Peptide Targeting of PDZ-Dependent Interactions as Pharmacological Intervention in Immune-Related Diseases
Abstract
:1. Introduction
2. Peptide Inhibitors for Targeting PDZ Protein–Protein Interactions
3. Role of Endogenous PDZ Interactions in Human Immune Processes
3.1. G Protein-Coupled Receptors (GPCRs)
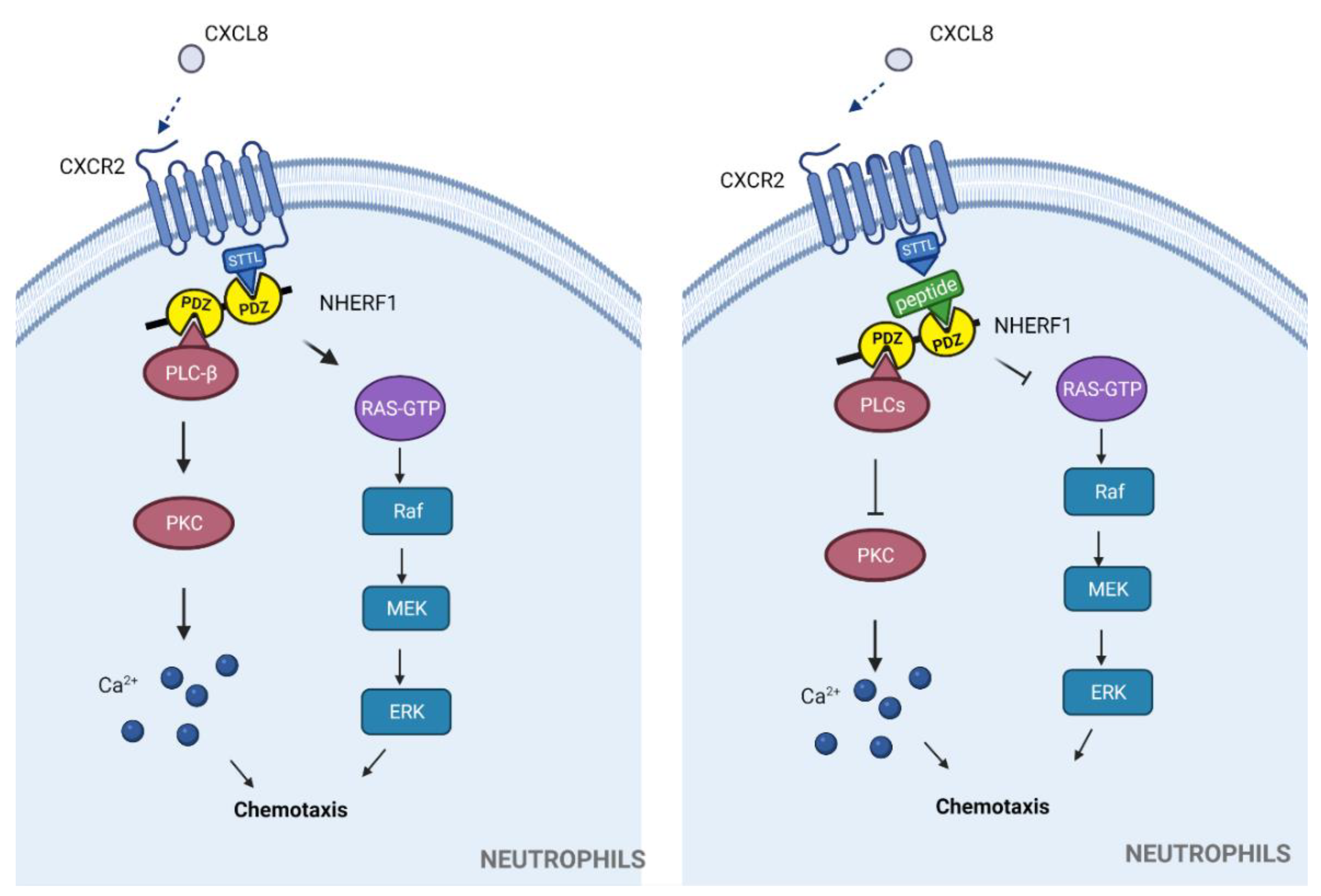
3.1.1. Chemokine Receptors
3.1.2. Other Chemokine Receptors with PDZbms
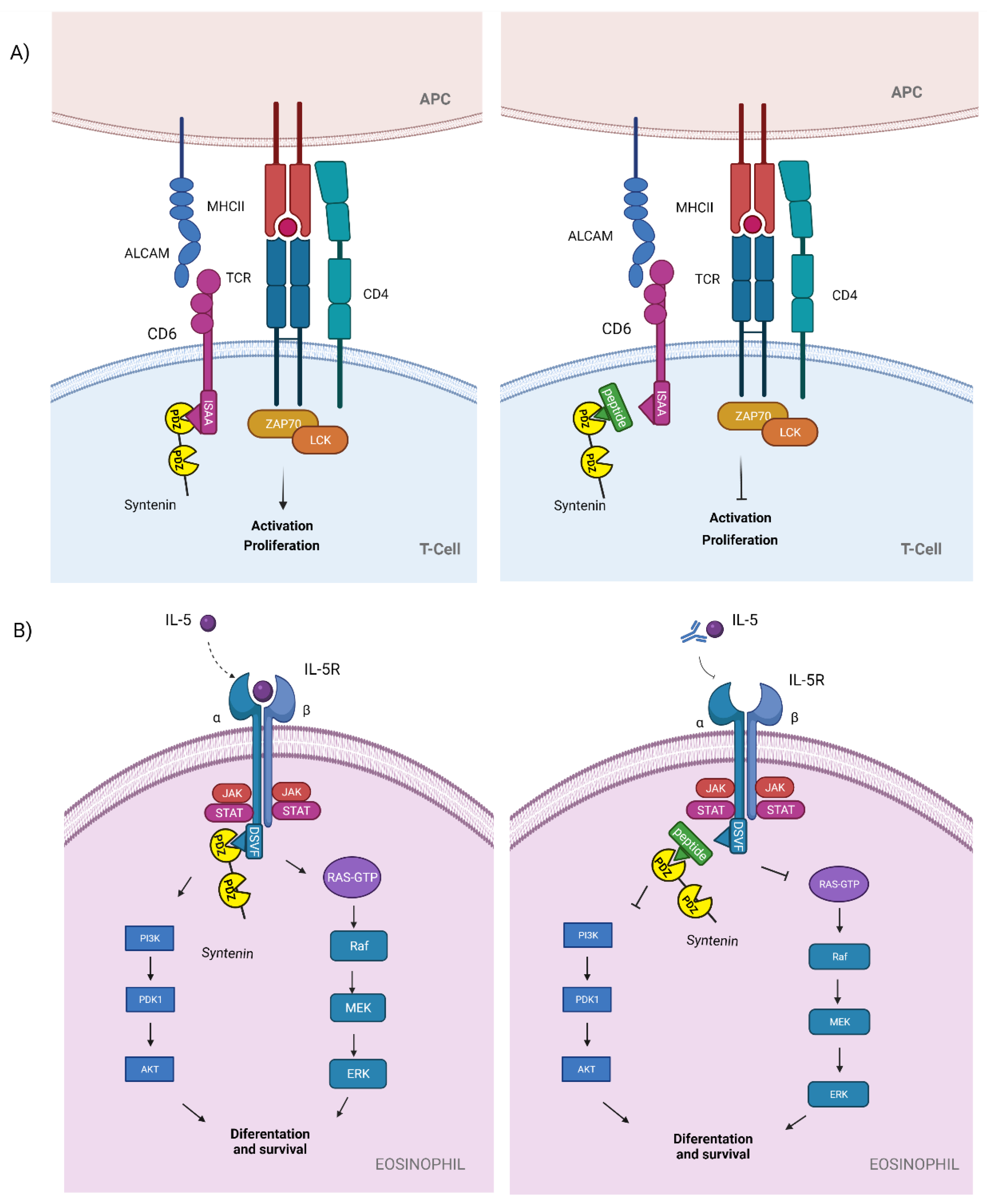
3.2. Syntenin
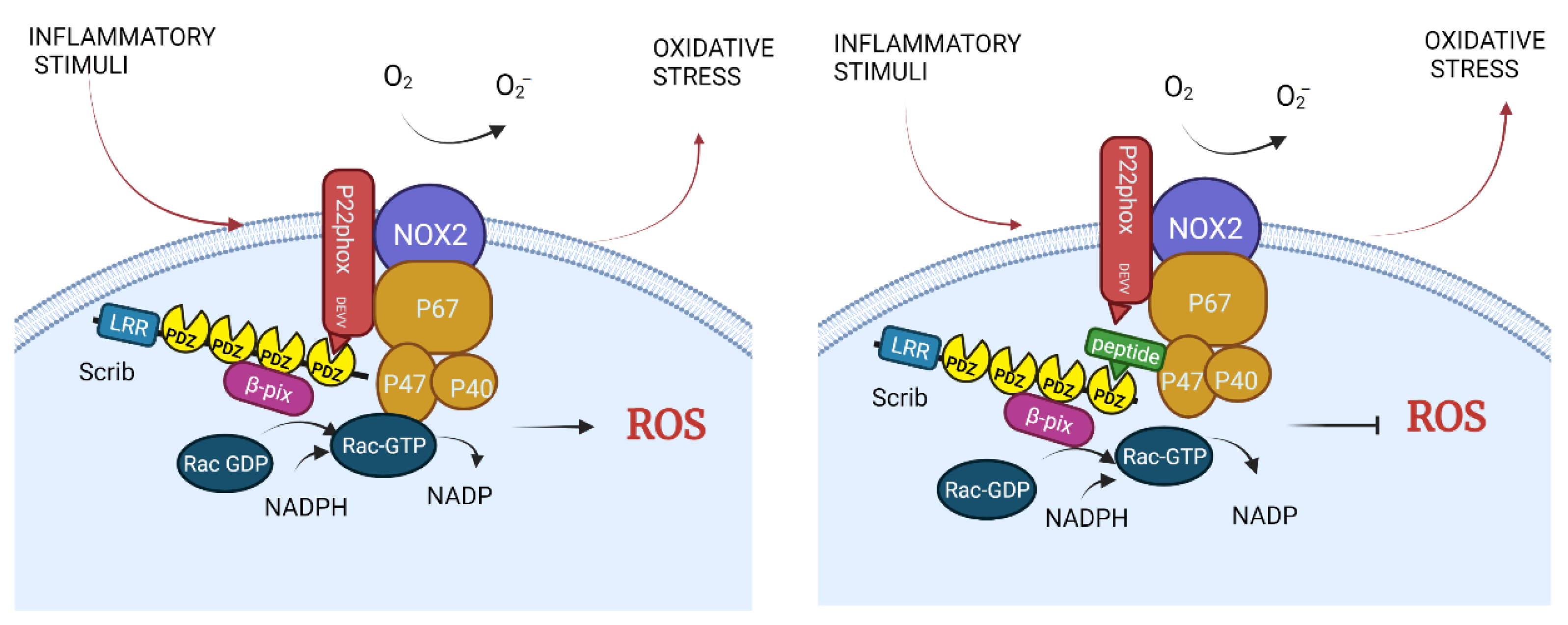
3.3. NADPH Oxidase
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de las Rivas, J.; Fontanillo, C. Protein-protein interactions essentials: Key concepts to building and analyzing interactome networks. PLoS Comput. Biol. 2010, 6, e1000807. [Google Scholar] [CrossRef] [Green Version]
- Rao, V.S.; Srinivas, K.; Sujini, G.N.; Kumar, G.N.S. Protein-Protein Interaction Detection: Methods and Analysis. Int. J. Proteom. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Stumpf, M.P.H.; Thorne, T.; de Silva, E.; Stewart, R.; An, H.J.; Lappe, M.; Wiuf, C. Estimating the size of the human interactome. Proc. Natl. Acad. Sci. USA 2008, 105, 6959–6964. [Google Scholar] [CrossRef] [Green Version]
- Ivarsson, Y.; Arnold, R.; McLaughlin, M.; Nim, S.; Joshi, R.; Ray, D.; Liu, B.; Teyra, J.; Pawson, T.; Moffat, J.; et al. Large-scale interaction profiling of PDZ domains through proteomic peptide-phage display using human and viral phage peptidomes. Proc. Natl. Acad. Sci. USA 2014, 111, 2542–2547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, N.R.; Čalyševa, J.; Fernandes, E.F.A.; Lüchow, S.; Clemmensen, L.S.; Haugaard-Kedström, L.M.; Strømgaard, K. PDZ Domains as Drug Targets. Adv. Ther. (Weinh) 2019, 2, 1800143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Zhou, Q.; He, J.; Jiang, Z.; Peng, C.; Tong, R.; Shi, J. Recent advances in the development of protein–protein interactions modulators: Mechanisms and clinical trials. Signal. Transduct. Target. Ther. 2020, 5, 213. [Google Scholar] [CrossRef] [PubMed]
- Erlendsson, S.; Madsen, K.L. Membrane binding and modulation of the PDZ domain of PICK1. Membranes 2015, 5, 597–615. [Google Scholar] [CrossRef] [Green Version]
- Chang, B.H.; Gujral, T.S.; Karp, E.S.; Bukhalid, R.; Grantcharova, V.P.; MacBeath, G. A systematic family-wide investigation reveals that ∼30% of mammalian PDZ domains engage in PDZ-PDZ interactions. Chem. Biol. 2011, 18, 1143–1152. [Google Scholar] [CrossRef] [Green Version]
- Thorsen, T.S.; Madsen, K.L.; Rebola, N.; Rathje, M.; Anggono, V.; Bach, A.; Moreira, I.S.; Stuhr-Hansen, N.; Dyhring, T.; Peters, D.; et al. Identification of a small-molecule inhibitor of the PICK1 PDZ domain that inhibits hippocampal LTP and LTD. Proc. Natl. Acad. Sci. USA 2010, 107, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Amacher, J.F.; Brooks, L.; Hampton, T.H.; Madden, D.R. Specificity in PDZ-peptide interaction networks: Computational analysis and review. J. Struct. Biol. X 2020, 4, 100022. [Google Scholar] [CrossRef]
- von Ossowski, I.; Oksanen, E.; von Ossowski, L.; Cai, C.; Sundberg, M.; Goldman, A.; Keinanen, K. Crystal structure of the second PDZ domain of SAP97 in complex with a GluR-A C-terminal peptide. FEBS J. 2006, 273, 5219–5229. [Google Scholar] [CrossRef]
- Dice, J.F. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem. Sci. 1990, 15, 305–309. [Google Scholar] [CrossRef]
- Zimmermann, P.; Meerschaert, K.; Reekmans, G.; Leenaerts, I.; Small, J.V.; Vandekerckhove, J.; David, G.; Gettemans, J. PIP2-PDZ Domain Binding Controls the Association of Syntenin with the Plasma Membrane. Mol. Cell 2002, 9, 1215–1225. [Google Scholar] [CrossRef] [Green Version]
- Ivarsson, Y. Plasticity of PDZ domains in ligand recognition and signaling. FEBS Lett. 2012, 586, 2638–2647. [Google Scholar] [CrossRef] [Green Version]
- Rousset, R.; Fabre, S.; Desbois, C.; Bantignies, F.; and Jalinot, P. The C-terminus of the HTLV-1 Tax oncoprotein mediates interaction with the PDZ domain of cellular proteins. Oncogene 1998, 16, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.S.; Weiss, R.S.; Javier, R.T. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. USA 1997, 94, 6670–6675. [Google Scholar] [CrossRef] [Green Version]
- Kiyono, T.; Hiraiwa, A.; Fujita, M.; Hayashi, Y.; Akiyama, T.; Ishibashi, M. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. USA 1997, 94, 11612–11616. [Google Scholar] [CrossRef] [Green Version]
- Sheng, M.; Sala, C. PDZ Domains and the Organization of Supramolecular Complexes. Annu. Rev. Neurosci. 2001, 24, 1–29. [Google Scholar] [CrossRef]
- Thomas, M.; Banks, L. Upsetting the Balance: When Viruses Manipulate Cell Polarity Control. J. Mol. Biol. 2018, 430, 3481–3503. [Google Scholar] [CrossRef] [PubMed]
- Tonikian, R.; Zhang, Y.; Sazinsky, S.L.; Currell, B.; Yeh, J.; Reva, B.; Held, H.A.; Appleton, B.A.; Evangelista, M.; Wu, Y.; et al. A Specificity Map for the PDZ Domain Family. PLoS Biol. 2008, 6, e60239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javier, R.T.; Rice, A.P. Emerging Theme: Cellular PDZ Proteins as Common Targets of Pathogenic Viruses. J. Virol. 2011, 85, 11544–11556. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-González, L.H.; Santos-Mendoza, T. Viral targeting of PDZ polarity proteins in the immune system as a potential evasion mechanism. FASEB J. 2019, 33, 10607–10617. [Google Scholar] [CrossRef]
- Valgardson, J.; Cosbey, R.; Houser, P.; Rupp, M.; Van Bronkhorst, R.; Lee, M.; Jagodzinski, F.; Amacher, J.F. MotifAnalyzer-PDZ: A computational program to investigate the evolution of PDZ-binding target specificity. Protein Sci. 2019, 28, 2127–2143. [Google Scholar] [CrossRef]
- Pelay-Gimeno, M.; Glas, A.; Koch, O.; Grossmann, T.N. Structure-Based Design of Inhibitors of Protein-Protein Interactions: Mimicking Peptide Binding Epitopes. Angew. Chem. Int. Ed. 2015, 54, 8896–8927. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, D.J.; Hus, J.C.; Banos, C.C.; Wildes, C.; Arduini, R.; Bergeron, C.; Hession, C.A.; Baker, D.P.; Lin, E.; Guckian, K.M. Lock and chop: A novel method for the generation of a PICK1 PDZ domain and piperidine-based inhibitor co-crystal structure. Protein Sci. 2018, 27, 672–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepley-McTaggart, A.; Sagum, C.A.; Oliva, I.; Rybakovsky, E.; DiGuilio, K.; Liang, J.; Bedford, M.T.; Cassel, J.; Sudol, M.; Mullin, J.M.; et al. SARS-CoV-2 Envelope (E) protein interacts with PDZ-domain-2 of host tight junction protein ZO1. PLoS ONE 2021, 16, e0251955. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shi, H.; Chang, L.; Zhang, C.C.; Si, M.; Li, N.; Zhu, D. nNOS-CAPON blockers produce anxiolytic effects by promoting synaptogenesis in chronic stress-induced animal models of anxiety. Br. J. Pharmacol. 2020, 177, 3674–3690. [Google Scholar] [CrossRef]
- Shabanzadeh, A.P.; D’Onofrio, P.M.; Magharious, M.; Choi, K.A.B.; Monnier, P.P.; Koeberle, P.D. Modifying PTEN recruitment promotes neuron survival, regeneration, and functional recovery after CNS injury. Cell Death Dis 2019, 10, 567. [Google Scholar] [CrossRef] [Green Version]
- Prehaud, C.; Wolff, N.; Terrien, E.; Lafage, M.; Megret, F.; Babault, N.; Cordier, F.; Tan, G.S.; Maitrepierre, E.; Menager, P.; et al. Attenuation of Rabies Virulence: Takeover by the Cytoplasmic Domain of Its Envelope Protein. Sci. Signal. 2010, 3, ra5. [Google Scholar] [CrossRef]
- Khan, Z.; Terrien, E.; Delhommel, F.; Lefebvre-Omar, C.; Bohl, D.; Vitry, S.; Bernard, C.; Ramirez, J.; Chaffotte, A.; Ricquier, K.; et al. Structure-based optimization of a PDZ-binding motif within a viral peptide stimulates neurite outgrowth. J. Biol. Chem. 2019, 294, 13755–13768. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorganic Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Ballarin, B.; Tymianski, M. Discovery and development of NA-1 for the treatment of acute ischemic stroke. Acta Pharmacol. Sin. 2018, 39, 661–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, C.Y.; Schulien, A.J.; Molyneaux, B.J.; Aizenman, E. Lessons from recent advances in ischemic stroke management and targeting kv2.1 for neuroprotection. Int. J. Mol. Sci. 2020, 21, 6107. [Google Scholar] [CrossRef]
- Zhang, Y.; Appleton, B.A.; Wiesmann, C.; Lau, T.; Costa, M.; Hannoush, R.N.; Sidhu, S.S. Inhibition of Wntsignaling by Dishevelled PDZ peptides. Nat. Chem. Biol. 2009, 5, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Christensen, N.R.; De Luca, M.; Lever, M.B.; Richner, M.; Hansen, A.B.; Noes-Holt, G.; Jensen, K.L.; Rathje, M.; Jensen, D.B.; Erlendsson, S.; et al. A high-affinity, bivalent PDZ domain inhibitor complexes PICK 1 to alleviate neuropathic pain. EMBO Mol. Med. 2020, 12, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Haresco, J.J.; Novak, K.A.P.; Stokoe, D.; Kuntz, I.D.; Guy, R.K. A selective irreversible inhibitor targeting a PDZ protein interaction domain. J. Am. Chem. Soc. 2003, 125, 12074–12075. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.D.; Goyal, M.; Menon, B.K.; Nogueira, R.G.; McTaggart, R.A.; Demchuk, A.M.; Poppe, A.Y.; Buck, B.H.; Field, T.S.; Dowlatshahi, D.; et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): A multicentre, double-blind, randomised controlled trial. Lancet 2020, 395. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Farooq, S.M.; Castelvetere, M.P.; Hou, Y.; Gao, J.L.; Navarro, J.V.; Oupicky, D.; Sun, F.; Li, C. A chemokine receptor CXCR2 macromolecular complex regulates neutrophil functions in inflammatory diseases. J. Biol. Chem. 2012, 287, 5744–5755. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Qu, L.; Zhou, W.; Huang, Y.; Jia, L.; Huang, X.; Qian, Z.; Xia, J.; Yu, Y. Syntenin-targeted peptide blocker inhibits progression of cancer cells. Eur. J. Med. Chem. 2018, 154, 354–366. [Google Scholar] [CrossRef]
- Kegelman, T.P.; Wu, B.; Das, S.K.; Talukdar, S.; Beckta, J.M.; Hu, B.; Emdad, L.; Valerie, K.; Sarkar, D.; Furnari, F.B.; et al. Inhibition of radiation-induced glioblastoma invasion by genetic and pharmacological targeting of MDA-9/Syntenin. Proc. Natl. Acad. Sci. USA 2017, 114, 370–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Consuegra-Fernández, M.; Lin, F.; Fox, D.A.; Lozano, F. Clinical and experimental evidence for targeting CD6 in immune-based disorders. Autoimmun. Rev. 2018, 17, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.M.; Henriques, S.N.; Santos, R.F.; Carmo, A.M. CD6, a rheostat-type signalosome that tunes T cell activation. Front. Immunol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Geijsen, N.; Uings, I.J.; Pals, C.; Armstrong, J.; McKinnon, M.; Raaijmakers, J.A.; Lammers, J.W.; Koenderman, L.; Coffer, P.J. Cytokine-specific transcriptional regulation through an IL-5Ralpha interacting protein. Science 2001, 293, 1136–1138. [Google Scholar] [CrossRef] [PubMed]
- Beekman, J.M.; Verhagen, L.P.; Geijsen, N.; Coffer, P.J. Regulation of myelopoiesis through syntenin-mediated modulation of IL-5 receptor output. Blood 2009, 114, 3917–3927. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Umitsu, M.; Jagan, I.; Tran, C.W.; Ishiyama, N.; Begora, M.; Araki, K.; Ohashi, P.S.; Ikura, M.; Muthuswamy, S.K. An interaction between Scribble and the NADPH oxidase complex controls M1 macrophage polarization and function. Nat. Cell Biol. 2016, 18, 1244–1252. [Google Scholar] [CrossRef]
- Cathcart, M.K. Regulation of Superoxide Anion Production by NADPH Oxidase in Monocytes/Macrophages. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Shimohama, S.; Tanino, H.; Kawakami, N.; Okamura, N.; Kodama, H.; Yamaguchi, T.; Hayakawa, T.; Nunomura, A.; Chiba, S.; Perry, G.; et al. Activation of NADPH Oxidase in Alzheimer’s Disease Brains. Biochem. Biophys. Res. Commun. 2000, 273, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, T.; Qin, L.; Gao, H.M.; Wilson, B.; Ali, S.F.; Zhang, W.; Hong, J.S.; Liu, B. Neuroprotective effect of dextromethorphan in the MPTP Parkinson’s disease model: Role of NADPH oxidase. FASEB J. 2004, 18, 589–591. [Google Scholar] [CrossRef]
- Conley, J.M.; Sun, H.; Ayers, K.L.; Zhu, H.; Chen, R.; Shen, M.; Hall, M.D.; Ren, H. Human GPR17 missense variants identified in metabolic disease patients have distinct downstream signaling profiles. J. Biol. Chem. 2021, 297, 100881. [Google Scholar] [CrossRef]
- Gorvin, C.M. Calcium-sensing receptor signaling—How human disease informs biology. Curr. Opin. Endocr. Metab. Res. 2021, 16, 10–18. [Google Scholar] [CrossRef]
- Abdulkareem, N.M.; Bhat, R.; Qin, L.; Vasaikar, S.; Gopinathan, A.; Mitchell, T.; Shea, M.J.; Nanda, S.; Thangavel, H.; Zhang, B.; et al. A novel role of ADGRF1 (GPR110) in promoting cellular quiescence and chemoresistance in human epidermal growth factor receptor 2-positive breast cancer. FASEB J. 2021, 35, e21719. [Google Scholar] [CrossRef]
- Dunn, H.A.; Ferguson, S.S.G. PDZ protein regulation of g protein-coupled receptor trafficking and signaling pathways. Mol. Pharmacol. 2015, 88, 624–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camp, N.D.; Lee, K.S.; Wacker-Mhyre, J.L.; Kountz, T.S.; Park, J.M.; Harris, D.A.; Estrada, M.; Stewart, A.; Wolf-Yadlin, A.; Hague, C. Individual protomers of a G protein-coupled receptor dimer integrate distinct functional modules. Cell Discov. 2015, 1, 1–12. [Google Scholar] [CrossRef]
- Olson, T.S.; Ley, K. Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R7–R28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55. [Google Scholar] [CrossRef]
- Gustine, J.N.; Jones, D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2021, 191, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, G.; Wu, Y.; Jiang, Y.; Wang, S.; Hou, Y.; Guan, X.; Brunzelle, J.; Sirinupong, N.; Sheng, S.; Li, C.; et al. Structural Insights into Neutrophilic Migration Revealed by the Crystal Structure of the Chemokine Receptor CXCR2 in Complex with the First PDZ Domain of NHERF1. PLoS ONE 2013, 8, e76219. [Google Scholar] [CrossRef] [Green Version]
- Baugher, P.J.; Richmond, A. The Carboxyl-terminal PDZ Ligand Motif of Chemokine Receptor CXCR2 Modulates Post-endocytic Sorting and Cellular Chemotaxis. J. Biol. Chem. 2008, 283, 30868–30878. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wu, Y.; Hou, Y.; Guan, X.; Castelvetere, M.P.; Oblak, J.J.; Banerjee, S.; Filtz, T.M.; Sarkar, F.H.; Chen, X.; et al. CXCR2 macromolecular complex in pancreatic cancer: A potential therapeutic target in tumor growth. Transl. Oncol. 2013, 6, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Wu, Y.; Farooq, S.M.; Guan, X.; Wang, S.; Liu, Y.; Oblak, J.J.; Holcomb, J.; Jiang, Y.; Strieter, R.M.; et al. A critical role of CXCR2 PDZ-mediated interactions in endothelial progenitor cell homing and angiogenesis. Stem Cell Res. 2015, 14, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, M.P.; Yu, Y. CXCR2 modulators: A patent review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Chen, W.; Debnath, B.; Wu, Y.; Nearmati, N. Synthesis, Structure-activity Relationship Studies and ADMET Properties of 3-aminocyclohex-2-en-1-ones as Chemokine Receptor 2 (CXCR2) Antagonists. ChemMedChem 2018, 13, 916–930. [Google Scholar] [CrossRef]
- Koenig, L.M.; Boehmer, D.F.R.; Metzger, P.; Schnurr, M.; Endres, S.; Rothenfusser, S. Blocking inflammation on the way: Rationale for CXCR2 antagonists for the treatment of COVID-19. J. Exp. Med. 2020, 217, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Geng, J.; Wang, F.; Chen, X.; Huang, Z.; Wang, Y. Role of IL-6 inhibitor in treatment of COVID-19-related cytokine release syndrome. Int. J. Med. Sci. 2021, 18, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Paing, M.M.; Temple, B.R.S.; Trejo, J.A. G protein-coupled receptor sorting to endosomes and lysosomes. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 601–629. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.E.; Paust, H.J.; Steinmetz, O.M.; Peters, A.; Riedel, J.H.; Erhardt, A.; Wegscheid, C.; Velden, J.; Fehr, S.; Mittrücker, H.W.; et al. CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J. Am. Soc. Nephrol. 2010, 21, 974–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirota, K.; Yoshitomi, H.; Hashimoto, M.; Maeda, S.; Teradaira, S.; Sugimoto, N.; Yamaguchi, T.; Nomura, T.; Ito, H.; Nakamura, T.; et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 2007, 204, 2803–2812. [Google Scholar] [CrossRef] [Green Version]
- Kadomoto, S.; Izumi, K.; Mizokami, A. The CCL20-CCR6 axis in cancer progression. Int. J. Mol. Sci. 2020, 21, 5186. [Google Scholar] [CrossRef]
- Rivas-Fuentes, S.; Salgado-Aguayo, A.; Arratia-Quijada, J.; Gorocica-Rosete, P. Regulation and biological functions of the CX3CL1-CX3CR1 axis and its relevance in solid cancer: A mini-review. J. Cancer 2020, 12, 571–583. [Google Scholar] [CrossRef]
- Cormican, S.; Griffin, M.D. Fractalkine (CX3CL1) and Its Receptor CX3CR1: A Promising Therapeutic Target in Chronic Kidney Disease? Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Grootjans, J.J.; Zimmermann, P.; Reekmans, G.; Smets, A.; Degeest, G.; Durr, J.; David, G. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc. Natl. Acad. Sci. USA 1997, 94, 13683–13688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, P.; Tomatis, D.; Rosas, M.; Grootjans, J.; Leenaerts, I.; Degeest, G.; Reekmans, G.; Coomans, C.; David, G. Characterization of Syntenin, a Syndecan-binding PDZ Protein, as a Component of Cell Adhesion Sites and Microfilaments. Mol. Biol. Cell 2001, 12, 339–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Beekman, J.M.; Coffer, P.J. The ins and outs of syntenin, a multifunctional intracellular adaptor protein. J. Cell Sci. 2008, 121, 1349–1355. [Google Scholar] [CrossRef] [Green Version]
- Shimada, T.; Yasuda, S.; Sugiura, H.; Yamagata, K. Syntenin: PDZ Protein Regulating Signaling Pathways and Cellular Functions. Int. J. Mol. Sci. 2019, 20, 4171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhoopathi, P.; Pradhan, A.K.; Bacolod, M.D.; Emdad, L.; Sarkar, D.; Das, S.K.; Fisher, P.B. Regulation of neuroblastoma migration, invasion, and in vivo metastasis by genetic and pharmacological manipulation of MDA-9/Syntenin. Oncogene 2019, 38, 6781–6793. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Maji, S.; Das, S.K.; Emdad, L.; Sarkar, D.; Fisher, P.B. MDA-9/Syntenin/SDCBP: New insights into a unique multifunctional scaffold protein. Cancer Metastasis Rev. 2020, 39, 769–781. [Google Scholar] [CrossRef]
- Garrido-Urbani, S.; Garg, P.; Ghossoub, R.; Arnold, R.; Lembo, F.; Sundell, G.N.; Kim, P.M.; Lopez, M.; Zimmermann, P.; Sidhu, S.S.; et al. Proteomic peptide phage display uncovers novel interactions of the PDZ1-2 supramodule of syntenin. FEBS Lett. 2016, 590, 3–12. [Google Scholar] [CrossRef]
- Das, S.K.; Kegelman, T.P.; Pradhan, A.K.; Shen, X.N.; Bhoopathi, P.; Talukdar, S.; Maji, S.; Sarkar, D.; Emdad, L.; Fisher, P.B. Suppression of prostate cancer pathogenesis using an MDA-9/syntenin (SDCBP) Pdz1 small-molecule inhibitor. Mol. Cancer Ther. 2019, 18, 1997–2007. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Maji, S.; Wechman, S.L.; Bhoopathi, P.; Pradhan, A.K.; Talukdar, S.; Sarkar, D.; Landry, J.; Guo, C.; Wang, X.; et al. MDA-9/Syntenin (SDCBP): Novel gene and therapeutic target for cancer metastasis. Pharmacol. Res. 2020, 155, 104695. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Wang, H.; Wang, B.; Zhao, K.; Jiang, W.; Bai, W.; Liu, J.; Yin, J. Syntenin1/MDA-9 (SDCBP) induces immune evasion in triple-negative breast cancer by upregulating PD-L1. Breast Cancer Res. Treat. 2018, 171, 345–357. [Google Scholar] [CrossRef]
- Sala-Valdés, M.; Gordón-Alonso, M.; Tejera, E.; Ibáñez, A.; Cabrero, J.R.; Ursa, A.; Mittelbrunn, M.; Lozano, F.; Sánchez-Madrid, F.; Yáñez-Mó, M. Association of syntenin-1 with M-RIP polarizes Rac-1 activation during chemotaxis and immune interactions. J. Cell Sci. 2012, 125, 1235–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimferrer, I.; Ibáñez, A.; Farnós, M.; Sarrias, M.-R.; Fenutría, R.; Roselló, S.; Zimmermann, P.; David, G.; Vives, J.; Serra-Pagès, C.; et al. The Lymphocyte Receptor CD6 Interacts with Syntenin-1, a Scaffolding Protein Containing PDZ Domains. J. Immunol. 2005, 175, 1406–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, D.; Grégoire, C.; Voisinne, G.; Celis-Gutierrez, J.; Aussel, R.; Girard, L.; Camus, M.; Marcellin, M.; Argenty, J.; Burlet-Schiltz, O.; et al. The T cell CD6 receptor operates a multitask signalosome with opposite functions in T cell activation. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Muñoz, J.M.; Gil-Martínez, M.; Sastre, B.; del Pozo, V. Emerging Evidence for Pleiotropism of Eosinophils. Int. J. Mol. Sci. 2021, 22, 7075. [Google Scholar] [CrossRef] [PubMed]
- Dorman, S.C.; Sehmi, R.; Gauvreau, G.M.; Watson, R.M.; Foley, R.; Jones, G.L.; Denburg, J.A.; Inman, M.D.; O’Byrne, P.M. Kinetics of Bone Marrow Eosinophilopoiesis and Associated Cytokines after Allergen Inhalation. Am. J. Respir. Crit. Care Med. 2004, 169, 565–572. [Google Scholar] [CrossRef]
- Kudo, M.; Ishigatsubo, Y.; Aoki, I. Pathology of asthma. Front. Microbiol. 2013, 4, 263. [Google Scholar] [CrossRef] [Green Version]
- Sehmi, R.; Lim, H.F.; Mukherjee, M.; Huang, C.; Radford, K.; Newbold, P.; Boulet, L.-P.; Dorscheid, D.; Martin, J.G.; Nair, P. Benralizumab attenuates airway eosinophilia in prednisone-dependent asthma. J. Allergy Clin. Immunol. 2018, 141, 1529–1532. [Google Scholar] [CrossRef] [Green Version]
- Herb, M.; Schramm, M.; Filosa, S. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Aguirre, J.; Lambeth, J.D. Nox enzymes from fungus to fly to fish and what they tell us about Nox function in mammals. Free Radic. Biol. Med. 2010, 49, 1342–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Lambeth, J.D.; Neish, A.S. Nox enzymes and new thinking on reactive oxygen: A double-edged sword revisited. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Bylund, J.; Brown, K.L.; Movitz, C.; Dahlgren, C.; Karlsson, A. Intracellular generation of superoxide by the phagocyte NADPH oxidase: How, where, and what for? Free Radic. Biol. Med. 2010, 49, 1834–1845. [Google Scholar] [CrossRef] [PubMed]
- Bánfi, B.; Malgrange, B.; Knisz, J.; Steger, K.; Dubois-Dauphin, M.; Krause, K.H. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004, 279, 46065–46072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalova, A.E.; Góngora, M.C.; Harrison, D.G.; Lambeth, J.D.; Dikalov, S.; Griendling, K.K. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am. J. Physiol. Hear. Circ. Physiol. 2010, 299, 673–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donkó, Á.; Ruisanchez, É.; Orient, A.; Enyedi, B.; Kapui, R.; Péterfi, Z.; De Deken, X.; Benyó, Z.; Geiszt, M. Urothelial cells produce hydrogen peroxide through the activation of Duox1. Free Radic. Biol. Med. 2010, 49, 2040–2048. [Google Scholar] [CrossRef]
- Gavazzi, G.; Banfi, B.; Deffert, C.; Fiette, L.; Schappi, M.; Herrmann, F.; Krause, K.H. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006, 580, 497–504. [Google Scholar] [CrossRef]
- Altenhöfer, S.; Radermacher, K.A.; Kleikers, P.W.M.; Wingler, K.; Schmidt, H.H.H.W. Evolution of NADPH Oxidase Inhibitors: Selectivity and Mechanisms for Target Engagement. Antioxid. Redox Signal. 2015, 23, 406–427. [Google Scholar] [CrossRef]
- Bubb, K.J.; Drummond, G.R.; Figtree, G.A. New opportunities for targeting redox dysregulation in cardiovascular disease. Cardiovasc. Res. 2020, 116, 532–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH Oxidases in Vascular Pathology. Antioxid. Redox Signal. 2014, 20, 2794–2814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosalski, R.; Guzik, T.J. Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.P.; Di Marco, E.; Okabe, J.; Szyndralewiez, C.; Heitz, F.; Montezano, A.C.; De Haan, J.B.; Koulis, C.; El-Osta, A.; Andrews, K.L.; et al. NADPH Oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 2013, 127, 1888–1902. [Google Scholar] [CrossRef] [Green Version]
- Inoguchi, T.; Nawata, H. NAD(P)H Oxidase Activation: A Potential Target Mechanism for Diabetic Vascular Complications, Progressive β-Cell Dysfunction and Metabolic Syndrome. Curr. Drug Targets 2005, 6, 495–501. [Google Scholar] [CrossRef]
- Wingler, K.; Hermans, J.; Schiffers, P.; Moens, A.; Paul, M.; Schmidt, H. NOX1, 2, 4, 5: Counting out oxidative stress. Br. J. Pharmacol. 2011, 164, 866–883. [Google Scholar] [CrossRef] [Green Version]
- Zekry, D.; Epperson, T.K.; Krause, K.-H. A Role for NOX NADPH Oxidases in Alzheimer’s Disease and Other Types of Dementia? IUBMB Life (Int. Union Biochem. Mol. Biol. Life) 2003, 55, 307–313. [Google Scholar] [CrossRef]
- Park, L. NADPH Oxidase-Derived Reactive Oxygen Species Mediate the Cerebrovascular Dysfunction Induced by the Amyloid Peptide. J. Neurosci. 2005, 25, 1769–1777. [Google Scholar] [CrossRef]
- Block, M.L. NADPH oxidase as a therapeutic target in Alzheimer’s disease. BMC Neurosci. 2008, 9, S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, B.; Cartier, L.; Dubois-Dauphin, M.; Li, B.; Serrander, L.; Krause, K.H. A key role for the microglial NADPH oxidase in APP-dependent killing of neurons. Neurobiol. Aging 2006, 27, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Laresgoiti-Servitje, E. A leading role for the immune system in the pathophysiology of preeclampsia. J. Leukoc. Biol. 2013, 94, 247–257. [Google Scholar] [CrossRef]
- Davidge, S. Oxidative Stress and Altered Endothelial Cell Function in Preeclampsia. Semin. Reprod. Med. 1998, 16, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Judkins, C.P.; Diep, H.; Broughton, B.R.S.; Mast, A.E.; Hooker, E.U.; Miller, A.A.; Selemidis, S.; Dusting, G.J.; Sobey, C.G.; Drummond, G.R. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am. J. Physiol. Hear. Circ. Physiol. 2010, 298, H24–H32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, R.; Acharya, R.; Delpachitra, P.; Hobson, S.; Sobey, C.G.; Drummond, G.R.; Wallace, E.M. Activin and NADPH-oxidase in preeclampsia: Insights from in vitro and murine studies. Am. J. Obstet. Gynecol. 2015, 212, 86.e1–86.e12. [Google Scholar] [CrossRef] [PubMed]
- Ghouleh, I.A.; Meijles, D.N.; Mutchler, S.; Zhang, Q.; Sahoo, S.; Gorelova, A.; Amaral, J.H.; Rodríguez, A.I.; Mamonova, T.; Song, G.J.; et al. Binding of EBP50 to Nox organizing subunit p47phox is pivotal to cellular reactive species generation and altered vascular phenotype. Proc. Natl. Acad. Sci. USA 2016, 113, E5308–E5317. [Google Scholar] [CrossRef] [Green Version]

| Peptide/Inhibitor | PDZ-Dependent Interaction (PDZbm) | Function of the PDZ-Dependent Interaction | Inhibitor Function | Study Phase | Disease | Reference |
|---|---|---|---|---|---|---|
| Peptide Pen-N3 * | DVL2-PDZ: Frizzled (Internal PDZbm) (KWYGW) | DVL facilitates Wnt signal, which leads to activation of b-catenin and T cell factor (TCF)-dependent transcription of developmental genes and genes associated with tumorigenesis | Inhibits canonical Wnt | Commercial: MERCK | Cancer | [35] |
| Tat-P4-(C5)2 * | PICK1-PDZ1: GluA2 (HWLKV) | PICK1 regulates PKC-dependent phosphorylation of S880 of AMPAR Glu A2 subunit in trafficking and plasticity | Interferes with excessive glutamate receptor transmission in pain; disrupts the interaction of PICK1 with AMPARs | Preclinic, in vivo | Neuropathic pain | [36] |
| 3-hydroxymethylindole ** | MAGI3-PDZ2: PTEN (YKQTSV) | Prevents PTEN recruitment to the plasma membrane and allows full activation of PKB | Blocks tumorigenic processes | Preclinic, in vitro | Cancer | [37] |
| NA-1 * | PDS-95-PDZ1: GluN2B (tSXV) | GluN2B activates nNOs in association with PSD95, promoting excitotoxicity in ischemic stroke | Inhibits neuronal excitotoxicity; penetrates the blood–brain barrier and interferes with GluN2B intersctions | Clinic, phase III | Ischemic stroke | [33,38] |
| Synthetic peptide: FVGSSSGHTSTTL * | NHERF1-CXCR2 (STTL) | Neutrophil migration in exacerbated inflammation | Proposed to be used in exacerbated inflammatory-related diseases | In vitro | Excessive neutrophil recruitment, tumorigenesis. | [39] |
| PDZ1i ** (CGSDKEϕϕV)2 * | Syntenin-CD6 (ISAA) | T cell activation and proliferation in autoimmune diseases: MS, EAE | Proposed to be used in autoimmune diseases | Theoretical | Autoimmune diseases | [40,41,42,43] |
| PDZ1i ** (CGSDKEϕϕV)2 * |
Syntenin-IL-5Ra (DSVF) | Eosinophil differentiation and survival in severe asthma | Proposed to be used in severe eosinophilic asthma | Theoretical | Severe eosinophilic asthma | [40,41,44,45] |
| To be designed. There is an inhibitor for PDZ1 and 3 domains but not for PDZ4. | Scrib-p22phox (DEVV) | ROS production in macrophages to destroy pathogens; probably exacerbated hypertension, Alzheimer’s and Parkinson’s diseases | Proposed to be used in hypertension, Alzheimer’s and Parkinson’s diseases | Theoretical | Hypertension, Alzheimer’s and Parkinson’s diseases | [46,47,48,49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-González, L.H.; Rivas-Fuentes, S.; Guzmán-Beltrán, S.; Flores-Flores, A.; Rosas-García, J.; Santos-Mendoza, T. Peptide Targeting of PDZ-Dependent Interactions as Pharmacological Intervention in Immune-Related Diseases. Molecules 2021, 26, 6367. https://doi.org/10.3390/molecules26216367
Gutiérrez-González LH, Rivas-Fuentes S, Guzmán-Beltrán S, Flores-Flores A, Rosas-García J, Santos-Mendoza T. Peptide Targeting of PDZ-Dependent Interactions as Pharmacological Intervention in Immune-Related Diseases. Molecules. 2021; 26(21):6367. https://doi.org/10.3390/molecules26216367
Chicago/Turabian StyleGutiérrez-González, Luis H., Selma Rivas-Fuentes, Silvia Guzmán-Beltrán, Angélica Flores-Flores, Jorge Rosas-García, and Teresa Santos-Mendoza. 2021. "Peptide Targeting of PDZ-Dependent Interactions as Pharmacological Intervention in Immune-Related Diseases" Molecules 26, no. 21: 6367. https://doi.org/10.3390/molecules26216367
APA StyleGutiérrez-González, L. H., Rivas-Fuentes, S., Guzmán-Beltrán, S., Flores-Flores, A., Rosas-García, J., & Santos-Mendoza, T. (2021). Peptide Targeting of PDZ-Dependent Interactions as Pharmacological Intervention in Immune-Related Diseases. Molecules, 26(21), 6367. https://doi.org/10.3390/molecules26216367






