Anti-Cancer Properties of Ginkgolic Acids in Human Nasopharyngeal Carcinoma CNE-2Z Cells via Inhibition of Heat Shock Protein 90
Abstract
:1. Introduction
2. Results
2.1. GAS Inhibit Hsp90 ATPase Activity
2.2. GAS Exhibit an Anti-Proliferative Activity in CNE-2Z Cells
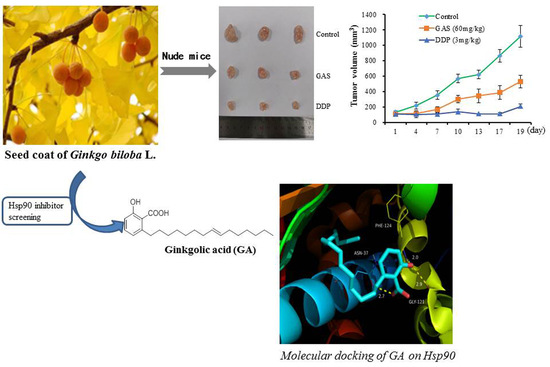
2.3. In Vivo Anti-Tumor Efficacy of GAS
2.4. GAS Suppress Migration and Invasion of CNE-2Z Cells
2.5. GAS Induce Apoptosis in CNE-2Z Cells
2.6. Molecular Docking of GA (15:1) on Hsp90
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Regents
4.3. Determination of Hsp90 ATPase Activity
4.4. Cell Lines and Cell Cultures
4.5. Determination of Cell Viability
4.6. Colony-Formation Assay
4.7. In Vivo Anti-Tumor Experiments
4.8. Wound-Healing Assay
4.9. Cell Migration Assay
4.10. Cell Invasion Assay
4.11. Western Blotting
4.12. Flow Cytometry with Annexin V/ PI Staining
4.13. DAPI Staining
4.14. Molecular Docking
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cao, S.M.; Xu, Y.J.; Lin, G.Z.; Huang, Q.H.; Wei, K.R.; Xie, S.H.; Liu, Q. Estimation of cancer burden in Guangdong Province, China in 2009. Chin. J. Cancer. 2015, 34, 594–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal carcinoma. Lancet. 2016, 387, 1012–1024. [Google Scholar] [CrossRef]
- Lee, A.W.; Ma, B.B.; Ng, W.T.; Chan, A.T. Management of nasopharyngeal carcinoma: Current practice and future perspective. J. Clin. Oncol. 2015, 33, 3356–3364. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, L.; Zhang, Y.; Li, W.F.; Mao, Y.P.; Liu, X.; Zhang, F.; Guo, R.; Liu, L.Z.; Tian, L.; et al. The tumour response to induction chemotherapy has prognostic value for long-term survival outcomes after intensity-modulated radiation therapy in nasopharyngeal carcinoma. Sci. Rep. 2016, 6, 24835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitesell, L.; Lindquist, S.L. Hsp90 and the chaperoning of cancer. Nat. Rev. Cancer. 2005, 5, 761–772. [Google Scholar] [CrossRef]
- Fen, J.; Xie, G.; Zhan, Y.; Lu, J.; Xu, L.; Fan, S.; Wang, W. Elevated Hsp90 associates with expression of HIF-1α and p-Akt and is predictive of poor prognosis in nasopharyngeal carcinoma. Histopathology. 2019, 75, 202–212. [Google Scholar]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the dynamic Hsp90 complex in cancer. Nat. Rev. Cancer. 2010, 10, 537–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backe, S.J.; Sager, R.A.; Woodford, M.R.; Makedon, A.M.; Mollapour, M. Post-translational modifications of Hsp90 and translating the chaperone code. J. Biol. Chem. 2020, 295, 11099–11117. [Google Scholar] [CrossRef]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The Hsp90 chaperone machinery. Nat. Rev. Mol. Cell. Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef]
- Kamal, A.; Thao, L.; Sensintaffar, J.; Zhang, L.; Boehm, M.F.; Fritz, L.C.; Burrows, F.J. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003, 425, 407–410. [Google Scholar] [CrossRef]
- Sanchez, J.; Carter, T.R.; Cohen, M.S.; Blagg, B.S. Old and new approaches to target the Hsp90 chaperone. Curr. Cancer Drug Targets. 2020, 20, 253–270. [Google Scholar] [CrossRef]
- Cao, C.; Peng, Y.N.; Wang, H.Z.; Fang, S.L.; Zhang, M.; Zhao, Q.; Liu, J. Inhibition of heat shock protein 90 as a novel platform for the treatment of cancer. Curr. Pharm. Des. 2019, 25, 849–855. [Google Scholar]
- Chan, K.C.; Ting, C.M.; Chan, P.S.; Lo, M.C.; Lo, K.W.; Curry, J.E.; Smyth, T.; Lee, A.W.; Ng, W.T.; Tsao, G.S.; et al. A novel Hsp90 inhibitor AT13387 induces senescence in EBV-positive nasopharyngeal carcinoma cells and suppresses tumor formation. Mol. Cancer. 2013, 12, 128. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Wang, L.; Yi, S.; Liu, Q.; Xu, X.; Su, M. Clinical and biological significances of heat shock protein 90 (Hsp90) in human nasopharyngeal carcinoma cells and anti-cancer effects of Hsp90 inhibitor. Biomed. Pharmacother. 2019, 120, 109533. [Google Scholar] [CrossRef]
- Li, H.M.; Li, B.; Sun, X.; Ma, H.; Zhu, M.; Dai, Y.; Ma, T.; Li, Y.; Hong, Y.S.; Wu, C.Z. Enzymatic biosynthesis and biological evaluation of novel 17-AAG glucoside as potential anti-cancer agents. Bioorg. Med. Chem. Lett. 2020, 30, 127282. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Gao, C.; Su, Y.; Wang, J.; Sun, J.; Chen, H.; Xu, A. Ginkgo biloba exocarp extracts inhibits angiogenesis and its effects on Wnt/β-catenin-VEGF signaling pathway in lewis lung cancer. J. Ethnopharmacol. 2016, 192, 406–412. [Google Scholar] [CrossRef]
- Jiang, L.; Si, Z.H.; Li, M.H.; Zhao, H.; Fu, Y.H.; Xing, Y.X.; Hong, W.; Ruan, L.Y.; Li, P.M.; Wang, J.S. 1H NMR-based metabolomics study of liver damage induced by ginkgolic acid (15:1) in mice. J. Pharm. Biomed. Anal. 2017, 136, 44–54. [Google Scholar] [CrossRef]
- Itokawa, H.; Totsuka, N.; Nakahara, K.; Takeya, K.; Lepoittevin, J.P.; Asakawa, Y. Antitumor principles from Ginkgo biloba L. Chem. Pharm. Bull 1987, 35, 3016–3020. [Google Scholar] [CrossRef] [Green Version]
- Kalkunte, S.S.; Singh, A.P.; Chaves, F.C.; Gianfagna, T.J.; Pundir, V.S.; Vorsa, A.K.; Jaiswal, N.; Sharma, S. Antidepressant and antistress activity of GC-MS characterized lipophilic extracts of Ginkgo biloba leaves. Phytother. Res. 2007, 21, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Ryu, S.Y.; Cho, M.H.; Lee, J. Ginkgolic acids and Ginkgo biloba extract inhibit Escherichia coli O157:H7 and Staphylococcus aureus biofilm formation. Int. J. Food. Microbiol. 2014, 174, 47–55. [Google Scholar] [CrossRef]
- Satyan, K.S.; Jaiswal, A.K.; Ghosal, S.; Bhattacharya, S.K. Anxiolytic activity of ginkgolic acid conjugates from Indian Ginkgo biloba. Psychopharmacology 1998, 136, 148–152. [Google Scholar] [CrossRef]
- Lu, J.M.; Yan, S.; Jamaluddin, S.; Weakley, S.M.; Liang, Z.; Siwak, E.B.; Yao, Q.; Chen, C. Ginkgolic acid inhibits HIV protease activity and HIV infection in vitro. Med. Sci. Monit. 2012, 18, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdoun, S.; Efferth, T. Ginkgolic acids inhibit migration in breast cancer cells by inhibition of NEMO sumoylation and NF-κB activity. Oncotarget. 2017, 8, 35103–35115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y.; et al. Ginkgolic acid inhibits invasion and migration and TGF-β-induced EMT of lung cancer cells through PI3K/Akt/mTOR inactivation. J. Cell. Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef]
- Ma, J.; Duan, W.; Han, S.; Lei, J.; Xu, Q.; Chen, X.; Jiang, Z.; Nan, L.; Li, J.; Chen, K. Ginkgolic acid suppresses the development of pancreatic cancer by inhibiting pathways driving lipogenesis. Oncotarget. 2015, 6, 20993–21003. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, B.; Zhang, L.; Cong, X.; Liu, Z.; Hu, Y.; Zhang, J.; Hu, H. Ginkgolic acid induces interplay between apoptosis and autophagy regulated by ROS generation in colon cancer. Biochem. Biophys. Res. Commun. 2018, 498, 246–253. [Google Scholar] [CrossRef]
- Li, H.; Meng, X.; Zhang, D.; Xu, X.; Li, S.; Li, Y. Ginkgolic acid suppresses the invasion of HepG2 cells via downregulation of HGF/c-Met signaling. Oncol. Rep. 2019, 41, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, Q.; Liu, X.; Liu, W.; Huang, W.; Mei, X.; Luo, J.; Shan, M.; Lin, R.; Zou, D.; et al. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: A review. J. Ethnopharmacol. 2018, 210, 318–339. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Zhang, L.; Gu, K.; Chai, N.; Ji, Q.; Zhou, L.; Wang, Y.; Ren, J.; Yang, L.; Zhang, B.; et al. YYFZBJS ameliorates colorectal cancer progression in ApcMin/+ mice by remodeling gut microbiota and inhibiting regulatory T-cell generation. Cell. Commun. Signal. 2020, 18, 113. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.W.; Tsang, C.M.; Lo, K.W. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2017, 372, 20160270. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002, 2, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Hance, M.W.; Dole, K.; Gopal, U.; Bohonowych, J.E.; Jezierska-Drutel, A.; Neumann, C.A.; Liu, H.; Garraway, I.P.; Isaacs, J.S. Secreted Hsp90 is a novel regulator of the epithelial to mesenchymal transition (EMT) in prostate cancer. J. Biol. Chem. 2012, 287, 37732–37744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.H.; Hao, L.J.; Hung, C.P.; Chen, J.W.; Leu, S.F.; Huang, B.M. Apoptotic effect of cisplatin and cordycepin on OC3 human oral cancer cells. Chin. J. Integr. Med. 2014, 20, 624–632. [Google Scholar] [CrossRef]
- Martinou, J.C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell. 2011, 21, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Olesen, S.H.; Ingles, D.J.; Zhu, J.Y.; Martin, M.P.; Betzi, S.; Georg, G.I.; Tash, J.S.; Schönbrunn, E. Stability of the human Hsp90- p50Cdc37 chaperone complex against nucleotides and Hsp90 inhibitors, and the influence of phosphorylation by casein kinase 2. Molecules. 2015, 20, 1643–1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stetz, G.; Astl, L.; Verkhivker, G.M. Exploring mechanisms of communication switching in the Hsp90-Cdc37 regulatory complexes with client kinases through allosteric coupling of phosphorylation sites: Perturbation-based modeling and hierarchical community analysis of residue interaction networks. J. Chem. Theory. Comput. 2020, 16, 4706–4725. [Google Scholar] [PubMed]
- Alaalm, L.; Crunden, J.L.; Butcher, M.; Obst, U.; Whealy, R.; Williamson, C.E.; O’Brien, H.E.; Schaffitzel, C.; Ramage, G.; Spencer, J.; et al. Identification and phenotypic characterization of hsp90 phosphorylation sites that modulate virulence traits in the major human fungal pathogen Candida albicans. Front. Cell Infect Microbiol. 2021, 11, 637836. [Google Scholar] [CrossRef]
- Hausen, B.M. The sensitizing capacity of ginkgolic acids in guinea pigs. Am. J. Contact. Dermat. 1998, 9, 146–148. [Google Scholar] [PubMed]
- Hecker, H.; Johannisson, R.; Koch, E.; Siegers, C.P. In vitro evaluation of the cytotoxic potential of alkylphenols from Ginkgo biloba L. Toxicology. 2002, 177, 167–177. [Google Scholar] [CrossRef]
- Ma, H.; Li, B.H.; Zhu, M.L.; Li, H.M.; Dai, Y.Q.; Chen, G.S.; Wu, C.Z. Study on the extraction process of ginkgolic acid from seed coat of Ginkgo biloba using alkaline extraction and acid precipitation. J. Bengbu. Med. Coll. 2019, 44, 1264–1267. [Google Scholar]
- Wu, C.Z.; Moon, A.N.; Choi, O.; Kang, S.Y.; Lee, J.J.; Lee, D.; Hwang, B.Y.; Kim, Y.H.; Lee, H.S.; Hong, Y.S. 6-Alkylsalicylic acid analogues inhibit in vitro ATPase activity of heat shock protein 90. Arch. Pharm. Res. 2010, 33, 1997–2001. [Google Scholar] [CrossRef] [PubMed]





| Compounds | Yeast Hsp90 ATPase (IC50) |
|---|---|
| GAS (μg·mL−1) | 32.76 ± 0.53 |
| GA (15:1) (μM) | 69.65 ± 1.02 |
| GM (μM) | 3.11 ± 0.21 |
| Compounds | CNE-2Z | MCF-7 | MDA-MB-231 | H1975 |
|---|---|---|---|---|
| GAS ( μg·mL−1) | 14.91 ± 1.03 | 23.81 ± 0.81 | 23.11 ± 0.42 | 17.52 ± 0.52 |
| GM (μM) a | 0.34 ± 0.05 | 0.19 ± 0.03 | 0.31 ± 0.02 | 0.41 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-M.; Ma, H.; Sun, X.; Li, B.; Cao, C.; Dai, Y.; Zhu, M.; Wu, C.-Z. Anti-Cancer Properties of Ginkgolic Acids in Human Nasopharyngeal Carcinoma CNE-2Z Cells via Inhibition of Heat Shock Protein 90. Molecules 2021, 26, 6575. https://doi.org/10.3390/molecules26216575
Li H-M, Ma H, Sun X, Li B, Cao C, Dai Y, Zhu M, Wu C-Z. Anti-Cancer Properties of Ginkgolic Acids in Human Nasopharyngeal Carcinoma CNE-2Z Cells via Inhibition of Heat Shock Protein 90. Molecules. 2021; 26(21):6575. https://doi.org/10.3390/molecules26216575
Chicago/Turabian StyleLi, Hong-Mei, Hui Ma, Xiaolong Sun, Bohan Li, Chengjiang Cao, Yiqun Dai, Meilin Zhu, and Cheng-Zhu Wu. 2021. "Anti-Cancer Properties of Ginkgolic Acids in Human Nasopharyngeal Carcinoma CNE-2Z Cells via Inhibition of Heat Shock Protein 90" Molecules 26, no. 21: 6575. https://doi.org/10.3390/molecules26216575