Biomass Valorization Using Natural Deep Eutectic Solvents: What’s New in France?
Abstract
:1. Introduction
2. Methodology
3. NaDES Design and Physicochemical Characterization
3.1. Computational Theoretical Tools
3.1.1. COSMO-RS
3.1.2. Hansen Solubility Parameters
3.2. NaDES Physicochemical Properties Study
3.2.1. Phase Diagram
3.2.2. Mass Spectrometry
3.2.3. Water Quantification: Karl Fischer (KF) Titration and Vibrational Spectroscopy
3.2.4. Impact of NaDES Physical Properties on Biomass Valorization
4. Biomass Pre-Treatment and Modification
5. Extraction
5.1. Biomass Type
5.1.1. Higher Plant
5.1.2. Microorganism
5.1.3. By-Product
5.2. Extraction Technique
5.2.1. Ultrasound Assisted NaDES Extraction (UAE)
5.2.2. Microwave Assisted NaDES Extraction (MAE)
5.2.3. Miscellaneous
6. Post-Extraction Step
6.1. Biological Evaluation
6.2. Toxicity Evaluation
6.3. Formulation
6.3.1. Bio-Compounds Encapsulation
6.3.2. Volatiles Organic Compounds Encapsulation
6.3.3. Drug Formulation
6.3.4. Enzyme Formulation
7. Industrial Development
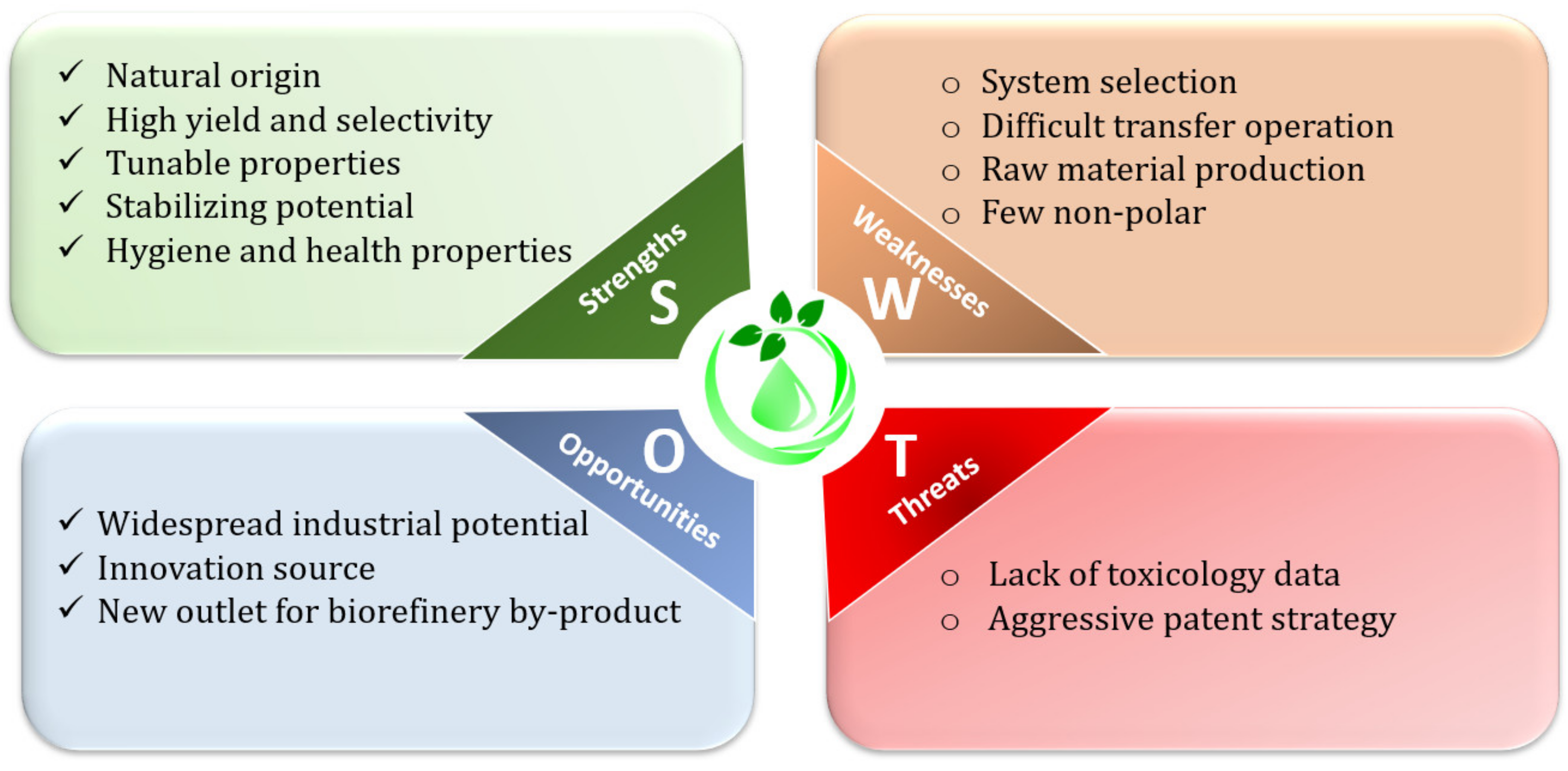
8. Strengths, Weaknesses, Opportunities and Threats (SWOTs) Analysis
9. Conclusions
Funding
Conflicts of Interest
References
- Li, Y.; Kunz, W.; Chemat, F. From Petroleum to Bio-Based Solvents: From Academia to Industry. In Plant Based “Green Chemistry 2.0”: Green Chemistry and Sustainable Technology; Li, Y., Chemat, F., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- De Los Angeles Fernandez, M.; Boiteux, J.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal. Chim. Acta 2018, 1038, 1–10. [Google Scholar] [CrossRef]
- Yang, Z. Natural Deep Eutectic Solvents and Their Applications in Biotechnology. In Application of Ionic Liquids in Biotechnology, 1st ed.; Itoh, T., Koo, Y.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 168, pp. 31–59. [Google Scholar] [CrossRef]
- Atilhan, M.; Aparicio, S. Review and Perspectives for Effective Solutions to Grand Challenges of Energy and Fuels Technologies via Novel Deep Eutectic Solvents. Energy Fuels 2021, 35, 6402–6419. [Google Scholar] [CrossRef]
- Chang, S.H. Utilization of green organic solvents in solvent extraction and liquid membrane for sustainable wastewater treatment and resource recovery—A review. Environ. Sci Pollut Res. 2020, 27, 32371–32388. [Google Scholar] [CrossRef] [PubMed]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef]
- Caprin, B.; Charton, V.; Vogelgesang, B. The use of NADES to support innovation in the cosmetic industry. In Advances in Botanical Research, 1st ed.; Verpoorte, R., Witkamp, G.-J., Choi, Y.H., Eds.; Elsevier: London, UK, 2021; Volume 97, pp. 309–332. [Google Scholar] [CrossRef]
- Balakrishnan, I.; Venkatachalam, S.; Datta, D.; Jawahar, N. A brief review on eutectic mixture and its role in pharmaceutical field. Int. J. Res. Pharm. Sci. 2020, 11, 3017–3023. [Google Scholar] [CrossRef]
- Abo-Hamad, A.; Hayyan, M.; Al Saadi, M.A.; Hashim, M.A. Potential applications of deep eutectic solvents in nanotechnology. Chem. Eng. J. 2015, 273, 551–567. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.-H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Parikh, B.S.; Liu, L.Z.; Cotta, M.A. Application of Natural Deep Eutectic Solvents in Biomass Pretreatment, Enzymatic Saccharification and Cellulosic Ethanol Production. Mater. Today Proc. 2018, 5, 23057–23063. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Dietz, C.H.J.T.; van Spronsen, J.; Kroon, M.C.; Gallucci, F.; van Sint Annaland, M.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Florindo, C.; Romero, L.; Rintoul, I.; Branco, L.C.; Marrucho, I.M. From Phase Change Materials to Green Solvents: Hydrophobic Low Viscous Fatty Acid–Based Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2018, 6, 3888–3895. [Google Scholar] [CrossRef]
- Wils, L.; Leman-Loubière, C.; Bellin, N.; Clément-Larosiére, B.; Pinault, M.; Chevalier, S.; Enguehard-Gueiffier, C.; Bodet, C.; Boudesocque-Delaye, L. Natural deep eutectic solvent formulations for spirulina: Preparation, intensification, and skin impact. Algal Res. 2021, 56, 102317. [Google Scholar] [CrossRef]
- La Biomasse Agricole: Quelles Ressources pour Quell Potentiel Énergétique? Available online: https://www.methaherbauges-corcoue.fr/wp-content/uploads/2021/09/14/fs-dt-biomasse-agricole-quelles-ressources-pour-quel-potentiel-energetique-29-07-21.pdf (accessed on 13 October 2021).
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Milani, G.; Vian, M.; Cavalluzzia, M.M.; Franchinia, C.; Corboa, F.; Lentinia, G.; Chemat, F. Ultrasound and deep eutectic solvents: An efficient combination to tune the mechanism of steviol glycosides extraction. Ultrason. Sonochem. 2020, 69, 105255–105266. [Google Scholar] [CrossRef] [PubMed]
- Chagnoleau, J.-B.; Papaiconomou, N.; Jamali, M.; Abranches, D.O.; Coutinho, J.A.P.; Fernandez, X.; Michel, T. Toward a Critical Evaluation of DES-Based Organic Biphasic Systems: Are Deep Eutectic Solvents so Critical? ACS Sustain. Chem. Eng. 2021, 9, 9707–9716. [Google Scholar] [CrossRef]
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green extraction of polyphenols from grapefruit peels using high voltage electrical discharges, deep eutectic solvents and aqueous glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Lecomte, J.; Villeneuve, P. From green chemistry to nature: The versatile role of low transition temperature mixtures. Biochimie 2016, 120, 119–123. [Google Scholar] [CrossRef]
- Guinet, Y.; Paccou, L.; Hédoux, A. Analysis of xylitol–citric acid system forming deep eutectic solvent: Application for dissolving poorly water-soluble drugs. A combination of calorimetric and Raman investigations. J. Mol. Liquid. 2020, 318, 114317–114325. [Google Scholar] [CrossRef]
- Percevault, L.; Delhaye, T.; Chaumont, A.; Schurhammer, R.; Paquin, L.; Rondeau, D. Cold-spray ionization mass spectrometry of the choline chloride-urea deep eutectic solvent (reline). J. Mass Spectrom. 2021, 56, e4725. [Google Scholar] [CrossRef] [PubMed]
- Elderderi, S.; Leman-Loubière, C.; Wils, L.; Henry, S.; Bertrand, D.; Byrne, H.J.; Chourpa, I.; Enguehard-Gueiffier, C.; Munniera, E.; Elbashi, A.A.; et al. ATR-IR spectroscopy for rapid quantification of water content in deep eutectic solvents. J. Mol. Liquid. 2020, 311, 113361–113370. [Google Scholar] [CrossRef]
- Elderderi, S.; Wils, L.; Leman-Loubière, C.; Henry, S.; Bertrand, D.; Byrne, H.J.; Chourpa, I.; Munniera, E.; Elbashi, A.A.; Boudesocque-Delaye, L.; et al. Comparison of Raman and attenuated total reflectance (ATR) infrared spectroscopy for water quantification in natural deep eutectic solvent. Anal. Bioanal. Chem. 2021, 413, 4785–4799. [Google Scholar] [CrossRef] [PubMed]
- Achkar, T.E.; Moufawad, T.; Achkar, T.E.; Ruellan, S.; Landy, D.; Greige-Gerges, H.; Fourmentin, S. Cyclodextrins: From solute to solvent. Chem. Commun. 2020, 56, 3385–3388. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, K.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M. Prediction of deep eutectic solvents densities at different temperatures. Thermochim. Acta 2011, 515, 67–72. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.Z.; Marrucho, I.M. Menthol-based Eutectic Mixtures: Hydrophobic Low Viscosity Solvents. ACS Sustain. Chem. Eng. 2015, 3, 2469–2477. [Google Scholar] [CrossRef]
- Caprin, B.; Charton, V.; Rodier, J.-D.; Vogelgesang, B.; Charlot, A.; Da Cruz-Boisson, F.; Fleury, E. Scrutiny of the supramolecular structure of bio-sourced fructose/glycerol/water ternary mixtures: Towards green low transition temperature mixtures. J. Mol. Liquid. 2021, 337, 116428. [Google Scholar] [CrossRef]
- Chen, M.; Lahaye, M. Natural deep eutectic solvents pretreatment as an aid for pectin extraction from apple pomace. Food Hydrocoll. 2021, 115, 106601–106612. [Google Scholar] [CrossRef]
- Chen, M.; Falourd, X.; Lahaye, M. Sequential natural deep eutectic solvent pretreatments of apple pomace: A novel way to promote water extraction of pectin and to tailor its main structural domains. Carbohydr. Polym. 2021, 266, 118113–118126. [Google Scholar] [CrossRef] [PubMed]
- Huet, G.; Hadad, C.; Gonzàlez-Domínguez, J.M.; Courty, M.; Jamali, A.; Cailleu, D.; van Nhien, A.N. IL versus DES: Impact on chitin pretreatment to afford high quality and highly functionalizable chitosan. Carbohydr. Polym. 2021, 269, 118332–118343. [Google Scholar] [CrossRef] [PubMed]
- Chemat, M.; Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano-Tixier, A.-S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delavault, A.; Ochs, K.; Gorte, O.; Syldatk, C.; Durand, E.; Ochsenreither, K. Microwave-Assisted One-Pot Lipid Extraction and Glycolipid Production from Oleaginous Yeast Saitozyma podzolica in Sugar Alcohol-Based Media. Molecules 2021, 26, 470. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.B.; Barouh, N.; Durand, E.; Baréa, B.; Robert, M.; Micard, V.; Lullien-Pellerin, V.; Villeneuve, P.L.; Ferreira, M.S.L.; Bourlieu-Lacanal, C. Metabolomics of Pigmented Rice Coproducts Applying Conventional or Deep Eutectic Extraction Solvents Reveal a Potential Antioxidant Source for Human Nutrition. Metabolites 2021, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Ruesgas-Ramón, M.; Suárez-Quiroz, M.L.; González-Ríos, O.; Baréa, B.; Cazals, G.; Figueroa-Espinoza, M.C.; Durand, E. Biomolecules extraction from coffee and cocoa by- and co-products using deep eutectic solvents. J. Sci. Food Agric. 2020, 100, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Nuutinen, E.-M.; Willberg-Keyriläinen, P.; Virtanen, T.; Mija, A.; Kuutti, L.; Lanttoa, R.; Jääskeläinena, A.-S. Green process to regenerate keratin from feathers with an aqueous deep eutectic solvent. RSC Adv. 2019, 9, 19720–19728. [Google Scholar] [CrossRef] [Green Version]
- Douard, L.; Bras, J.; Encinas, T.; Belgacem, M.N. Natural acidic deep eutectic solvent to obtain cellulose nanocrystals using the design of experience approach. Carbohydr. Polym. 2021, 252, 117136. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Bi, W.; Zhang, H.; Row, K.H. Deep Eutectic Solvent-Based HS-SME Coupled with GC for the Analysis of Bioactive Terpenoids in Chamaecyparis obtusa Leaves. Chromatographia 2014, 77, 373–377. [Google Scholar] [CrossRef]
- Miličević, N.; Panić, M.; Valinger, D.; Bubalo, M.C.; Benković, M.; Jurina, T.; Kljusurić, J.G.; Radojčić Redovniković, I.; Tušek, A.J. Development of continuously operated aqueous two-phase microextraction process using natural deep eutectic solvents. Sep. Purif. Technol. 2020, 244, 116746–116754. [Google Scholar] [CrossRef]
- Triaux, Z.; Petitjean, H.; Marchioni, E.; Boltoeva, M.; Marcic, C. Deep eutectic solvent–based headspace single-drop microextraction for the quantification of terpenes in spices. Anal. Bioanal. Chem. 2020, 412, 933–948. [Google Scholar] [CrossRef] [PubMed]
- Basar, A.O.; Prieto, C.; Durand, E.; Villeneuve, P.; Sasmazel, H.T.; Lagaron, J. Encapsulation of β-Carotene by Emulsion Electrospraying Using Deep Eutectic Solvents. Molecules 2020, 25, 981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pietro, M.E.; Dugoni, G.C.; Ferro, M.; Mannu, A.; Castiglione, F.; Costa Gomes, M.; Fourmentin, S.; Mele, A. Do Cyclodextrins Encapsulate Volatiles in Deep Eutectic Systems? ACS Sustain. Chem. Eng. 2019, 7, 17397–17405. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Benlebna, M.; Ruesgas-Ramon, M.; Bonafos, B.; Fouret, G.; Casas, F.; Coudray, C.; Durand, E.; Cruz Figueroa-Espinoza, M.; Feillet-Coudray, C. Toxicity of Natural Deep Eutectic Solvent Betaine:Glycerol in Rats. J. Agric. Food Chem. 2018, 66, 6205–6212. [Google Scholar] [CrossRef] [PubMed]
- Shamseddin, A.; Crauste, C.; Durand, E.; Villeneuve, P.; Dubois, G.; Durand, T.; Vercauteren, J.; Veas, F. Resveratrol formulated with a natural deep eutectic solvent inhibits active matrix metalloprotease-9 in hormetic conditions: Resveratrol NADES formulation and hormesis. Eur. J. Lipid Sci. Technol. 2017, 119, 1700171–1700180. [Google Scholar] [CrossRef] [Green Version]
- Durand, E.; Lecomte, J.; Upasani, R.; Chabi, B.; Bayrasy, C.; Baréa, B.; Jublanc, E.; Clarke, M.J.; Moore, D.J.; Crowther, J.; et al. Evaluation of the ROS Inhibiting Activity and Mitochondrial Targeting of Phenolic Compounds in Fibroblast Cells Model System and Enhancement of Efficiency by Natural Deep Eutectic Solvent (NADES) Formulation. Pharm. Res. 2017, 34, 1134–1146. [Google Scholar] [CrossRef]
- Durand, E.; Villeneuve, P.; Bourlieu-lacanal, C.; Carrière, F. Natural deep eutectic solvents: Hypothesis for their possible roles in cellular functions and interaction with membranes and other organized biological systems. In Advances in Botanical Research, 1st ed.; Verpoorte, R., Witkamp, G.-J., Choi, Y.H., Carrière, F., Eds.; Elsevier: London, UK, 2021; Volume 97, pp. 133–158. [Google Scholar] [CrossRef]
- Delorme, A.E.; Andanson, J.-M.; Verney, V. Improving laccase thermostability with aqueous natural deep eutectic solvents. Int. J. Biol. Macromol. 2020, 163, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.W.; Ng, T.B.; Deng, X.; Lin, J.; Ye, X. Laccases: Production, Expression Regulation, and Applications in Pharmaceutical Biodegradation. Front. Microbiol. 2017, 8, 1–24. [Google Scholar] [CrossRef]
- Base de Formulation Cosmétique d’Origine Naturelle ou Végétale et Composition Cosmétique FR 3 017 292-A1. Available online: https://patents.google.com/patent/FR3017292A1/fr (accessed on 15 July 2021).
- Utilisation Cosmétique d’un Solvant Eutectique pour Améliorer l’Aspect de la Peau FR 3 046 352-A1. Available online: https://patents.google.com/patent/FR3046352A1/en (accessed on 15 July 2021).
- Cosmetic Use of Vegetable Ivory Extract (Phytelephas sp.) WO 2019/081859-A1. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019081859 (accessed on 15 July 2021).
- Cosmetic Use of Syringa vulgaris L. Meristematic Cells for a Soothing and/or Softening Action on the Skin WO 2019/063929 A1. Available online: https://worldwide.espacenet.com/patent/search/family/061027844/publication/WO2019063929A1?q=WO%202019%2F063929%20A1 (accessed on 15 July 2021).
- Sennelier, B.; Laugier-Cassin, F.; Mervoyer, C. Cosmetic Use of Syringa vulgaris L. Meristematic Cells for Anti-Ageing Action on the Skin WO 2019/063927-A1. 2019. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019063927 (accessed on 15 July 2021).
- Laperdrix, C.; Lubrano, C.; Portet, B. Utilisation d’un Extrait de Réglisee (Glycyrrhiza glabra L.) pour une Action d’Hydratation de la Peau, de ses Annexes ou des Muqueuses FR 3 042 415-A1. 2017. Available online: https://patentimages.storage.googleapis.com/96/d5/60/a6449402bc74da/FR3042415A1.pdf (accessed on 15 July 2021).
- Extraits Végétaux Destinés à la Cosmétique, Solvants et Procédés pour les Obtenir FR 3036618 A1. Available online: https://worldwide.espacenet.com/patent/search/family/053524883/publication/FR3036618A1?q=FR%203036618%20A1 (accessed on 15 July 2021).
- Caprin, B.; Charton, V.; Demargne, F. Solvants Eutectiques pour la Dissolution de Stilbenoides ou Leurs Dérivés FR 3068352-A1. 2019. Available online: https://patents.google.com/patent/FR3036618A1/fr (accessed on 15 July 2021).
- Extraits de Withania Somnifera Pour Lutter Contre les Effets Nocifs des Rayonnements Visibles sur la Peau FR 3067939 A1. Available online: https://worldwide.espacenet.com/patent/search/family/060138461/publication/FR3067939A1?q=FR%203067939%20A1 (accessed on 15 July 2021).
- Lavaud, A.; Laguerre, M.; Birtic, S.; Fabiano-Tixier, A.S.; Roller, M.; Chemat, F.; Bily, A.C. Solvant Eutectique d’Extraction, Procédé d’Extraction par Eutectigénèse Utilisant Ledit Solvant, et Extrait Issu Dudit Procédé FR 3034625-A1. 2016. Available online: https://patents.google.com/patent/FR3034625A1/fr (accessed on 15 July 2021).
- Laguerre, M.; Harris, R.; Lavaud, A.; Tenon, M.; Birtic, S.; Bily, A.C.; Abbott, A.P. Eutectic Extract Formation and Purification WO 2019/219774-A2. 2019. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019219774 (accessed on 15 July 2021).
- Extraits d’Helichrysum italicum Obtenus au Moyen de Solvants Eutectiques Profonds FR 3067604-A1. Available online: https://worldwide.espacenet.com/patent/search/family/057349046/publication/FR3067604A1?q=FR%203067604%20A1 (accessed on 15 July 2021).
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y. Natural Deep Eutectic Solvents and Their Application in Natural Product Research and Development. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 2013. [Google Scholar]



| NaDES | Ratio | Used Processes | Matrix | Metabolite | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Component 1 | Component 2 | Component 3 | ||||||
| Pre-treatement | Choline Chloride | lactic acid | - | 1:2 | stirring and heating | apple pomace | pectine | [33] |
| urea | - | 1:2 | ||||||
| oxalic acid | - | 1:2 | ||||||
| Choline Chloride | lactic acid | - | 1:2 | NaDES extraction followed by EtOH and acetone wash | apple pomace | pectine | [32] | |
| Glycerol | - | 1:2 | ||||||
| Choline Chloride | lactic acid | - | 1:3 | high voltage electrical discharges | grapefruit peels | polyphenols (naringin) | [22] | |
| tartaric acid | - | 1:3 | ||||||
| Glucose | Lactic acid | - | 1:5 | |||||
| Glycine | Lactic acid | - | 1:3 | |||||
| Extraction | Betaine | Glycerol | - | 1:2, 1:4, 1:8 | ultrasound-assisted extraction | spirulina | fatty acids and pigments | [17] |
| Glucose | Glycerol | - | 1:2, 1:3, 1:4, 1:5 | |||||
| Glycerol | Water | 1:2:2, 1:2:4 | ||||||
| Glycerol | Betaine | 1:2:4 | ||||||
| Lactic acid | Betaine | - | 2:1 | |||||
| Glycerol | - | 1:1 | ||||||
| Menthol | Lactic acid | - | 1:2 | |||||
| Levulinic acid | - | 1:2 | ||||||
| Octanoic acid | - | 1:1 | ||||||
| Octanoic acid | Lauric acid | - | 3:1 | |||||
| Nonanoic acid | Lauric acid | - | 3:1 | |||||
| Nonanoic acid | Decanoic acid | Lauric acid | 3:2:1 | |||||
| Choline Chloride | Lactic acid | Water | 1:2:1.5 | ultrasound-assisted extractionstirring and heating extraction | coffee and cocoaby- and co-products | gallic acid, chlorogenic acid, caffeine, theobromine, furfural | [39] | |
| Glycerol | 1:2:1.5 | |||||||
| 1,4-Butanediol | 1:2:1.1 | |||||||
| Betaine | Lactic acid | 1:2:1.5 | ||||||
| Glycerol | 1:2:1 | |||||||
| 1,4-Butanediol | 1:2:1 | |||||||
| Choline Chloride | Arabinose | - | 1:1 | microwave-one pot assisted extraction | yeast Saitozyma podzolica | lipid extraction and glycolipid production | [37] | |
| Glucose | - | 2:1 | ||||||
| Urea | - | 1:2 | ||||||
| Glycerol | - | 1:2 | ||||||
| 1,2-Propanediol | - | 1:1 | ||||||
| Saccharose | - | 4:1 | ||||||
| Xylitol | - | 1:1 | ||||||
| Sorbitol | - | 1:1 | ||||||
| Betaine | Glycerol | - | 1:2 | |||||
| 1,4-Butanediol | - | 1:4 | ||||||
| Choline Chloride | 1.2-propanediol | Water | 1:1:1 | orbital agitation | rice co-products | phenolic compounds | [38] | |
| Lactic acid | - | 1:10 | ||||||
| Choline Chloride | Oxalic acid | - | 1:1 | glass reactor | cotton fiber | cellulose nanocrystals | [41] | |
| Microextraction | Choline Chloride | Urea | - | 1:2 | single-drop microextraction | cinnamon, cumin, fennel, clove, thyme, and nutmeg | terpenes | [44] |
| Lactic acid | - | 2:3 | ||||||
| Encapsulation | Choline Chloride | propanediol | water | 1:1:1 | encapsulation by emulsion electrospraying in whey protein concentrate | - | β-carotene | [45] |
| Glucose | 5:2:5 | |||||||
| Glycerol | - | 1:2 | ||||||
| butanediol | - | 1:2 | ||||||
| Choline Chloride | Urea | Cyclodextrin | 1:2 (10%) | - | volatile compounds | [46] | ||
| Company | Patent | NaDES Raw Materials | Biomass/Metabolites | Market | Ref |
|---|---|---|---|---|---|
| Yves Rocher | FR 3017292-A1 | Hexoses Organic acids Polyols Aminoacids | active cosmetic ingredient | Cosmetics | [54] |
| FR 3046352-A | [55] | ||||
| WO 2019/081859-A1 | Phytelephas sp. | [56] | |||
| WO 2019/063929-A1 | Syringa vulgaris | [57] | |||
| WO 2019/063927-A1 | [58] | ||||
| FR 3042415-A1 | Glycyrrhiza glabra | [59] | |||
| Gattefossé | FR 3036618-A1 | Sugar Polyols Aminoacids (Betain) | Plants | Cosmetics | [60] |
| FR 3068352-A1 | Stilbenoids | [61] | |||
| FR 3067939-A1 | Fructose/Glycerol/water | Withania somnifera | [62] | ||
| Givaudan-Naturex | FR 3034625-A1 | Betain Polyols Organic acids | plant, animal, procaryote | Cosmetic Food Pharmaceutic | [63] |
| FR 3049864-A1 | Aerva sp. | [64] | |||
| WO 2019/219774-A2 | Exogenic amine | plant, animal, procaryote | Food Pharmaceutic | [65] | |
| Laboratoires M&L | FR 3067604-A1 | Organic acids Sugars Polyols Choline salts Aminoacids | Helichrysum italicum | Cosmetics | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wils, L.; Hilali, S.; Boudesocque-Delaye, L. Biomass Valorization Using Natural Deep Eutectic Solvents: What’s New in France? Molecules 2021, 26, 6556. https://doi.org/10.3390/molecules26216556
Wils L, Hilali S, Boudesocque-Delaye L. Biomass Valorization Using Natural Deep Eutectic Solvents: What’s New in France? Molecules. 2021; 26(21):6556. https://doi.org/10.3390/molecules26216556
Chicago/Turabian StyleWils, Laura, Soukaina Hilali, and Leslie Boudesocque-Delaye. 2021. "Biomass Valorization Using Natural Deep Eutectic Solvents: What’s New in France?" Molecules 26, no. 21: 6556. https://doi.org/10.3390/molecules26216556
APA StyleWils, L., Hilali, S., & Boudesocque-Delaye, L. (2021). Biomass Valorization Using Natural Deep Eutectic Solvents: What’s New in France? Molecules, 26(21), 6556. https://doi.org/10.3390/molecules26216556







