Resveratrol Hinders Postovulatory Aging by Modulating Oxidative Stress in Porcine Oocytes
Abstract
1. Introduction
2. Results
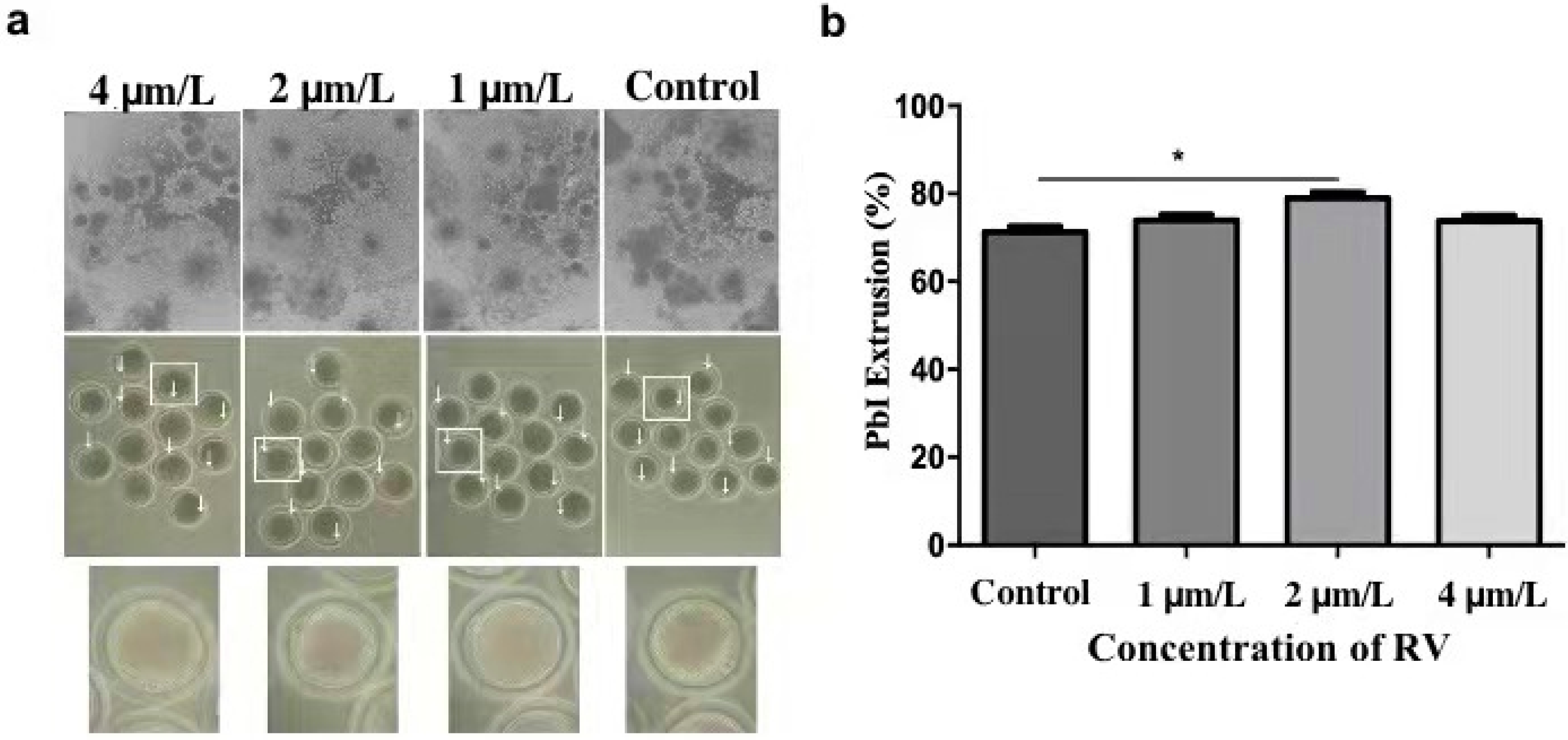
2.1. RV Treatment Reverses Aging-Induced Impairment in Aged Porcine Oocytes
2.2. RV Suppresses the Increasing Perivitaline Space (PVS) in Aged Porcine Oocytes
2.3. RV Reduces the Apoptosis Extent in Aged Porcine Oocytes
2.4. RV Alleviates the Oxidative Stress in Aged Porcine Oocytes
2.5. RV Rescues the Mitochondrial Membrane Potential in Aged Porcine Oocytes
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Reagents
4.3. Oocytes Collection and IVM
4.4. RV Concentration and In Vitro Aging
4.5. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.6. Measurement of Reactive Oxygen Species (ROS) Intensity
4.7. Determination of Intracellular GSH Contents
4.8. Mitochondrial Membrane Potential Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ma, W.; Zhang, D.; Hou, Y.; Li, Y.-H.; Sun, Q.-Y.; Sun, X.-F.; Wang, W.-H. Reduced Expression of MAD2, BCL2, and MAP Kinase Activity in Pig Oocytes after In Vitro Aging Are Associated with Defects in Sister Chromatid Segregation During Meiosis II and Embryo Fragmentation After Activation. Biol. Reprod. 2005, 72, 373–383. [Google Scholar] [CrossRef]
- Mukherjee, A.; Malik, H.; Saha, A.P.; Dubey, A.; Singhal, D.K.; Boateng, S.; Saugandhika, S.; Kumar, S.; De, S.; Guha, S.K.; et al. Resveratrol treatment during goat oocytes maturation enhances developmental competence of parthenogenetic and hand-made cloned blastocysts by modulating intracellular glutathione level and embryonic gene expression. J. Assist. Reprod. Genet. 2014, 31, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Koike, K.; Saito, T.; Nohara, M.; Kawagoe, S.; Hiroi, M. Aging Changes in the Alignment of Chromosomes after Human Chorionic Gonadotropin Stimulation May Be a Possible Cause of Decreased Fertility in Mice. Horm. Res. 1993, 39, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Díaz, H.; Esponda, P. Ageing-induced changes in the cortical granules of mouse eggs. Zygote 2004, 12, 95–103. [Google Scholar] [CrossRef][Green Version]
- Goud, P.; Goud, A.; Laverge, H.; De Sutter, P.; Dhont, M. Effect of post-ovulatory age and calcium in the injection medium on the male pronucleus formation and metaphase entry following injection of human spermatozoa into golden hamster oocytes. Mol. Hum. Reprod. 1999, 5, 227–233. [Google Scholar] [CrossRef]
- Díaz, H.; Esponda, P. Postovulatory ageing induces structural changes in the mouse zona pellucida. J. Submicrosc. Cytol. Pathol. 2004, 36, 211. [Google Scholar]
- Kovacic, P.; Somanathan, R. Multifaceted Approach to Resveratrol Bioactivity: Focus on Antioxidant Action, Cell Signaling and Safety. Oxid. Med. Cell. Longev. 2010, 3, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Tarín, J.J.; Pérez-Albalá, S.; Aguilar, A.; Miñarro, J.; Hermenegildo, C.; Cano, A. Long-term effects of postovulatory aging of mouse oocytes on offspring: A two-generational study. Biol. Reprod. 1999, 61, 1347–1355. [Google Scholar] [CrossRef]
- Lord, T.; Aitken, R.J. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction 2013, 146, R217–R227. [Google Scholar] [CrossRef]
- Mailhes, J.B.; Young, D.; London, S.N. Postovulatory Ageing of Mouse Oocytes in Vivo and Premature Centromere Separation and Aneuploidy. Biol. Reprod. 1998, 58, 1206–1210. [Google Scholar] [CrossRef]
- Dankert, D.; Demond, H.; Trapphoff, T.; Heiligentag, M.; Rademacher, K.; Eichenlaub-Ritter, U.; Horsthemke, B.; Grümmer, R. Pre- and Postovulatory Aging of Murine Oocytes Affect the Transcript Level and Poly(A) Tail Length of Maternal Effect Genes. PLoS ONE 2014, 9, e108907. [Google Scholar] [CrossRef]
- Huang, J.-C.; Yan, L.-Y.; Lei, Z.-L.; Miao, Y.-L.; Shi, L.-H.; Yang, J.-W.; Wang, Q.; Ouyang, Y.-C.; Sun, Q.-Y.; Chen, D.-Y. Changes in Histone Acetylation During Postovulatory Aging of Mouse Oocyte. Biol. Reprod. 2007, 77, 666–670. [Google Scholar] [CrossRef]
- Trapphoff, T.; Heiligentag, M.; Dankert, D.; Demond, H.; Deutsch, D.; Fröhlich, T.; Arnold, G.; Grümmer, R.; Horsthemke, B.; Eichenlaub-Ritter, U. Postovulatory aging affects dynamics of mRNA, expression and localization of maternal effect proteins, spindle integrity and pericentromeric proteins in mouse oocytes. Hum. Reprod. 2016, 31, 133–149. [Google Scholar] [CrossRef]
- Wang, T.; Gao, Y.-Y.; Chen, L.; Nie, Z.-W.; Cheng, W.; Liu, X.; Schatten, H.; Zhang, X.; Miao, Y.-L. Melatonin prevents postovulatory oocyte aging and promotes subsequent embryonic development in the pig. Aging 2017, 9, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.; Mei, Z.; Hassan, F.; Saeed, M.; Alagawany, M.; Shar, A.; Rajput, I. Lycopene: A natural antioxidant for prevention of heat-induced oxidative stress in poultry. World’s Poult. Sci. J. 2018, 74, 89–100. [Google Scholar] [CrossRef]
- Kim, W.-J.; Lee, S.-E.; Park, Y.-G.; Jeong, S.-G.; Kim, E.-Y.; Park, S.-P. Antioxidant hesperetin improves the quality of porcine oocytes during aging in vitro. Mol. Reprod. Dev. 2019, 86, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sato, E.F.; Nishikawa, M.; Park, A.-M.; Kira, Y.; Imada, I.; Utsumi, K. Mitochondrial Generation of Reactive Oxygen Species and its Role in Aerobic Life. Curr. Med. Chem. 2003, 10, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fiskum, G.; Schubert, D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002, 80, 780–787. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta BBA Bioenerg. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Liang, S.; Guo, J.; Choi, J.-W.; Shin, K.-T.; Wang, H.-Y.; Jo, Y.-J.; Kim, N.-H.; Cui, X.-S. Protein phosphatase 2A regulatory subunit B55α functions in mouse oocyte maturation and early embryonic development. Oncotarget 2017, 8, 26979–26991. [Google Scholar] [CrossRef] [PubMed]
- Ramalho-Santos, J.; Varum, S.; Amaral, S.; Mota, P.C.; Sousa, A.P.; Amaral, A. Mitochondrial functionality in reproduction: From gonads and gametes to embryos and embryonic stem cells. Hum. Reprod. Updat. 2009, 15, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Babayev, E.; Wang, T.; Szigeti-Buck, K.; Lowther, K.; Taylor, H.S.; Horvath, T.; Seli, E. Reproductive aging is associated with changes in oocyte mitochondrial dynamics, function, and mtDNA quantity. Maturitas 2016, 93, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Han, J.; Duan, X.; Xiong, B.; Cui, X.-S.; Kim, N.-H.; Liu, H.-L.; Sun, S.-C. The toxic effects and possible mechanisms of Bisphenol A on oocyte maturation of porcine in vitro. Oncotarget 2016, 7, 32554–32565. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, W.; Li, Y.-H.; Hou, Y.; Li, S.-W.; Meng, X.-Q.; Sun, X.-F.; Sun, Q.-Y.; Wang, W.-H. Intra-oocyte Localization of MAD2 and Its Relationship with Kinetochores, Microtubules, and Chromosomes in Rat Oocytes During Meiosis. Biol. Reprod. 2004, 71, 740–748. [Google Scholar] [CrossRef]
- Suzuki, Y.; Imai, Y.; Nakayama, H.; Takahashi, K.; Takio, K.; Takahashi, R. A Serine Protease, HtrA2, Is Released from the Mitochondria and Interacts with XIAP, Inducing Cell Death. Mol. Cell 2001, 8, 613–621. [Google Scholar] [CrossRef]
- Verhagen, A.M.; Ekert, P.; Pakusch, M.; Silke, J.; Connolly, L.M.; Reid, G.; Moritz, R.L.; Simpson, R.; Vaux, D.L. Identification of DIABLO, a Mammalian Protein that Promotes Apoptosis by Binding to and Antagonizing IAP Proteins. Cell 2000, 102, 43–53. [Google Scholar] [CrossRef]
- Fico, A.; Manganelli, G.; Cigliano, L.; Bergamo, P.; Abrescia, P.; Franceschi, C.; Martini, G.; Filosa, S. 2-deoxy-d-ribose induces apoptosis by inhibiting the synthesis and increasing the efflux of glutathione. Free Radic. Biol. Med. 2008, 45, 211–217. [Google Scholar] [CrossRef]
- Papaliagkas, V.; Anogianaki, A.; Anogianakis, G.; Ilonidis, G. The proteins and the mechanisms of apoptosis: A mini-review of the fundamentals. Hippokratia 2007, 11, 108–113. [Google Scholar]
- Hoffmann, P.R.; Decathelineau, A.M.; Ogden, C.A.; Leverrier, Y.; Bratton, D.L.; Daleke, D.L.; Ridley, A.; Fadok, V.A.; Henson, P.M. Phosphatidylserine (PS) induces PS receptor–mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 2001, 155, 649–660. [Google Scholar] [CrossRef]
- Pervaiz, S.; Holme, A.L. Resveratrol: Its Biologic Targets and Functional Activity. Antioxid. Redox Signal. 2009, 11, 2851–2897. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.-Y.; Xiang, D.-C.; Shao, Q.-Y.; Zhang, B.; Liu, S.-N.; Hong, Q.-H.; Quan, G.-B.; Wu, G.-Q. Inhibitory effects of astaxanthin on postovulatory porcine oocyte aging in vitro. Sci. Rep. 2020, 10, 20217. [Google Scholar] [CrossRef] [PubMed]
- Nutt, L.K.; Gogvadze, V.; Uthaisang, W.; Mirnikjoo, B.; McConkey, D.J.; Orrenius, S. Research paper indirect effects of Bax and Bak initiate the mitochondrial alterations that lead to cytochrome c release during arsenic trioxide-induced apoptosis. Cancer Biol. Ther. 2005, 4, 459–467. [Google Scholar] [CrossRef]
- May-Panloup, P.; Boucret, L.; De La Barca, J.-M.C.; Desquiret-Dumas, V.; Ferré-L’Hotellier, V.; Morinière, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Updat. 2016, 22, 725–743. [Google Scholar] [CrossRef]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.-A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.-S.; Cheong, S.-A.; Jeon, Y.; Lee, E.; Choi, K.-C.; Jeung, E.-B.; Hyun, S.-H. The effects of resveratrol on porcine oocyte in vitro maturation and subsequent embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology 2012, 78, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front. Pharmacol. 2021, 11, 617843. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Therond, P. Oxidative stress and damages to biomolecules (lipids, proteins, DNA). Ann. Pharm. Fr. 2006, 64, 383–389. [Google Scholar] [CrossRef]
- Liang, Q.-X.; Lin, Y.-H.; Zhang, C.-H.; Sun, H.-M.; Zhou, L.; Schatten, H.; Sun, Q.-Y.; Qian, W.-P. Resveratrol increases resistance of mouse oocytes to postovulatory aging in vivo. Aging 2018, 10, 1586–1596. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, H.; Misra, B.R.; Mahaney, J.E.; Li, Y.; Misra, H.P. EPR studies on the superoxide-scavenging capacity of the nutraceutical resveratrol. Mol. Cell. Biochem. 2008, 313, 187–194. [Google Scholar] [CrossRef] [PubMed]
- De La Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Choi, Y.-J.; Suh, S.-I.; Baek, W.-K.; Suh, M.-H.; Jin, I.-N.; Min, D.S.; Woo, J.-H.; Chang, J.-S.; Passaniti, A.; et al. Bcl-2 overexpression attenuates resveratrol-induced apoptosis in U937 cells by inhibition of caspase-3 activity. Carcinogenesis 2001, 22, 1633–1639. [Google Scholar] [CrossRef]
- Wenzel, E.; Soldo, T.; Erbersdobler, H.; Somoza, V. Bioactivity and metabolism of trans-resveratrol orally administered to Wistar rats. Mol. Nutr. Food Res. 2005, 49, 482–494. [Google Scholar] [CrossRef]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef]
- Meng, Q.; Guo, T.; Li, G.; Sun, S.; He, S.; Cheng, B.; Shi, B.; Shan, A. Dietary resveratrol improves antioxidant status of sows and piglets and regulates antioxidant gene expression in placenta by Keap1-Nrf2 pathway and Sirt1. J. Anim. Sci. Biotechnol. 2018, 9, 34. [Google Scholar] [CrossRef]
- Wu, C.-C.; Huang, Y.-S.; Chen, J.-S.; Huang, C.-F.; Su, S.-L.; Lu, K.-C.; Lin, Y.-F.; Chu, P.; Lin, S.-H.; Sytwu, H.-K. Resveratrol Ameliorates Renal Damage, Increases Expression of Heme Oxygenase-1, and Has Anti-Complement, Anti-Oxidative, and Anti-Apoptotic Effects in a Murine Model of Membranous Nephropathy. PLoS ONE 2015, 10, e0125726. [Google Scholar] [CrossRef]
- Movahed, A.; Yu, L.; Thandapilly, S.J.; Louis, X.; Netticadan, T. Resveratrol protects adult cardiomyocytes against oxidative stress mediated cell injury. Arch. Biochem. Biophys. 2012, 527, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Soeur, J.; Eilstein, J.; Léreaux, G.; Jones, C.; Marrot, L. Skin resistance to oxidative stress induced by resveratrol: From Nrf2 activation to GSH biosynthesis. Free Radic. Biol. Med. 2015, 78, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Bigagli, E.; Luceri, C.; Manni, E.M.; Zaid, M. Protective Effect of Resveratrol against Oxidation Stress Induced by 2-Nitropropane in Rat Liver. Pharmacol. Pharm. 2011, 2, 127–135. [Google Scholar] [CrossRef]
- Gebauer, F.; Xu, W.; Cooper, G.; Richter, J. Translational control by cytoplasmic polyadenylation of c-mos mRNA is necessary for oocyte maturation in the mouse. EMBO J. 1994, 13, 5712–5720. [Google Scholar] [CrossRef] [PubMed]
- Yew, N.; Mellini, M.L.; Martinez, C.K.; Woude, G.F.V. Meiotic initiation by the mos protein in Xenopus. Nature 1992, 355, 649–652. [Google Scholar] [CrossRef]
- Pascuali, N.; Scotti, L.; Abramovich, D.; Irusta, G.; Di Pietro, M.; Bas, D.; Tesone, M.; Parborell, F. Inhibition of platelet-derived growth factor (PDGF) receptor affects follicular development and ovarian proliferation, apoptosis and angiogenesis in prepubertal eCG-treated rats. Mol. Cell. Endocrinol. 2015, 412, 148–158. [Google Scholar] [CrossRef]
- Tong, Z.; Xie, Y.; He, M.; Ma, W.; Zhou, Y.; Lai, S.; Meng, Y.; Liao, Z. VDAC1 deacetylation is involved in the protective effects of resveratrol against mitochondria-mediated apoptosis in cardiomyocytes subjected to anoxia/reoxygenation injury. Biomed. Pharmacother. 2017, 95, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Brunelle, J.K.; Letai, A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 2009, 122, 437–441. [Google Scholar] [CrossRef]
- Shi, F.; Li, W.; Zhao, H.; He, Y.; Jiang, Y.; Ni, J.; Abbasi, B.; Rui, R.; Ju, S. Microcystin-LR exposure results in aberrant spindles and induces apoptosis in porcine oocytes. Theriogenology 2020, 158, 358–367. [Google Scholar] [CrossRef]




| Target Gene | Forward and Reverse Sequence | Product Size (bp) | Accession Number |
|---|---|---|---|
| GAPDH | F: 5′-GTCGGTTGTGGATCTGACCT-3′ R: 5′-TTGACGAAGTGGTCGTTGAG-3′ | 207 | NM_001206359 |
| Caspase-3 | F: 5′-CGTGCTTCTAAGCCATGGTG-3′ R: 5′-GTCCCACTGTCCGTCTCAAT-3′ | 186 | NM_214131 |
| Bcl-2 | F:5′-AGGGCATTCAGTGACCTGAC-3′ R: 5′-CGATCCGACTCACCAATACC-3′ | 193 | NM_214285 |
| Bax | F:5′-TGCCTCAGGATGCATCTACC-3′ R: 5′-AAGTAGAAAAGCGCGACCAC-3′ | 199 | XM_003127290 |
| CAT | F-5′-AACTGTCCCTTCCGTGCTA-3′ R-5′-CCTGGGTGACATTATCTTCG-3′ | 195 | XM_021081498 |
| GPX | F:GAGCCCTTCAACCTGTCCTC R:GTCGGACGTACTTCAGGCAA | 210 | NC_010455.5 |
| SOD1 | F-5′-ACCTGGGCAATGTGACTG-3′ R-5′-TCCAGCATTTCCCGTCT-3 | 131 | NM_001190422 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi, B.; Dong, Y.; Rui, R. Resveratrol Hinders Postovulatory Aging by Modulating Oxidative Stress in Porcine Oocytes. Molecules 2021, 26, 6346. https://doi.org/10.3390/molecules26216346
Abbasi B, Dong Y, Rui R. Resveratrol Hinders Postovulatory Aging by Modulating Oxidative Stress in Porcine Oocytes. Molecules. 2021; 26(21):6346. https://doi.org/10.3390/molecules26216346
Chicago/Turabian StyleAbbasi, Benazir, Yan Dong, and Rong Rui. 2021. "Resveratrol Hinders Postovulatory Aging by Modulating Oxidative Stress in Porcine Oocytes" Molecules 26, no. 21: 6346. https://doi.org/10.3390/molecules26216346
APA StyleAbbasi, B., Dong, Y., & Rui, R. (2021). Resveratrol Hinders Postovulatory Aging by Modulating Oxidative Stress in Porcine Oocytes. Molecules, 26(21), 6346. https://doi.org/10.3390/molecules26216346





