The Antibiofilm Nanosystems for Improved Infection Inhibition of Microbes in Skin
Abstract
:1. Introduction
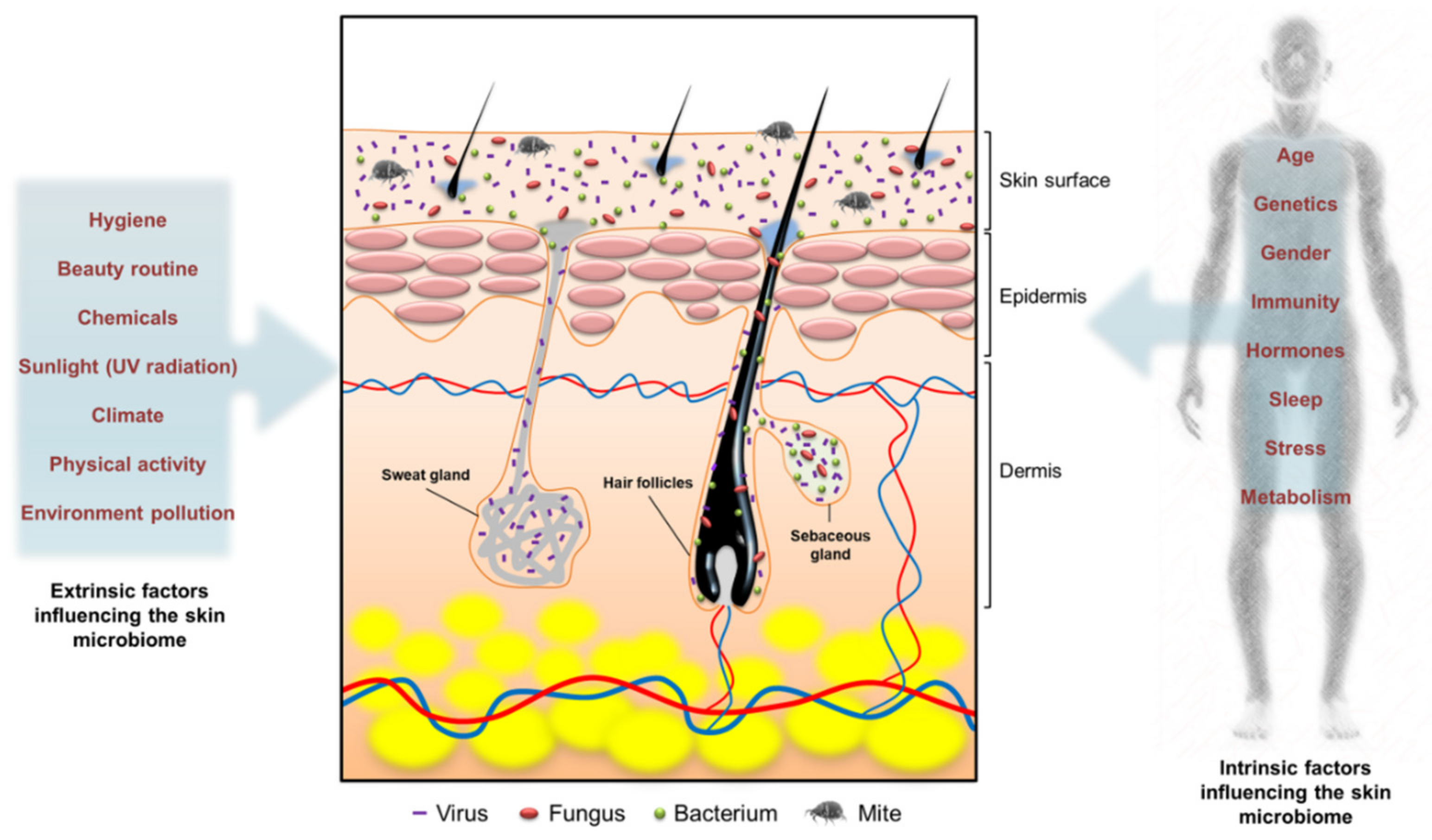
2. Cutaneous Microbiome
3. Cutaneous Microbiome-Associated Skin Diseases
3.1. Atopic Dermatitis (AD)
3.2. Acne
3.3. Skin Wounds
4. Biofilms
4.1. The Formation and Dispersion of Biofilms
4.2. The Components and Structure of Biofilms
4.3. Treatment Strategies for Biofilm Eradication
5. Different Types of Nanoparticles for Biofilm Eradication
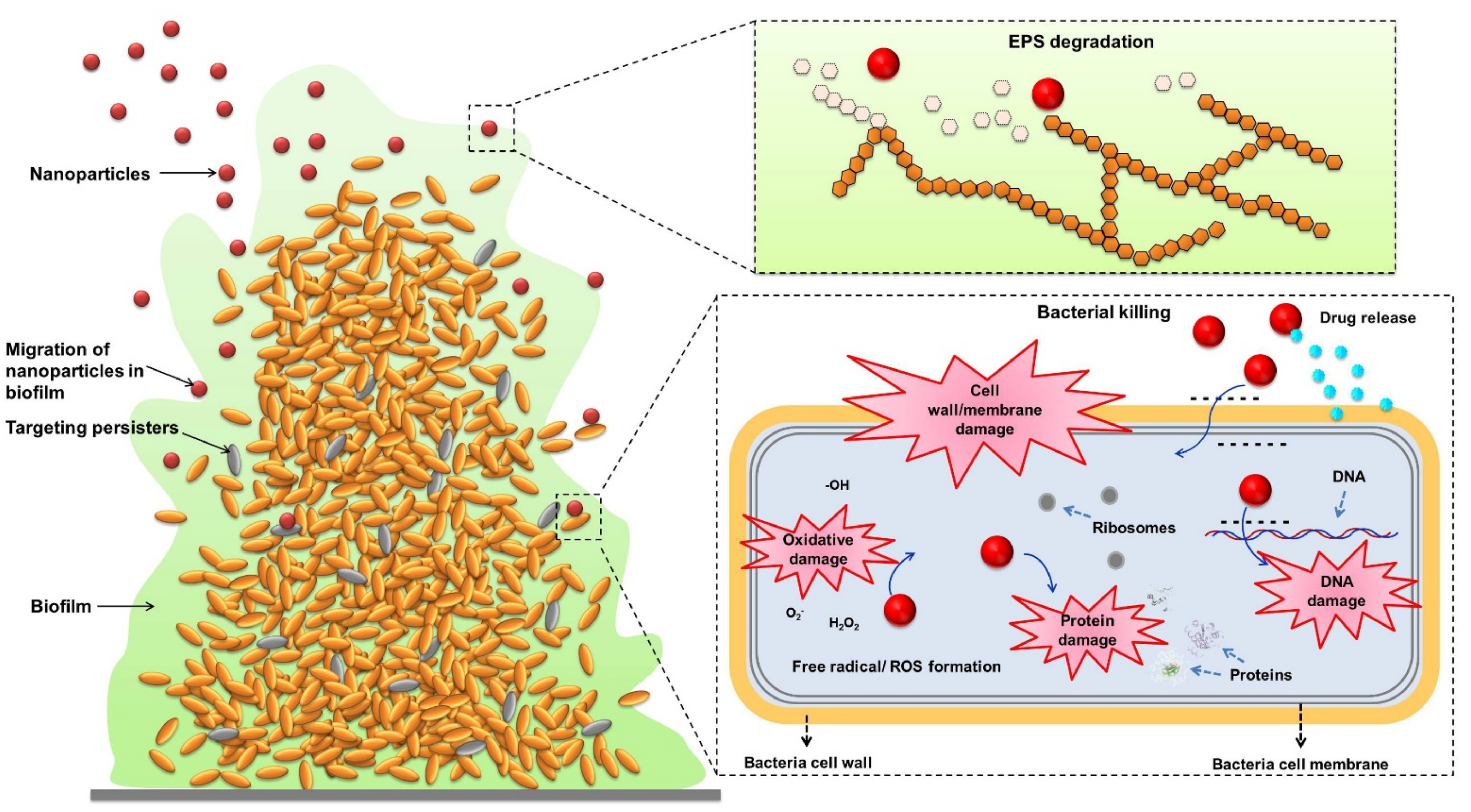
5.1. The Antibiofilm Mechanisms of Nanoparticles
5.2. Metallic Nanoparticles
5.3. Polymer Nanoparticles
5.4. Lipid Nanoparticles
6. Topically Applying Nanoparticles to Treat Cutaneous Biofilms
6.1. Metallic Nanoparticles
6.2. Polymeric Nanoparticles
6.3. Lipid-Based Nanoparticles
7. The Safety of Nanoparticles on Skin
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Capone, K.A.; Dowd, S.E.; Stamatas, G.N.; Nikolovski, J. Diversity of the human skin microbiome early in life. J. Investig. Dermatol. 2011, 131, 2026–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dye, C. After 2015: Infectious diseases in a new era of health and development. Philos. Trans. R. Soc. B 2014, 369, 20130426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luepke, K.H.; Suda, K.J.; Boucher, H.; Russo, R.L.; Bonney, M.W.; Hunt, T.D.; Mohr, J.F. Past, present, and future of antibacterial economics: Increasing bacterial resistance, limited antibiotic pipeline, and societal implications. Pharmacotherapy 2017, 37, 71–84. [Google Scholar] [CrossRef]
- Eisenreich, W.; Rudel, T.; Heesemann, J.; Goebel, W. How viral and intracellular bacterial pathogens reprogram the metabolism of host cells to allow their intracellular replication. Front. Cell Infect. Microbiol. 2019, 9, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dyck, K.; Pinto, R.M.; Pully, D.; Van Dijck, P. Microbial interkingdom biofilms and the quest for novel therapeutic strategies. Microorganisms 2021, 9, 412. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef] [Green Version]
- Sønderholm, M.; Bjarnsholt, T.; Alhede, M.; Kolpen, M.; Jensen, P.Ø.; Kühl, M.; Kragh, K.N. The consequences of being in an infectious biofilm: Microenvironmental conditions governing antibiotic tolerance. Int. J. Mol. Sci. 2017, 18, 2688. [Google Scholar] [CrossRef] [Green Version]
- Chung, P.Y.; Toh, Y.S. Anti-biofilm agents: Recent breakthrough against multi-drug resistant Staphylococcus aureus. Pathog. Dis. 2014, 70, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Abouelhassan, Y.; Zhang, Y.; Jin, S.; Huigens, R.W. Transcript profiling of MRSA biofilms treated with a halogenated phenazine eradicating agent: A platform for defining cellular targets and pathways critical to biofilm survival. Angew. Chem. Int. Ed. 2018, 57, 15523–15528. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial biofilm eradication agents: A current review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Xia, G.; Shi, C.; Wan, J.; Liu, L.; Chen, Y.; Wu, Y.; Zhang, W.; Zhou, M.; He, H.; et al. Therapeutic strategies against bacterial biofilms. Fundam. Res. 2021, 1, 193–212. [Google Scholar] [CrossRef]
- Nag, M.; Lahiri, D.; Sarkar, T.; Ghosh, S.; Dey, A.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbial fabrication of nanomaterial and its role in disintegration of exopolymeric matrices of biofilm. Front. Chem. 2021, 9, 690590. [Google Scholar] [CrossRef]
- Rosenberg, M.; Visnapuu, M.; Vija, H.; Kisand, V.; Kasemets, K.; Kahru, A.; Ivask, A. Selective antibiofilm properties and biocompatibility of nan-ZnO and nano-ZnO/Ag coated surfaces. Sci. Rep. 2020, 10, 13478. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Lin, Y.K.; Wang, P.W.; Alalaiwe, A.; Yang, Y.C.; Yang, S.C. The droplet-size effect of squalene@cetylpyridinium chloride nanoemulsions on antimicrobial potency against planktonic and biofilm MRSA. Int. J. Nanomed. 2019, 14, 8133–8147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmowafy, M. Skin penetration/permeation success determinants of nanocarriers: Pursuit of a perfect formulation. Colloids Surf. B Biointerfaces 2021, 203, 111748. [Google Scholar] [CrossRef] [PubMed]
- Güngör, S.; Kahraman, E. Nanocarriers mediated cutaneous drug delivery. Eur. J. Pharm. Sci. 2021, 158, 105638. [Google Scholar] [CrossRef] [PubMed]
- Bellu, E.; Medici, S.; Coradduzza, D.; Cruciani, S.; Amler, E.; Maioli, M. Nanomaterials in skin regeneration and rejuvenation. Int. J. Mol. Sci. 2021, 22, 7095. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human skin microbiome: Impact of intrinsic and extrinsic factors on skin microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef]
- Cundell, A.M. Microbial ecology of the human skin. Microb. Ecol. 2018, 76, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Flowers, L.; Grice, E.A. The skin microbiota: Balancing risk and reward. Cell Host Microbe 2020, 28, 190–200. [Google Scholar] [CrossRef]
- Lousada, M.B.; Lachnit, T.; Edelkamp, J.; Rouillé, T.; Ajdic, D.; Uchida, Y.; Di Nardo, A.; Bosch, T.C.G.; Paus, R. Exploring the human hair follicle microbiome. Br. J. Dermatol. 2021, 184, 802–815. [Google Scholar] [CrossRef]
- Lange-Asschenfeldt, B.; Marenbach, D.; Lang, C.; Patzelt, A.; Ulrich, M.; Maltusch, A.; Terhorst, D.; Stockfleth, E.; Sterry, W.; Lademann, J. Distribution of bacteria in the epidermal layers and hair follicles of the human skin. Skin Pharmacol. Physiol. 2011, 24, 305–311. [Google Scholar] [CrossRef]
- Fang, C.L.; Aljuffali, I.A.; Li, Y.C.; Fang, J.Y. Delivery and targeting of nanoparticles into hair follicles. Ther. Deliv. 2014, 5, 991–1006. [Google Scholar] [CrossRef]
- Kong, H.H.; Segre, J.A. Skin microbiome: Looking back to move forward. J. Investig. Dermatol. 2012, 132, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Brown, M.M.; Horswill, A.R. Staphylococcus epidermidis-Skin friend or foe? PLoS Pathog. 2020, 16, e1009026. [Google Scholar] [CrossRef]
- Cau, L.; Williams, M.R.; Butcher, A.M.; Nakatsuji, T.; Kavanaugh, J.S.; Cheng, J.Y.; Shafiq, F.; Higbee, K.; Hata, T.R.; Horswill, A.R.; et al. Staphylococcus epidermidis protease EcpA can be a deleterious component of the skin microbiome in atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 955–966. [Google Scholar] [CrossRef]
- del Giudice, P. Skin infections caused by Staphylococcus aureus. Acta Derm. Venereol. 2020, 100, adv00110. [Google Scholar] [CrossRef]
- Goyal, N.; Miller, A.; Tripathi, M.; Parvizi, J. Methicillin-resistant Staphylococcus aureus (MRSA). Bone Jt. J. 2013, 95, 4–9. [Google Scholar] [CrossRef]
- Platsidaki, E.; Dessinioti, C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Research 2018, 7, 1953. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, J.; Watterson, S.; Layton, A.M.; Bjourson, A.J.; Barnard, E.; McDowell, A. Propionibacterium acnes and acne vulgaris: New insights from the integration of population genetic, multi-omic, biochemical and host-microbe studies. Microorganisms 2019, 7, 128. [Google Scholar] [CrossRef] [Green Version]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Hay, R.J. Malassezia, dandruff and seborrhoeic dermatitis: An overview. Br. J. Dermatol. 2011, 165 (Suppl. 2), 2–8. [Google Scholar] [CrossRef]
- Williams, M.R.; Gallo, R.L. Evidence that human skin microbiome dysbiosis promotes atopic dermatitis. J. Investig. Dermatol. 2017, 137, 2460–2461. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef]
- Alexander, H.; Paller, A.S.; Traidl-Hoffmann, C.; Beck, L.A.; De Benedetto, A.; Dhar, S.; Girolomoni, G.; Irvine, A.D.; Spuls, P.; Su, J.; et al. The role of bacterial skin infections in atopic dermatitis: Expert statement and review from the International Eczema Council Skin Infection Group. Br. J. Dermatol. 2020, 182, 1331–1342. [Google Scholar] [CrossRef] [Green Version]
- Ogonowska, P.; Gilaberte, Y.; Barańska-Rybak, W.; Nakonieczna, J. Colonization with Staphylococcus aureus in atopic dermatitis patients: Attempts to reveal the unknown. Front. Microbiol. 2021, 11, 567090. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, L.; Ren, Y.; Tan, X.; Liu, W.; Liu, Z. Applications of human skin microbiota in the cutaneous disorders for ecology-based therapy. Front. Cell. Infect. Microbiol. 2020, 10, 570261. [Google Scholar] [CrossRef] [PubMed]
- Chng, K.R.; Tay, A.S.; Li, C.; Ng, A.H.; Wang, J.; Suri, B.K.; Matta, S.A.; McGovern, N.; Janela, B.; Wong, X.F.; et al. Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat. Microbiol. 2016, 1, 16106. [Google Scholar] [CrossRef] [PubMed]
- Heng, A.H.S.; Chew, F.T. Systematic review of the epidemiology of acne vulgaris. Sci. Rep. 2020, 10, 5754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodarzi, A.; Mozafarpoor, S.; Bodaghabadi, M.; Mohamadi, M. The potential of probiotics for treating acne vulgaris: A review of literature on acne and microbiota. Dermatol. Ther. 2020, 33, e13279. [Google Scholar] [CrossRef] [PubMed]
- Jahns, A.C.; Lundskog, B.; Ganceviciene, R.; Palmer, R.H.; Golovleva, I.; Zouboulis, C.C.; McDowell, A.; Patrick, S.; Alexeyev, O.A. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: A case-control study. Br. J. Dermatol. 2012, 167, 50–58. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.M.; Gallo, R.L. Host-microbiome interactions and recent progress into understanding the biology of acne vulgaris. Microbiome 2018, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Huitema, L.; Phillips, T.; Alexeev, V.; Tomic-Canic, M.; Pastar, I.; Igoucheva, O. Intracellular escape strategies of Staphylococcus aureus in persistent cutaneous infections. Exp. Dermatol. 2020, 30, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Preda, M.; Lungu, I.; Gestal, M.C.; Popa, M.I.; Holban, A.M. Nanocoatings for chronic wound repair-modulation of microbial colonization and biofilm formation. Int. J. Mol. Sci. 2018, 19, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.K.; Cheng, N.C.; Cheng, C.M. Biofilms in chronic wounds: Pathogenesis and diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Díaz, M.; Alvarado-Gomez, E.; Magaña-Aquino, M.; Sánchez-Sánchez, R.; Velasquillo, C.; Gonzalez, C.; Ganem-Rondero, A.; Martínez-Castañon, G.; Zavala-Alonso, N.; Martinez-Gutierrez, F. Anti-biofilm activity of chitosan gels formulated with silver nanoparticles and their cytotoxic effect on human fibroblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.M.; Zenilman, J.M.; Lazarus, G.S. Molecular microbiology: New dimensions for cutaneous biology and wound healing. J. Investig. Dermatol. 2010, 130, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond risk: Bacterial biofilms and their regulating approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Tang, K.W.; Yang, S.H.; Alalaiwe, A.; Yang, Y.C.; Tseng, C.H.; Yang, S.C. Synthetic naphthofuranquinone derivatives are effective in eliminating drug-resistant Candida albicans in hyphal, biofilm, and intracellular forms: An application for skin-infection treatment. Front. Microbiol. 2020, 11, 2053. [Google Scholar] [CrossRef] [PubMed]
- Kernien, J.F.; Snarr, B.D.; Sheppard, D.C.; Nett, J.E. The interface between fungal biofilms and innate immunity. Front. Immunol. 2018, 8, 1968. [Google Scholar] [CrossRef] [Green Version]
- Neu, T.R.; Lawrence, J.R. Innovative techniques, sensors, and approaches for imaging biofilms at different scales. Trends Microbiol. 2014, 23, 233–242. [Google Scholar] [CrossRef]
- Erskine, E.; MacPhee, C.E.; Stanley-Wall, N.R. Functional amyloid and other protein fibers in the biofilm matrix. J. Mol. Biol. 2018, 430, 3642–3656. [Google Scholar] [CrossRef] [PubMed]
- Billings, N.; Birjiniuk, A.; Samad, T.S.; Doyle, P.S.; Ribbeck, K. Material properties of biofilms–a review of methods for understanding permeability and mechanics. Rep. Prog. Phys. 2015, 78, 036601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Kang, F.; Yang, B.; Zhang, W.; Qin, C.; Gao, Y. Extracellular polymeric substances acting as a permeable barrier hinder the lateral transfer of antibiotic resistance genes. Front. Microbiol. 2019, 10, 736. [Google Scholar] [CrossRef] [Green Version]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisonneuve, E.; Gerdes, K. Molecular mechanisms underlying bacterial persisters. Cell 2014, 157, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, V.C.; Souza, M.T.; Zanotto, E.D.; Watanabe, E.; Coraça-Huber, D. Biofilm formation and expression of virulence genes of microorganisms grown in contact with a new bioactive glass. Pathogens 2020, 9, 927. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Huang, T.H.; Yang, S.C.; Chen, C.C.; Fang, J.Y. Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: A review of recent advances. Front. Chem. 2020, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Geng, M.; Bai, L. Targeting biofilms therapy: Current research strategies and development hurdles. Microorganisms 2020, 8, 1222. [Google Scholar] [CrossRef]
- Sahni, K.; Khashai, F.; Forghany, A.; Krasieva, T.; Wilder-Smith, P. Exploring mechanisms of biofilm removal. Dentistry 2016, 6, 371. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, H.; Bahador, A.; Pourhajibagher, M.; Alikhani, M.Y. Antimicrobial photodynamic therapy: An effective alternative approach to control bacterial infections. J. Lasers Med. Sci. 2018, 9, 154–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Huang, Y.Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Li, L.; Hu, Q. The recent progress in photothermal-triggered bacterial eradication. Biomater. Sci. 2021, 9, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Jiménez, C.; Niño-Martínez, N.; Patiño-Marín, N.; Martínez-Gutiérrez, F.; Ruiz, F.; Bach, H.; Martínez-Castañón, G. Effective control of biofilms by photothermal therapy using a gold nanorod hydrogel. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 333–342. [Google Scholar] [CrossRef]
- Okshevsky, M.; Regina, V.R.; Meyer, R.L. Extracellular DNA as a target for biofilm control. Curr. Opin. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Liu, J. Cleaving DNA by nanozymes. J. Mater. Chem. B 2020, 8, 7135–7142. [Google Scholar] [CrossRef]
- Shukla, S.K.; Rao, T.S. Dispersal of Bap-mediated Staphylococcus aureus biofilm by proteinase K. J. Antibiot. 2013, 66, 55–60. [Google Scholar] [CrossRef]
- Nguyen, U.T.; Burrows, L.L. DNase I and proteinase K impair Listeria monocytogenes biofilm formation and induce dispersal of pre-existing biofilms. Int. J. Food Microbiol. 2014, 187, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Mlynek, K.D.; Hettiarachchi, H.; Alamneh, Y.A.; Biggemann, L.; Zurawski, D.V.; Black, C.C.; Bane, C.E.; Kim, R.K.; Granick, M.S. Extracellular polymeric substance (EPS)-degrading enzymes reduce staphylococcal surface attachment and biocide resistance on pig skin in vivo. PLoS ONE 2018, 13, e0205526. [Google Scholar] [CrossRef]
- Bhagwat, A.; Collins, C.H.; Dordick, J.S. Selective antimicrobial activity of cell lytic enzymes in a bacterial consortium. Appl. Microbiol. Biotechnol. 2019, 103, 7041–7054. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, C.; Han, Z.; Kalas, V.; Klein, R.; Pinkner, J.S.; Ford, B.; Binkley, J.; Cusumano, C.K.; Cusumano, Z.; Mydock-McGrane, L.; et al. Antivirulence isoquinolone mannosides: Optimization of the biaryl aglycone for FimH lectin binding affinity and efficacy in the treatment of chronic UTI. ChemMedChem 2016, 11, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Ridyard, K.E.; Overhage, J. The potential of human peptide LL-37 as an antimicrobial and anti-biofilm agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef]
- Flüh, G.; Seifert, H.; Kaasch, A.J. Oritavancin: An update. Future Microbiol. 2018, 13, 727–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, L.Q.S.; de Almeida Vaucher, R.; Giongo, J.L.; Gündel, A.; Santos, R.C.V. Characterisation and anti-biofilm activity of glycerol monolaurate nanocapsules against Pseudomonas aeruginosa. Microb. Pathog. 2019, 130, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhou, Z.; Dong, J.; Zhang, J.; Xia, Y.; Shu, R. Antibacterial and antibiofilm activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against periodontopathic bacteria. Microb. Pathog. 2016, 99, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Obłąk, E.; Piecuch, A.; Rewak-Soroczyńska, J.; Paluch, E. Activity of gemini quaternary ammonium salts against microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial quorum sensing and microbial community interactions. mBio 2018, 9, e02331-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Bouton, J.; Van Hecke, K.; Rasooly, R.; Van Calenbergh, S. Synthesis of pyrrolidine-based hamamelitannin analogues as quorum sensing inhibitors in Staphylococcus aureus. Beilstein J. Org. Chem. 2018, 14, 2822–2828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Jagasia, R.; Kaufmann, G.F.; Mathison, J.C.; Ruiz, D.I.; Moss, J.A.; Meijler, M.M.; Ulevitch, R.J.; Janda, K.D. Infection control by antibody disruption of bacterial quorum sensing signaling. Chem. Biol. 2007, 14, 1119–1127. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.; Qiu, S.; Jiang, Q.; Sun, H.; Xue, T.; Cai, G.; Sun, B. AI-2 quorum sensing negatively regulates rbf expression and biofilm formation in Staphylococcus aureus. Int. J. Med. Microbiol. 2017, 307, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle-biofilm interactions: The role of the EPS matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef] [PubMed]
- Ikuma, K.; Decho, A.W.; Lau, B.L. When nanoparticles meet biofilms-interactions guiding the environmental fate and accumulation of nanoparticles. Front. Microbiol. 2015, 6, 591. [Google Scholar] [CrossRef]
- Gómez-Núñez, M.F.; Castillo-López, M.; Sevilla-Castillo, F.; Roque-Reyes, O.J.; Romero-Lechuga, F.; Medina-Santos, D.I.; Martínez-Daniel, R.; Peón, A.N. Nanoparticle-based devices in the control of antibiotic resistant bacteria. Front. Microbiol. 2020, 11, 563821. [Google Scholar] [CrossRef]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative strategies toward the disassembly of the EPS matrix in bacterial biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.K.; Biswas, R.; Biswas, L. An update on recent developments in the prevention and treatment of Staphylococcus aureus biofilms. Int. J. Med. Microbiol. 2019, 309, 1–12. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Wang, L.S.; Gupta, A.; Rotello, V.M. Nanomaterials for the treatment of bacterial biofilms. ACS Infect. Dis. 2016, 2, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.M.; Tran, H.; Booth, M.A.; Fox, K.E.; Nguyen, T.H.; Tran, N.; Tran, P.A. Nanomaterials for treating bacterial biofilms on implantable medical devices. Nanomaterials 2020, 10, 2253. [Google Scholar] [CrossRef]
- Khan, S.T.; Ahamed, M.; Musarrat, J.; Al-Khedhairy, A.A. Anti-biofilm and antibacterial activities of zinc oxide nanoparticles against the oral opportunistic pathogens Rothia dentocariosa and Rothia mucilaginosa. Eur. J. Oral Sci. 2014, 122, 397–403. [Google Scholar] [CrossRef]
- Hou, J.; Li, T.; Miao, L.; You, G.; Xu, Y.; Liu, S. Effects of titanium dioxide nanoparticles on algal and bacterial communities in periphytic biofilms. Environ. Pollut. 2019, 251, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nickel, R.; Wu, J.; Lin, F.; van Lierop, J.; Liu, S. A new tool to attack biofilms: Driving magnetic iron-oxide nanoparticles to disrupt the matrix. Nanoscale 2019, 11, 6905–6915. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Herdade, A.M.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Paranhos, A.; Veiga, F. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. 2021, 597, 120311. [Google Scholar] [CrossRef]
- Albisa, A.; Espanol, L.; Prieto, M.; Sebastian, V. Polymeric nanomaterials as nanomembrane entities for biomolecule and drug delivery. Curr. Pharm. Des. 2017, 23, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.P.; Lin, Y.K.; Chen, C.H.; Fang, J.Y. Recent advances in polymeric nanosystems for treating cutaneous melanoma and its metastasis. Curr. Pharm. Des. 2017, 23, 5301–5314. [Google Scholar] [CrossRef] [PubMed]
- Birk, S.E.; Boisen, A.; Nielsen, L.H. Polymeric nano- and microparticulate drug delivery systems for treatment of biofilms. Adv. Drug Deliv. Rev. 2021, 174, 30–52. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of nanoparticles as permeation enhancers and targeted delivery options for skin: Advantages and disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef]
- Del Pozo-Rodríguez, A.; Solinís, M.Á.; Rodríguez-Gascón, A. Applications of lipid nanoparticles in gene therapy. Eur. J. Pharm. Biopharm. 2016, 109, 184–193. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef]
- Wang, Y. Liposome as a delivery system for the treatment of biofilm-mediated infections. J. Appl. Microbiol. 2021. [Google Scholar] [CrossRef]
- Pircalabioru, G.G.; Chifiriuc, M.C. Nanoparticulate drug-delivery systems for fighting microbial biofilms: From bench to bedside. Future Microbiol. 2020, 15, 679–698. [Google Scholar] [CrossRef]
- Barik, S.K.; Singh, B.N. Nanoemulsion-loaded hydrogel coatings for inhibition of bacterial virulence and biofilm formation on solid surfaces. Sci. Rep. 2019, 9, 6520. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.C.; Hwang, T.L.; Huang, T.H.; Tahara, K.; Trousil, J.; Fang, J.Y. Monovalent antibody-conjugated lipid-polymer nanohybrids for active targeting to desmoglein 3 of keratinocytes to attenuate psoriasiform inflammation. Theranostics 2021, 11, 4567–4584. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Buttaro, B.A.; Xue, H.Y.; Tran, N.T.; Wong, H.L. Lipid-polymer hybrid nanoparticles carrying linezoid improve treatment of methicillin-resistant Staphylococcus aureus (MRSA) harbored inside bone cells and biofilms. Eur. J. Pharm. Biopharm. 2020, 151, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Facal, P.; Thomas, N.; Vandecandelaere, I.; Ramezanpour, M.; Cooksley, C.; Prestidge, C.A.; Coenye, T.; Wormald, P.J.; Vreugde, S. Taking the silver bullet colloidal silver particles for the topical treatment of biofilm-related infections. ACS Appl. Mater. Interfaces 2017, 9, 21631–21638. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.; Ahumada, M.; Franco, W.; Mah, T.F.; Seymour, R.; Suuronen, E.J.; Alarcon, E.I. Sprayable peptide-modified silver nanoparticles as a barrier against bacterial colonization. Nanoscale 2016, 8, 19200–19203. [Google Scholar] [CrossRef] [PubMed]
- Lazurko, C.; Khatoon, Z.; Goel, K.; Sedlakova, V.; Eren Cimenci, C.; Ahumada, M.; Zhang, L.; Mah, T.F.; Franco, W.; Suuronen, E.J.; et al. Multifunctional nano and collagen-based therapeutic materials for skin repair. ACS Biomater. Sci. Eng. 2020, 6, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Wang, Y.; Vuong, L.N.; Fisher, J.M.; Farinelli, W.A.; Anderson, R.R. Reconstitution of full-thickness skin by microcolumn grafting. J. Tissue Eng. Regen. Med. 2017, 11, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Gomez, E.; Martínez-Castañon, G.; Sanchez-Sanchez, R.; Ganem-Rondero, A.; Yacaman, M.J.; Martinez-Gutierrez, F. Evaluation of anti-biofilm and cytotoxic effect of a gel formulation with Pluronic F-127 and silver nanoparticles as a potential treatment for skin wounds. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrogi, V.; Pietrella, D.; Donnadio, A.; Latterini, L.; Di Michele, A.; Luffarelli, I.; Ricci, M. Biocompatible alginate silica supported silver nanoparticles composite films for wound dressing with antibiofilm activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110863. [Google Scholar] [CrossRef] [PubMed]
- Paterson, T.E.; Bari, A.; Bullock, A.J.; Turner, R.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C.; MacNeil, S.; Shepherd, J. Multifunctional copper-containing mesoporous glass nanoparticles as antibacterial and proangiogenic agents for chronic wounds. Front. Bioeng. Biotechnol. 2020, 8, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.; Lee, J.M.; Oh, S.B.; Choi, Y.; Jung, H.S.; Choi, J. Development of antibiofilm nanocomposites: Ag/Cu bimetallic nanoparticles synthesized on the surface of grapheme oxide nanosheets. ACS Appl. Mater. Interfaces 2020, 12, 35826–35834. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Jiang, X. Aminosaccharide-gold nanoparticle assemblies as narrow-spectrum antibiotics against methicillin-resistant Staphylococcus aureus. Nano Res. 2018, 11, 6237–6243. [Google Scholar] [CrossRef]
- Yang, X.; Wei, Q.; Shao, H.; Jiang, X. Multivalent aminosaccharide-based gold nanoparticles as narrow-spectrum antibiotics in vivo. ACS Appl. Mater. Interfaces 2019, 11, 7725–7730. [Google Scholar] [CrossRef]
- Raghuwanshi, N.; Kumari, P.; Srivastava, A.K.; Vashisth, P.; Yadav, T.C.; Prasad, R.; Pruthi, V. Synergistic effects of Woodfordia fruticosa gold nanoparticles in preventing microbial adhesion and accelerating wound healing in Wistar albino rats in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Amresh, G.; Sahu, P.K.; Mishra, N.; Rao, C.V.; Singh, A.P. Wound healing potential of flowers extracts of Woodfordia fruticosa Kurz. Indian J. Biochem. Biophys. 2013, 50, 296–304. [Google Scholar] [PubMed]
- Pati, R.; Mehta, R.K.; Mohanty, S.; Padhi, A.; Sengupta, M.; Vaseeharan, B.; Goswami, C.; Sonawane, A. Topical application of zinc oxide nanoparticles reduces bacterial skin infection in mice and exhibits antibacterial activity by inducing oxidative stress response and cell membrane disintegration in macrophages. Nanomed.-Nanotechnol. Biol. Med. 2014, 10, 1195–1208. [Google Scholar] [CrossRef]
- Rayyif, S.M.I.; Mohammed, H.B.; Curuțiu, C.; Bîrcă, A.C.; Grumezescu, A.M.; Vasile, B.Ș.; Dițu, L.M.; Lazăr, V.; Chifiriuc, M.C.; Mihăescu, G.; et al. ZnO nanoparticles-modified dressings to inhibit wound pathogens. Materials 2021, 14, 3084. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Yamayoshi, I.; Mathew, S.; Lin, H.; Nayfach, J.; Simon, S.I. Magnetic nanoparticle targeted hyperthermia of cutaneous Staphylococcus aureus infection. Ann. Biomed. Eng. 2013, 41, 598–609. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, H.; Wang, B.; Ye, Z.; Lei, W.; Jia, F.; Jin, Q.; Ren, K.F.; Ji, J. Surface-adaptive gold nanoparticles with effective adherence and enhanced photothermal ablation of methicillin-resistant Staphylococcus aureus biofilm. ACS Nano 2017, 11, 9330–9339. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Li, W.; Tang, Y.; Elzatahry, A.; Lu, G.; Zhao, D. Mesoporous organosilica hollow nanoparticles: Synthesis and applications. Adv. Mater. 2019, 31, 1707612. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Ge, W.; Yin, J.; Yang, D.; Wang, W.; Song, X.; Hu, Y.; Yin, J.; Dong, X. Mesoporous silica supported silver-bismuth nanoparticles as photothermal agents for skin infection synergistic antibacterial therapy. Small 2020, 16, 2000436. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jana, D.; Zhao, Y. Metal-organic framework derived nanozymes in biomedicine. Acc. Chem. Res. 2020, 53, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Hu, Y.; Xiao, Y.; Zhang, Y.; Sun, K.; Wu, T.; Lv, N.; Wang, W.; Ding, W.; Li, F.; et al. Near-infrared-controlled nanoplatform exploiting photothermal promotion of peroxidase-like and OXD-like activities for potent antibacterial and anti-biofilm therapies. ACS Appl. Mater. Interfaces 2020, 12, 50260–50274. [Google Scholar] [CrossRef] [PubMed]
- Vendramini, Y.; Salles, A.; Portella, F.F.; Brew, M.C.; Steier, L.; de Figueiredo, J.A.P.; Bavaresco, C.S. Antimicrobial effect of photodynamic therapy on intracanal biofilm: A systematic review of in vitro studies. Photodiagnosis. Photodyn. Ther. 2020, 32, 102025. [Google Scholar] [CrossRef] [PubMed]
- Sherwani, M.A.; Tufail, S.; Khan, A.A.; Owais, M. Gold nanoparticle-photosensitizer conjugate based photodynamic inactivation of biofilm producing cells: Potential for treatment of C. albicans infection in BALB/c mice. PLoS ONE 2015, 10, 0131684. [Google Scholar]
- Xiao, Y.; Xu, M.; Lv, N.; Cheng, C.; Huang, P.; Li, J.; Hu, Y.; Sun, M. Dual stimuli-responsive metal-organic framework-based nanosystem for synergistic photothermal/pharmacological antibacterial therapy. Acta Biomater. 2021, 122, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Hung, C.F.; Hsu, C.Y.; Lin, Z.C.; Fang, J.Y. Prodrugs in combination with nanocarriers as a strategy for promoting antitumoral efficiency. Future Med. Chem. 2019, 11, 2131–2150. [Google Scholar] [CrossRef]
- El-Deeb, N.M.; Abo-Eleneen, M.A.; Al-Madboly, L.A.; Sharaf, M.M.; Othman, S.S.; Ibrahim, O.M.; Mubarak, M.S. Biogenically synthesized polysaccharides-capped silver nanoparticles: Immunomodulatory and antibacterial potentialities against resistant Pseudomonas aeruginosa. Front. Bioeng. Biotechnol. 2020, 8, 643. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chen, M.; Gong, H.; Thamphiwatana, S.; Eckmann, L.; Gao, W.; Zhang, L. A bioadhesive nanoparticle-hydrogel hybrid system for localized antimicrobial drug delivery. ACS Appl. Mater. Interfaces 2016, 8, 18367–18374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waite, R.D.; Stewart, J.E.; Stephen, A.S.; Allaker, R.P. Activity of a nitric oxide-generating wound treatment system against wound pathogen biofilms. Int. J. Antimicrob. Agents 2018, 52, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Cao, J.; Lee, J.; Naeem, M.; Hlaing, S.P.; Kim, J.; Jung, Y.; Lee, B.L.; Yoo, J.W. PEI-NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109741. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Hyaluronic acid and controlled release: A review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef] [PubMed]
- Kłodzińska, S.N.; Pletzer, D.; Rahanjam, N.; Rades, T.; Hancock, R.E.W.; Nielsen, H.M. Hyaluronic acid-based nanogels improve in vivo compatibility of the anti-biofilm peptide DJK-5. Nanomed.-Nanotechnol. Biol. Med. 2019, 20, 102022. [Google Scholar] [CrossRef]
- Wu, D.; Wei, D.; Du, M.; Ming, S.; Ding, Q.; Tan, R. Targeting antibacterial effect and promoting of skin wound healing after infected with methicillin-resistant Staphylococcus aureus for the novel polyvinyl alcohol nanoparticles. Int. J. Nanomed. 2021, 16, 4031–4044. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.M. Chitosan and their derivatives: Antibiofilm drugs against pathogenic bacteria. Colloids Surf. B Biointerfaces 2020, 185, 110627. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Mir, M.; Utomo, E.; Donnelly, R.F. Bacterially sensitive nanoparticle-based dissolving microneedles of doxyxyxline for enhanced treatment of bacterial biofilm skin infection: A proof of concept study. Int. J. Pharm. X 2020, 2, 100047. [Google Scholar] [PubMed]
- Mir, M.; Permana, A.D.; Tekko, I.A.; McCarthy, H.O.; Ahmed, N.; Rehman, A.U.; Donnelly, R.F. Microneedle liquid injection system assisted delivery of infection responsive nanoparticles: A promising approach for enhanced site-specific delivery of carvacrol against polymicrobial biofilms-infected wounds. Int. J. Pharm. 2020, 587, 119643. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef]
- Singh, N.; Romero, M.; Travanut, A.; Monteiro, P.F.; Jordana-Lluch, E.; Hardie, K.R.; Williams, P.; Alexander, M.R.; Alexander, C. Dual bioresponsive antibiotic and quorum sensing inhibitor combination nanoparticles for treatment of Pseudomonas aeruginosa biofilms in vitro and ex vivo. Biomater. Sci. 2019, 7, 4099–4111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Tøndervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Onsøyen, E.; Myrvold, R.; Howe, R.A.; Walsh, T.R.; Hill, K.E.; et al. Overcoming drug resistance with alginate oligosaccharides able to potentiate the action of selected antibiotics. Antimicrob. Agents Chemother. 2012, 56, 5134–5141. [Google Scholar] [CrossRef] [Green Version]
- Aljuffali, I.A.; Sung, C.T.; Shen, F.M.; Huang, C.T.; Fang, J.Y. Squarticles as a lipid nanocarrier for delivering diphencyprone and minoxidil to hair follicles and human dermal papilla cells. AAPS J. 2014, 16, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Eroğlu, İ.; Aslan, M.; Yaman, Ü.; Gultekinoglu, M.; Çalamak, S.; Kart, D.; Ulubayram, K. Liposome-based combination therapy for acne treatment. J. Liposome Res. 2020, 30, 263–273. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Huang, X.; Wang, X.; Liao, G.; Chen, Z. Preparation and characterization of flexible nanoliposomes loaded with daptomycin, a novel antibiotic, for topical skin therapy. Int. J. Nanomed. 2013, 8, 1285–1292. [Google Scholar] [CrossRef] [Green Version]
- Popp, J.A.; Layon, A.J.; Nappo, R.; Richards, W.T.; Mozingo, D.W. Hospital-acquired infections and thermally injured patients: Chlorhexidine gluconate baths work. Am. J. Infect. Control 2014, 42, 129–132. [Google Scholar] [CrossRef]
- Song, Z.; Sun, H.; Yang, Y.; Jing, H.; Yang, L.; Tong, Y.; Wei, C.; Wang, Z.; Zou, Q.; Zeng, H. Enhanced efficacy and anti-biofilm activity of novel nanoemulsions against skin burn wound multi-drug resistant MRSA infections. Nanomed.-Nanotechnol. Biol. Med. 2016, 12, 1543–1555. [Google Scholar] [CrossRef]
- Lewińska, A.; Jaromin, A.; Jezierska, J. Role of architecture of N-oxide surfactants in the design of nanoemulsions for Candida skin infection. Colloids Surf. B Biointerfaces 2020, 187, 110639. [Google Scholar] [CrossRef]
- Lin, M.H.; Hung, C.F.; Aljuffali, I.A.; Sung, C.T.; Huang, C.T.; Fang, J.Y. Cationic amphiphile in phospholipid bilayer or oil-water interface of nanocarriers affects planktonic and biofilm bacteria killing. Nanomed.-Nanotechnol. Biol. Med. 2017, 13, 353–361. [Google Scholar] [CrossRef]
- Alalaiwe, A.; Wang, P.W.; Lu, P.L.; Chen, Y.P.; Fang, J.Y.; Yang, S.C. Synergistic anti-MRSA activity of cationic nanostructured lipid carriers in combination with oxacillin for cutaneous application. Front. Microbiol. 2018, 9, 1493. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Surekha, D.B.; Tripathi, M.; Anjum, M.M.; Muthu, M.S.; Tilak, R.; Agrawal, A.K.; Singh, S. Antibiofilm potential of silver sulfadiazine-loaded nanoparticle formulations: A study on the effect of DNase-I on microbial biofilm and wound healing activity. Mol. Pharm. 2019, 16, 3916–3925. [Google Scholar] [CrossRef]
- Hassan, D.; Omolo, C.A.; Fasiku, V.O.; Mocktar, C.; Govender, T. Novel chitosan-based pH-responsive lipid-polymer hybrid nanovesicles (OLA-LPHVs) for delivery of vancomycin against methicillin-resistant Staphylococcus aureus infections. Int. J. Biol. Macromol. 2020, 147, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Riebeling, C.; Luch, A.; Tralau, T. Skin toxicology and 3Rs–Current challenges for public health protection. Exp. Dermatol. 2018, 27, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Osmond, M.J.; McCall, M.J. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15–41. [Google Scholar] [CrossRef]
- Kim, H.; Choi, J.; Lee, H.; Park, J.; Yoon, B.I.; Jin, S.M.; Park, K. Skin corrosion and irritation test of nanoparticles using reconstructed three-dimensional human skin model, EpiDermTM. Toxicol. Res. 2016, 32, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Vinardell, M.P.; Llanas, H.; Marics, L.; Mitjans, M. In vitro comparative skin irritation induced by nano and non-nano zinc oxide. Nanomaterials 2017, 7, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Wu, M.; Jiang, S.; Zhang, Y.; Li, R.; Lu, Y.; Liu, L.; Wu, G.; Liu, Y.; Xie, L.; et al. Skin toxicity assessment of silver nanoparticles in a 3D epidermal model compared to 2D keratinocytes. Int. J. Nanomed. 2019, 14, 9707–9719. [Google Scholar] [CrossRef] [Green Version]
- Roque, L.V.; Dias, I.S.; Cruz, N.; Rebelo, A.; Roberto, A.; Rijo, P.; Reis, C.P. Design of finasteride-loaded nanoparticles for potential treatment of alopecia. Skin Pharmacol. Physiol. 2017, 30, 197–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.H.; Jeon, Y.E.; Kang, S.; Lee, J.Y.; Lee, K.W.; Kim, K.T.; Kim, D.D. Lipid nanoparticles for enhancing the physicochemical stability and topical skin delivery of orobol. Pharmaceutics 2020, 12, 845. [Google Scholar] [CrossRef] [PubMed]
- Felippi, C.; Oliveira, D.; Ströher, A.; Carvalho, A.R.; Van Etten, E.A.M.A.; Bruschi, M.; Raffin, R.P. Safety and efficacy of antioxidants-loaded nanoparticles for an anti-aging application. J. Biomed. Nanotechnol. 2012, 8, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Dreher, F.; Walde, P.; Luisi, P.L.; Elsner, P. Human skin irritation studies of a lecithin microemulsion gel and of lecithin liposomes. Skin Pharmacol. 1996, 9, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Pierre, M.B.R.; dos Santos Miranda Costa, I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch. Dermatol. Res. 2011, 303, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Kuroda, E.; Hirai, T.; Tsutsumi, Y.; Ishii, K.J. Allergic responses induced by the immunomodulatory effects of nanomaterials upon skin exposure. Front. Immunol. 2017, 8, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winnicka, K.; Wroblewska, M.; Sosnowska, K.; Car, H.; Kasacka, I. Evaluation of cationic polyamidoamine dendrimers’ dermal toxicity in the rat skin model. Drug Des. Dev. Ther. 2015, 9, 1367–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| The Strategy | Classification | The Mechanism of Eradication |
|---|---|---|
| Water spray | Mechanic force | The physical removal of biofilm by local delivery of mechanic forces |
| Water-based jet | Mechanic force | The physical removal of biofilm by local delivery of mechanic forces |
| Photodynamic therapy | Physical method | The combination of specific light irradiation and photosensitizers to produce oxidative stress |
| Photothermal therapy | Physical method | The combination of near-infrared irradiation and photothermal agents to produce local hyperthermia |
| DNase | Enzymatic degradation | Hydrolysis of the extracellular DNA |
| Proteinase K | Enzymatic degradation | Cleavage of the C-terminal peptide bond for protein digestion |
| Dispersin B | Enzymatic degradation | Biofilm-releasing enzyme from Aggregatibacter actinomycetecomitans to eliminate biofilm |
| Lysostaphin | Enzymatic degradation | Cleavage of pentaglycine cross-bridge in peptidoglycan |
| Mannosides | Adhesin inhibition | Target to bacterial adhesin FimH for prevent bacterial binding to surface |
| LL-37 | Antimicrobial peptide | Prevention of bacterial attachment to surface |
| Oritavancin | Antimicrobial peptide | Prevention of bacterial attachment to surface |
| Glycerol monolaurate | Antimicrobial lipid | The amphiphile nature to disrupt biofilm structure |
| Free fatty acids | Antimicrobial lipid | The amphiphile nature to disrupt biofilm structure |
| Quaternary ammonium compounds | Surfactant | The amphiphile nature to disrupt biofilm structure |
| Autoinducing peptides | QS inhibitor | Reactivates agr QS in biofilm to disassemble biofilm |
| Hammelitannin | QS inhibitor | QS inhibitor RNAIII-inhibiting peptide to reduce S. aureus attachment |
| AP4 antibody | QS inhibitor | Biofilm inhibition in mouse abscess infection model |
| AI-2 | QS inhibitor | Reduction of proportion of adherent bacteria and dispersal |
| Nanoparticle Type | Average Size | Infection Model | The Microorganisms Tested | Antibiofilm Efficacy | Reference |
|---|---|---|---|---|---|
| Silver | 40, 70, or 140 nm | C. elegans | P. aeruginosa, S. aureus, and MRSA | Biofilm elimination by >96% | Richter et al. [114] |
| Silver | 750 nm | Full-thickness skin wound in mice | P. aeruginosa | A 8-log reduction of bacterial colony in biofilm | McLaughlin et al. [115] |
| Silver | 5−12 nm | Full-thickness skin wound in diabetic mice | P. aeruginosa and S. aureus | Bacterial number reduction in skin open wound | Lazurko et al. [116] |
| Silver | 9 nm | In vitro drip flow reactor model | P. aeruginosa and S. aureus | A 2-log reduction of bacterial colony in biofilm | Alvarado-Gomez et al. [118] |
| Silver | 8−20 nm | In vitro static biofilm assay | P. aeruginosa and S. aureus | Elimination of biomass determined by crystal violet assay | Ambrogi et al. [120] |
| Copper | 100−150 nm | 3D tissue engineered infection skin model | P. aeruginosa and S. aureus | Elimination of biomass and biofilm metabolic activity | Paterson et al. [121] |
| Copper and silver | 7 nm | Full-thickness skin wound in mice | P. aeruginosa | Biofilm area reduction by 70% | Jang et al. [122] |
| Gold | 4 nm | MRSA-infected skin wound in rats | S. aureus and MRSA | A 93% killing of bacterial number in biofilm | Yang et al. [124] |
| Gold | 10−20 nm | Full-thickness skin wound in rats | C. albicans and C. neoformans | The biofilm is disrupted, scattered, and distorted | Raghuwanshi et al. [125] |
| Zinc | 50 and 500 nm | Intradermal injection of bacteria in mice | S. aureus | The biofilm is disintegrated | Pati et al. [127] |
| Zinc | 40 nm | In vitro static biofilm assay | P. aeruginosa, S. aureus, E. faecalis, and E. coli | Biofilm growth suppression | Rayyif et al. [128] |
| Ferrous oxide with hyperthermia | About 100 nm | S. aureus-infected skin wound in mice | S. aureus | A 3-log reduction of bacterial conoly in biofilm | Kim et al. [129] |
| Gold with PPT | 14 nm | MRSA-induced abscess in rabbits | MRSA | Most of MRSA in the biofilm is killed | Hu et al. [131] |
| Bismuth-silver with PPT | 15 nm | MRSA-induced abscess in mice | MRSA | Biofilm elimination by 70% | Cao et al. [133] |
| Quantum dot with PTT | 11 nm | VISA-infected skin abscess in mice | VISA | Complete disruption of biofilm | Xu et al. [135] |
| Gold with PDT | 10−20 nm | Cutaneous infection in mice | C. albicans | A 80% killing of fungal number in biofilm | Sherwani et al. [137] |
| Zeolite with PTT | About 170 nm | VISA-infected skin abscess in mice | VISA | Biofilm elimination by 76% | Xiao et al. [138] |
| Nanoparticle Type | Average Size | Infection Model | The Microorganisms Tested | Antibiofilm Efficacy | Reference |
|---|---|---|---|---|---|
| Algal polysaccharides | About 10 nm | P. aeruginosa infection in rat skin | P. aeruginosa, S. aureus, S. mutans, and S. enterica | Biofilm elimination by 60% | El-Deeb et al. [140] |
| PLGA | 151 nm | Biofilm under the flow condition | E. coli | A 20-fold reduction of bacterial colony | Zhang et al. [141] |
| PLGA | 240 nm | Biofilm-infected skin wound in diabetic mice | MRSA | Elimination of biomass by 67% | Hasan et al. [143] |
| Hyaluronic acid | 174−194 nm | P. aeruginosa abscess model in mice | P. aeruginosa | A 4-fold reduction of bacterial colony in abscess | Kłodzińska et al. [145] |
| PLGA and chitosan | 230 nm | MRSA-infected full-thickness wound in mice | MRSA | A 80% reduction of bacterial colony in skin wound | Wu et al. [146] |
| PLGA, PCL, and chitosan | 217−263 nm | Ex vivo model of biofilm on pig skin | P. aeruginosa and S. aureus | More than 99% of bacteria is killed | Permana et al. [149] |
| PCL | 199 nm | Ex vivo model of biofilm on pig skin | P. aeruginosa, S. aureus and MRSA | A 88−100% killing of bacterial aamount | Mir et al. [150] |
| Alginate | 179 nm | Ex vivo model of biofilm on pig skin | P. aeruginosa | A reduction of bacterial viability in biofilm | Singh et al. [152] |
| Nanoparticle Type | Average Size | Infection Model | The Microorganisms Tested | Antibiofilm Efficacy | Reference |
|---|---|---|---|---|---|
| Liposomes | 111 nm | In vitro biofilm susceptibility test | S. aureus and Streptococcus epidermidis | Biofilm growth inhibition | Eroğlu et al. [155] |
| Liposomes | 55 nm | Subcutaneous infection in mouse skin | S. aureus | A 8-log reduction of bacterial colony in biofilm | Li et al. [156] |
| Nanoemulsions | Not determined | Burn wound in mouse skin | MRSA | A 84% killing of bacterial number in biofilm | Song et al. [158] |
| Nanoemulsions | 78 and 85 nm | In vitro biofilm disk assay | C. albicans | Biofilm elimination by 80% | Lewińska et al. [159] |
| Liposomes and nanoemulsions | 75 and 214 nm | Subcutaneous infection in mouse skin | S. aureus, S. epidermidis, and MRSA | A 2.4-fold reduction of biofilm thickness | Lin et al. [160] |
| NLCs | 177 nm | Subcutaneous infection in mouse skin | MRSA | A 4-log reduction of bacterial colony in abscess | Alalaiwe et al. [161] |
| SLNs | About 300 nm | Burn wound healing study in rats | P. aeruginosa | Removal of 79% of biomass | Patel et al. [162] |
| Lipid-polymer nanohybrids | 14 nm | Intradermal MRSA infection on mice | MRSA | A significant biofilm elimination determined by live/dead staining | Hassan et al. [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-K.; Yang, S.-C.; Hsu, C.-Y.; Sung, J.-T.; Fang, J.-Y. The Antibiofilm Nanosystems for Improved Infection Inhibition of Microbes in Skin. Molecules 2021, 26, 6392. https://doi.org/10.3390/molecules26216392
Lin Y-K, Yang S-C, Hsu C-Y, Sung J-T, Fang J-Y. The Antibiofilm Nanosystems for Improved Infection Inhibition of Microbes in Skin. Molecules. 2021; 26(21):6392. https://doi.org/10.3390/molecules26216392
Chicago/Turabian StyleLin, Yin-Ku, Shih-Chun Yang, Ching-Yun Hsu, Jui-Tai Sung, and Jia-You Fang. 2021. "The Antibiofilm Nanosystems for Improved Infection Inhibition of Microbes in Skin" Molecules 26, no. 21: 6392. https://doi.org/10.3390/molecules26216392
APA StyleLin, Y.-K., Yang, S.-C., Hsu, C.-Y., Sung, J.-T., & Fang, J.-Y. (2021). The Antibiofilm Nanosystems for Improved Infection Inhibition of Microbes in Skin. Molecules, 26(21), 6392. https://doi.org/10.3390/molecules26216392






