Evaluation of the Inhibitory Effects of Pyridylpyrazole Derivatives on LPS-Induced PGE2 Productions and Nitric Oxide in Murine RAW 264.7 Macrophages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
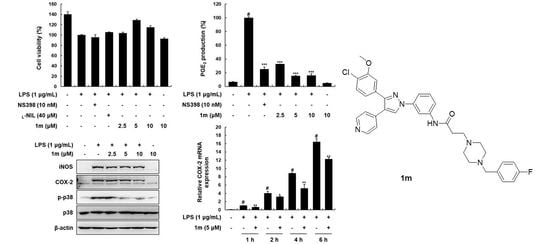
2.2. Biological Evaluation
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Experimental
Conflicts of Interest
Sample Availability
References
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860. [Google Scholar] [CrossRef] [PubMed]
- Qui, H.; Johansson, A.S.; Sjostrom, M.; Wan, M.; Schroder, O.; Palmbald, J.; Haeggstrom, J.Z. Differential induction of BLT receptor expression on human endothelial cells by lipopolysaccharide, cytokines, and leukotriene B4. Proc. Natl. Acad. Sci. USA 2006, 103, 6913. [Google Scholar]
- Sung, B.; Prasad, S.; Yadav, V.R.; Lavasanifar, A.; Aggarwal, B.B. Cancer and diet: How are they related? Free Radical Res. 2011, 45, 864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, I.-A.; Bae, E.-A.; Hyun, Y.-J.; Kim, D.-H. Dextran sulfate sodium and 2,4,6-trinitrobenzene sulfonic acid induce lipid peroxidation by the proliferation of intestinal gram-negative bacteria in mice. J. Inflamm. 2010, 7, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochberg, M.C. Semin. Changes in the incidence and prevalence of rheumatoid arthritis in England and Wales, 1970-1982. Arthritis Rheum. 1990, 19, 294. [Google Scholar] [CrossRef]
- Sastre, M.; Richardson, J.C.; Gentleman, S.M.; Brooks, D.J. Inflammatory risk factors and pathologies associated with Alzheimer’s disease. Curr. Alzheimer Res. 2011, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Brune, K.J. Cyclooxygenase-2–10 years later. Pharmacol. Exp. Ther. 2002, 300, 367. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Batchu, S.N.; Kaur, J.; Huang, Z.; Seubert, J.M.; Knaus, E.E. Cardiovascular properties of a nitric oxide releasing rofecoxib analogue: beneficial anti-hypertensive activity and enhanced recovery in an ischemic reperfusion injury model. ChemMedChem 2012, 7, 1365. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Huang, Z.; Kaur, J.; Knaus, E.E. Rofecoxib analogues possessing a nitric oxide donor sulfohydroxamic acid (SO2NHOH) cyclooxygenase-2 pharmacophore: synthesis, molecular modeling, and biological evaluation as anti-inflammatory agents. ChemMedChem 2012, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Bhardwaj, A.; Huang, Z.; Knaus, E.E. Aspirin analogues as dual cyclooxygenase-2/5-lipoxygenase inhibitors: synthesis, nitric oxide release, molecular modeling, and biological evaluation as anti-inflammatory agents. ChemMedChem 2011, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.Y.; Dawson, V.L.; Dawson, T.M. Neurobiology of nitric oxide. Crit. Rev. Neurobiol. 1996, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.K.; Kaur, N.; Bansal, Y.; Bansal, G. Novel coumarin-benzimidazole derivatives as antioxidants and safer anti-inflammatory agents. Acta Pharm. Sin. B. 2014, 4, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azelmat, J.; Fiorito, S.; Taddeo, V.A.; Genovese, S.; Epifano, F.; Grenier, D. Synthesis and evaluation of antibacterial and anti-inflammatory properties of naturally occurring coumarins. Phytochem. Lett. 2015, 13, 399. [Google Scholar] [CrossRef]
- Wei, W.; Wu, X.-W.; Deng, G.-G.; Yang, X.-W. Anti-inflammatory coumarins with short- and long-chain hydrophobic groups from roots of Angelica dahurica cv. Hangbaizhi. Phytochemistry 2016, 123, 58. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Vyas, V.K.; Variya, B.; Patel, P.; Qureshi, G.; Ghate, M. Synthesis, anti-inflammatory, analgesic, 5-lipoxygenase (5-LOX) inhibition activities, and molecular docking study of 7-substituted coumarin derivatives. Bioorg. Chem. 2016, 67, 130. [Google Scholar] [CrossRef] [PubMed]
- Gamal El-Din, M.M.; El-Gamal, M.I.; Abdel-Maksoud, M.S.; Yoo, K.H.; Oh, C.-H. Design, synthesis, in vitro potent antiproliferative activity, and kinase inhibitory effects of new triarylpyrazole derivatives possessing different heterocycle terminal moieties. J. Enz. Inhibit. Med. Chem. 2019, 34, 1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Gamal, M.I.; Abdel-Maksoud, M.S.; Gamal El-Din, M.M.; Shin, J.-S.; Lee, K.-T.; Yoo, K.H.; Oh, C.-H. Synthesis, in vitro Antiproliferative and Antiinflammatory Activities, and Kinase Inhibitory effects of New 1,3,4-triarylpyrazole Derivatives. Anti-Cancer Agents Med. Chem. 2017, 17, 75. [Google Scholar] [CrossRef]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enz. Inhibit. Med. Chem. 2007, 22, 527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madkour, M.M.; Anbar, H.S.; El-Gamal, M.I. Current status and future prospects of p38α/MAPK14 kinase and its inhibitors. Eur. J. Med. Chem. 2021, 213, 113216. [Google Scholar] [CrossRef] [PubMed]






| Compound No. | n | R | Cell Viability (%) | |
|---|---|---|---|---|
| 1 μM a | 10 μM a | |||
| 1a | 1 |  | 99 ± 5.9 | 10 ± 1.2 |
| 1b | 1 |  | 92 ± 4.5 | 65 ± 2.8 |
| 1c | 1 |  | 78 ± 3.3 | 5 ± 0.8 |
| 1d | 1 |  | 75 ± 2.9 | 3 ± 0.4 |
| 1e | 1 |  | 87 ± 4.1 | 65 ± 4.1 |
| 1f | 1 |  | 66 ± 1.8 | 107± 8.5 |
| 1g | 2 |  | 87 ± 3.8 | 5 ± 1.1 |
| 1h | 2 |  | 99 ± 2.8 | 75 ± 1.7 |
| 1i | 2 |  | 99 ± 3.4 | 5 ± 1.8 |
| 1j | 2 |  | 98 ± 3.1 | 5 ± 0.8 |
| 1k | 2 |  | 89 ± 2.8 | 112 ± 7.6 |
| 1l | 2 |  | 86 ± 3.7 | 34 ± 1.1 |
| 1m | 2 |  | 90 ± 4.1 | 97 ± 3.9 |
| Compound | Inhibition Rate (%) | |
|---|---|---|
| 1 (μM) | 10 (μM) | |
| 1f | 0 | 11.06 ± 1.5 |
| 1m | 0 | 37.19 ± 3.4 |
| L-NIL | 3.9 ± 2.1 | 31.32 ± 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamal El-Din, M.M.; El-Gamal, M.I.; Kwon, Y.-D.; Kim, S.-Y.; Han, H.-S.; Park, S.-E.; Oh, C.-H.; Lee, K.-T.; Kim, H.-K. Evaluation of the Inhibitory Effects of Pyridylpyrazole Derivatives on LPS-Induced PGE2 Productions and Nitric Oxide in Murine RAW 264.7 Macrophages. Molecules 2021, 26, 6489. https://doi.org/10.3390/molecules26216489
Gamal El-Din MM, El-Gamal MI, Kwon Y-D, Kim S-Y, Han H-S, Park S-E, Oh C-H, Lee K-T, Kim H-K. Evaluation of the Inhibitory Effects of Pyridylpyrazole Derivatives on LPS-Induced PGE2 Productions and Nitric Oxide in Murine RAW 264.7 Macrophages. Molecules. 2021; 26(21):6489. https://doi.org/10.3390/molecules26216489
Chicago/Turabian StyleGamal El-Din, Mahmoud M., Mohammed I. El-Gamal, Young-Do Kwon, Su-Yeon Kim, Hee-Soo Han, Sang-Eun Park, Chang-Hyun Oh, Kyung-Tae Lee, and Hee-Kwon Kim. 2021. "Evaluation of the Inhibitory Effects of Pyridylpyrazole Derivatives on LPS-Induced PGE2 Productions and Nitric Oxide in Murine RAW 264.7 Macrophages" Molecules 26, no. 21: 6489. https://doi.org/10.3390/molecules26216489
APA StyleGamal El-Din, M. M., El-Gamal, M. I., Kwon, Y. -D., Kim, S. -Y., Han, H. -S., Park, S. -E., Oh, C. -H., Lee, K. -T., & Kim, H. -K. (2021). Evaluation of the Inhibitory Effects of Pyridylpyrazole Derivatives on LPS-Induced PGE2 Productions and Nitric Oxide in Murine RAW 264.7 Macrophages. Molecules, 26(21), 6489. https://doi.org/10.3390/molecules26216489