. Adv. Catal. 1986, 34, 131. [Google Scholar] [CrossRef]
- Datka, J.; Broctawik, E.; Gil, B. IR spectroscopic studies and quantum chemical calculations concerning the O-H dissociation energies in zeolites NaHX and NaHY. J. Phys. Chem. 1994, 98, 5622. [Google Scholar] [CrossRef]
- Beran, S. Quantum chemical study of the effect of the structural characteristics of zeolites on the properties of their bridging of groups. J. Mol. Catal. 1984, 26, 31. [Google Scholar] [CrossRef]
- Beran, S. Quantum chemical study of the physical characteristics of HZSM-5 zeolites and comparison with faujasites. Zeitschrift für Physikalische Chemie 1983, 137, 89–97. [Google Scholar] [CrossRef]
- Schroder, K.P.; Sauer, J.; Leslie, M.; Catlow, C.R. Siting of Al and bridging hydroxyl groups in ZSM-5: A computer simulation study. Zeolites 1992, 12, 20. [Google Scholar] [CrossRef]
- Sauer, J.; Kolmel, C.; Hasse, F.; Ahlrichs, R. Proton transfer from acidic sites to water, methanol and ammonia. A comparative Ab initio study. In Proceedings of the 9th International Zeolite Conference, Montreal, QC, Canada, 5–10 July 1992; von Ballmoos, R., Higgins, J.B., Treacy, M.M.J., Eds.; Butterworth Heinemann: Stoneham, MA, USA, 1993; p. 679. [Google Scholar]
- Fyfe, C.A.; Strobl, H.; Kokotailo, G.T.; Kennedy, G.J.; Barlow, G.E. Ultra-high-resolution 29Si solid-state MAS NMR investigation of sorbate and temperature-induced changes in the lattice structure of zeolite ZSM-5. J. Am. Chem. Soc. 1988, 110, 3373–3380. [Google Scholar] [CrossRef]
- Datka, J.; Gil, B.; Baran, P. Heterogeneity of OH groups in HZSM-5 zeolites: Splitting of OH and OD bands in low-temperature IR spectra. Microporous Mesoporous Mater. 2003, 58, 291–294. [Google Scholar] [CrossRef]
- Datka, J.; Gil, B. Heterogeneity of OH groups in HZSM-5 zeolites. IR studies of ammonia adsorption and desorption. J. Mol. Struct. 2001, 596, 41–45. [Google Scholar] [CrossRef]
- Haag, W.O. Zeolites and related microporous materials: State of Art. Stud. Surf. Sci. Catal. 1994, 84, 1375. [Google Scholar] [CrossRef]
- Kotrel, S.; Lunsford, J.H.; Knozinger, H.J. Characterizing zeolite acidity by spectroscopic and catalytic means: A comparison. Phys. Chem. B 2001, 105, 3917. [Google Scholar] [CrossRef]
- Mintova, S.; Valtchev, V.; Onfroy, T.; Marichal, C.; Knozinger, H.; Bein, T. Variation of the Si/Al ratio in nanosized zeolite Beta crystals. Microporous Mesoporous Mater. 2006, 90, 237. [Google Scholar] [CrossRef]
- Góra-Marek, K.; Datka, J.; Dzwigaj, S.; Che, M. Influence of V content on the nature and strength of acidic sites in VSiβ zeolite evidenced by IR spectroscopy. J. Phys. Chem. B 2006, 110, 6763. [Google Scholar] [CrossRef]
- Bisio, C.; Massiani, P.; Fajerwerg, K.; Sordelli, L.; Stievano, L.; Silva, E.R.; Coluccia, S.; Martra, G. Identification of cationic and oxidic cesium species in basic Cs-overloaded BEA zeolites. Microporous Mesoporous Mater. 2006, 90, 175. [Google Scholar] [CrossRef]
- Van Bokhoven, J.A.; Koningsberger, D.C.; Kunkeler, P.; Van Bekkum, H.; Kentgens, A.P.M. Stepwise Dealumination of zeolite βeta at specific t-sites observed with 27Al MAS and 27Al MQ MAS NMR. J. Am. Chem. Soc. 2000, 122, 12842–12847. [Google Scholar] [CrossRef]
- Mintova, S.; Barrier, N. Verified Syntheses of Zeolitic Materials, 3rd ed.; Synthesis Commission of the International Zeolite Association: Rio de Janeiro, Brazil, 2016; ISSN 978-0-692-68539-6. [Google Scholar]
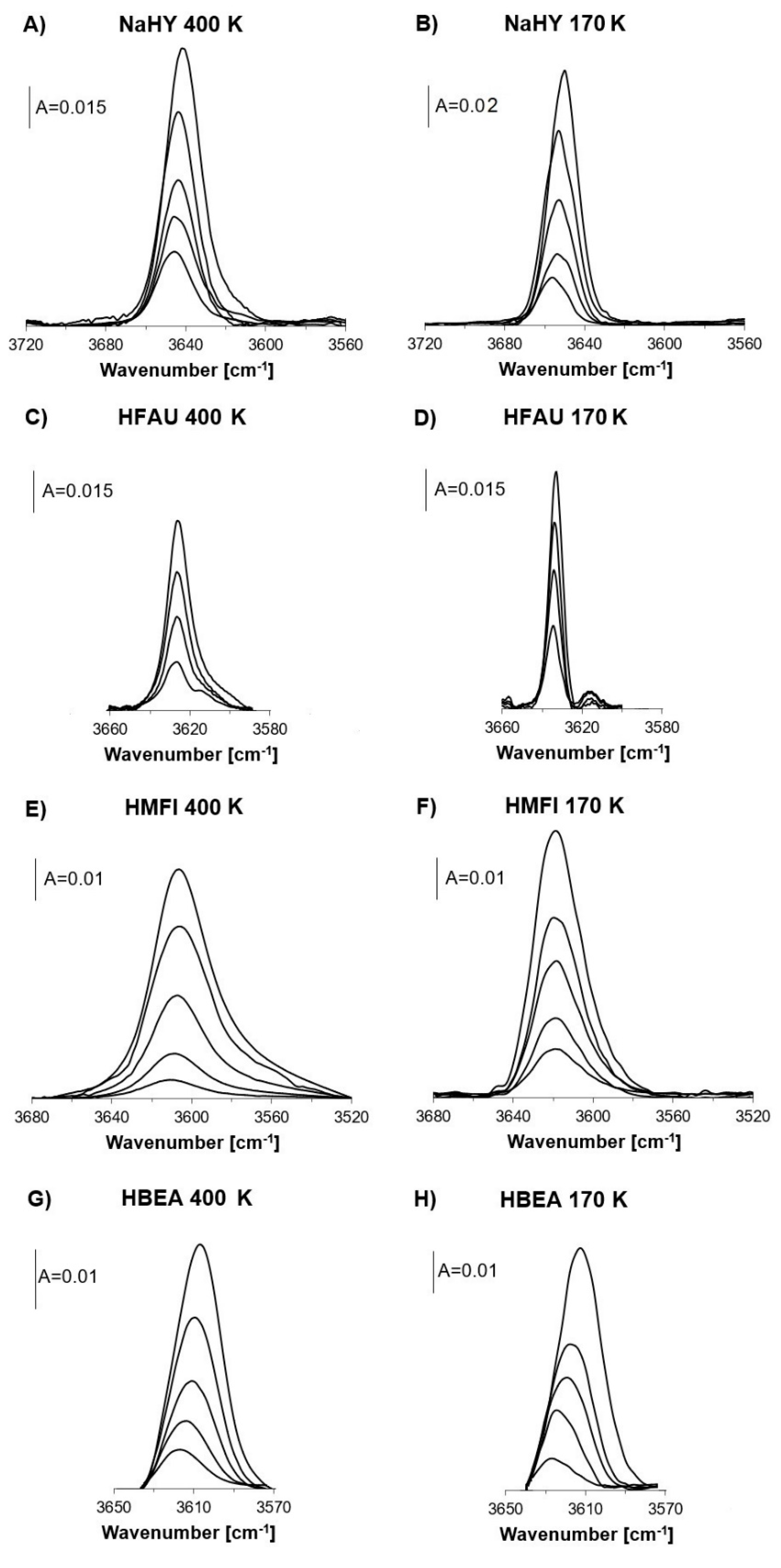
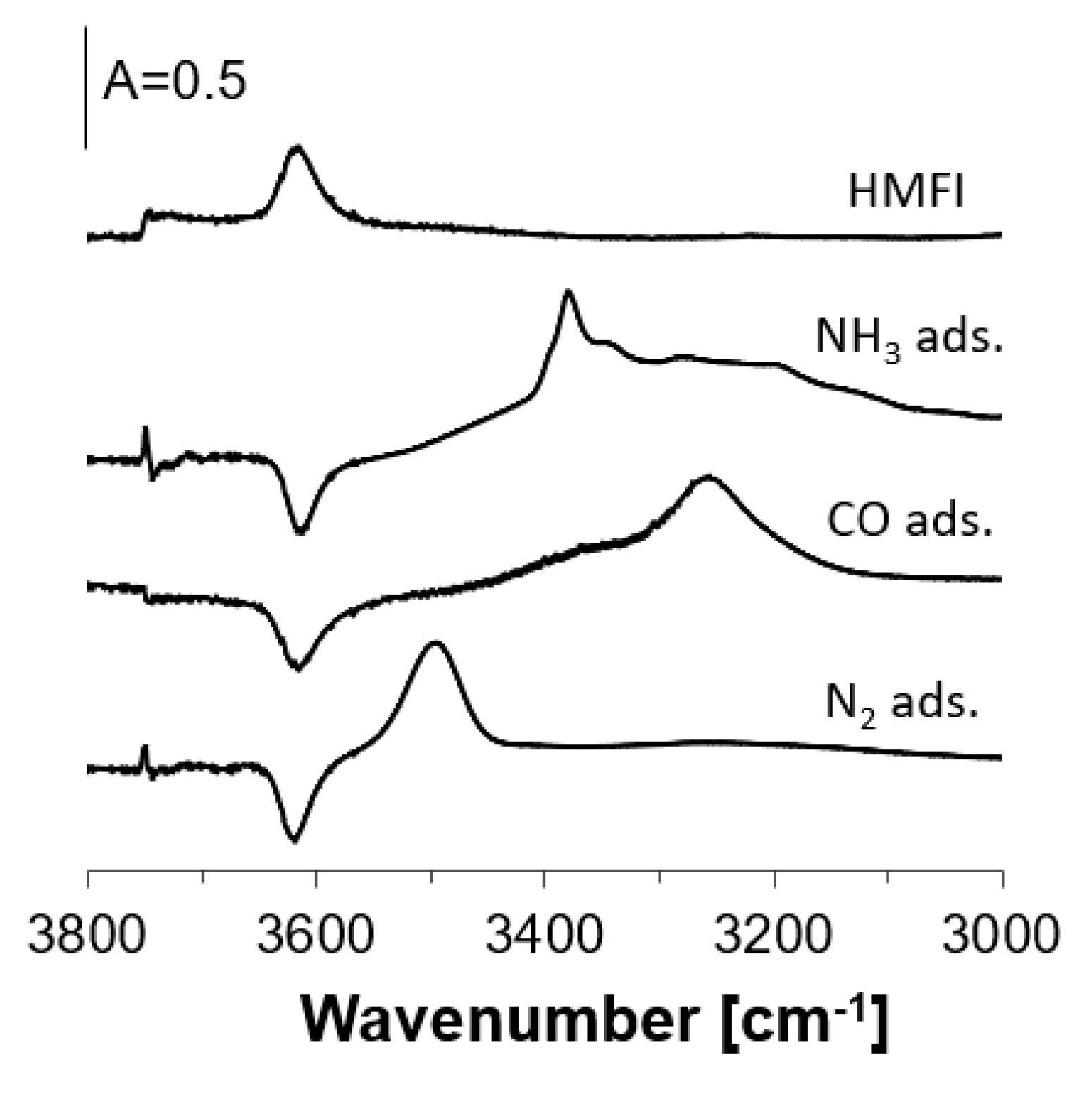
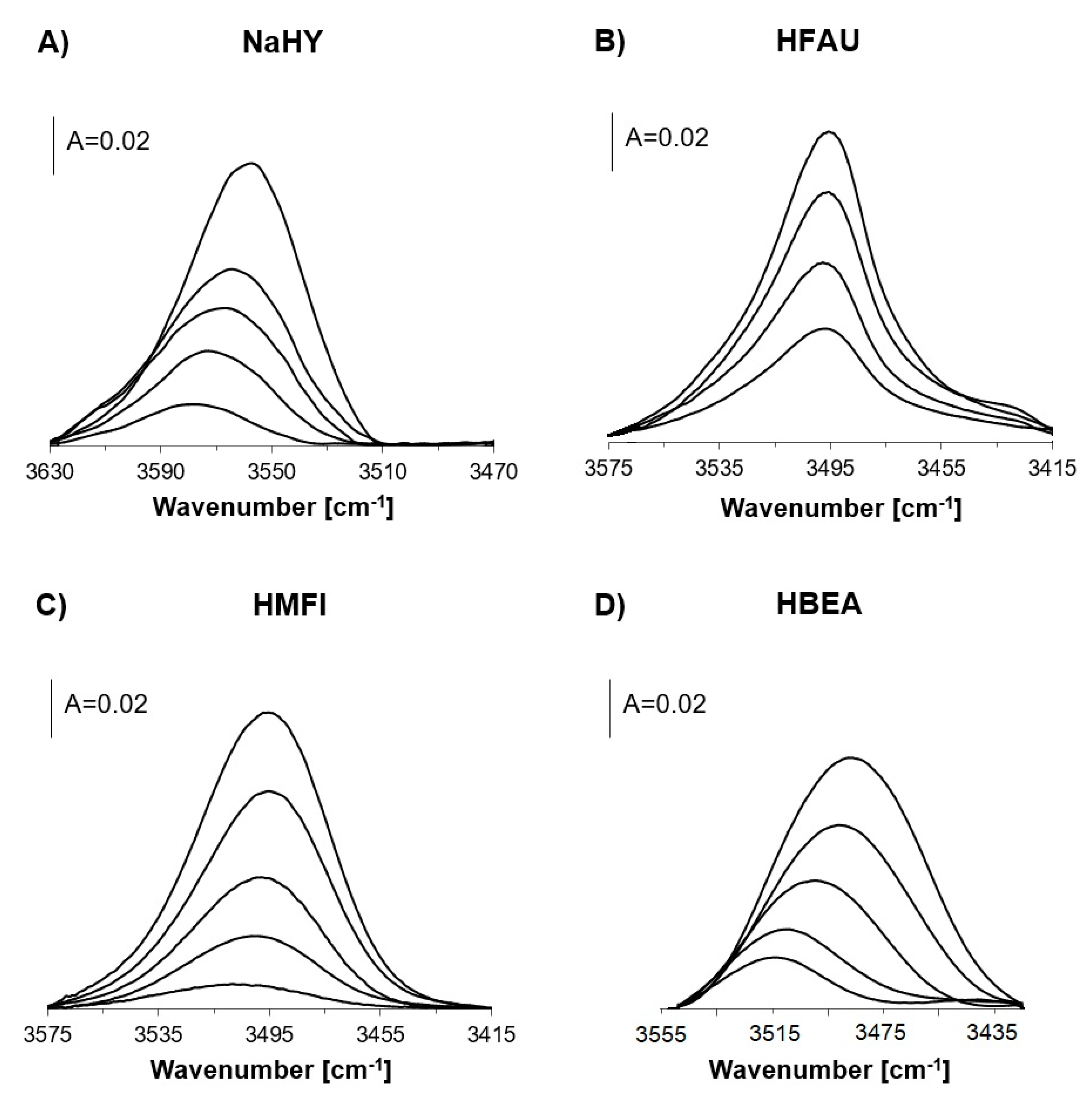
| NH3 Desorption Temperature | νOH | νOH,,,N2 | ΔνOH,,,N2 | ||
|---|---|---|---|---|---|
| 400 K | 170 K | ||||
| NaHY | 418 K | 3646 | 3656 | 3580 | 76 |
| 433 K | 3645 | 3654 | 3572 | 82 | |
| 443 K | 3644 | 3653 | 3568 | 85 | |
| 458 K | 3644 | 3653 | 3564 | 91 | |
| without NH3 | 3641 | 3650 | 3558 | 92 | |
| HFAU | 473 K | 3626 | 3634 | 3496 | 138 |
| 483 K | 3626 | 3634 | 3495 | 139 | |
| 505 K | 3627 | 3634 | 3496 | 138 | |
| without NH3 | 3627 | 3633 | 3495 | 138 | |
| HMFI | 473 K | 3611 | 3619 | 3507 | 112 |
| 483 K | 3610 | 3620 | 3500 | 120 | |
| 503 K | 3608 | 3619 | 3496 | 125 | |
| 518 K | 3607 | 3619 | 3495 | 124 | |
| without NH3 | 3607 | 3619 | 3495 | 124 | |
| HBEA | 443 K | 3617 | 3627 | 3515 | 112 |
| 463 K | 3615 | 3625 | 3511 | 114 | |
| 483 K | 3615 | 3619 | 3500 | 119 | |
| 503 K | 3610 | 3618 | 3491 | 127 | |
| without NH3 | 3607 | 3613 | 3486 | 127 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuterasiński, Ł.; Gackowski, M.; Podobiński, J.; Rutkowska-Zbik, D.; Datka, J. Nitrogen as a Probe Molecule for the IR Studies of the Heterogeneity of OH Groups in Zeolites. Molecules 2021, 26, 6261. https://doi.org/10.3390/molecules26206261
Kuterasiński Ł, Gackowski M, Podobiński J, Rutkowska-Zbik D, Datka J. Nitrogen as a Probe Molecule for the IR Studies of the Heterogeneity of OH Groups in Zeolites. Molecules. 2021; 26(20):6261. https://doi.org/10.3390/molecules26206261
Chicago/Turabian StyleKuterasiński, Łukasz, Mariusz Gackowski, Jerzy Podobiński, Dorota Rutkowska-Zbik, and Jerzy Datka. 2021. "Nitrogen as a Probe Molecule for the IR Studies of the Heterogeneity of OH Groups in Zeolites" Molecules 26, no. 20: 6261. https://doi.org/10.3390/molecules26206261
APA StyleKuterasiński, Ł., Gackowski, M., Podobiński, J., Rutkowska-Zbik, D., & Datka, J. (2021). Nitrogen as a Probe Molecule for the IR Studies of the Heterogeneity of OH Groups in Zeolites. Molecules, 26(20), 6261. https://doi.org/10.3390/molecules26206261






