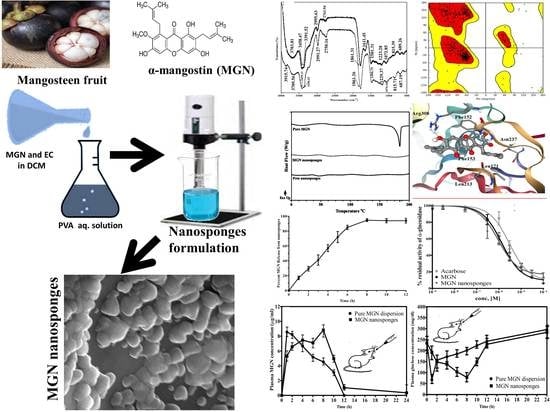
Fabrication and Biological Assessment of Antidiabetic ?-Mangostin Loaded Nanosponges: In Vitro, In Vivo, and In Silico Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Characterization
2.1.1. Fourier-Transform Infra-Red (FTIR) Spectroscopic Analysis
2.1.2. Differential Scanning Calorimetric (DSC) Analysis
2.1.3. Scanning Electron Microscopic (SEM) Analysis
2.1.4. Nanosponges Size Analysis
2.1.5. Entrapment Efficiency (%EE)
2.1.6. In Vitro Dissolution Release and Release Kinetics
2.2. In Vivo Studies
2.3. In Vitro Enzyme Inhibition Studies
α-Glucosidase Inhibitory Activity
2.4. Molecular Docking Studies
3. Materials and Methods
3.1. General
3.2. Animals
3.3. Development of MGN Nanosponges
3.4. Physical Characterization of Nanosponges
3.4.1. Fourier Transform Infra-Red (FTIR) Spectroscopic Analysis
3.4.2. Differential Scanning Calorimetric (DSC) Analysis
3.4.3. Scanning Electron Microscopic (SEM) Analysis
3.4.4. Particle Size Estimation
3.4.5. Determination of Entrapment Efficiency (%EE)
3.4.6. Determination of Production Yield (%)
3.4.7. In Vitro Dissolution Studies
3.5. In Vitro Enzyme Inhibition Studies
α-Glucosidase Inhibitory Activity
3.6. In Vivo Anti-Diabetic Activity
3.6.1. Induction of Type 2 Diabetes in Rats
3.6.2. Experimental Design and Blood Sampling
3.6.3. HPLC Assay Method
3.7. Molecular Docking Studies
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arabian J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Fatmawati, S.; Ersam, T.; Shimizu, K. The inhibitory activity of aldose reductase in vitro by constituents of Garcinia mangostana Linn. Phytomedicine 2015, 22, 49–51. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Siriwatanametanon, N.; Heinrich, M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother. Res. 2009, 23, 1047–1065. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.A.N.; Amaq, F.; Suhailah, H.; Kuncoroningrat, S.R.J.; Bilqis, I.; Dwi, W.; Akhmad, H.S. A review on medicinal properties of mangosteen (Garcinia mangostana L.). Res. J. Pharm. Technol. 2020, 13, 974–982. [Google Scholar] [CrossRef]
- Chin, Y.-W.; Kinghorn, A.D. Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Mini-Rev. Org. Chem. 2008, 5, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.-Y.; Wang, Y.-T.; Lin, L.-G. New insights into the anti-obesity activity of xanthones from Garcinia mangostana. Food Funct. 2015, 6, 383–393. [Google Scholar] [CrossRef]
- Panda, S.S.; Chand, M.; Sakhuja, R.; Jain, S. Xanthones as potential antioxidants. Curr. Med. Chem. 2013, 20, 4481–4507. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Sukatta, U.; Takenaka, M.; Ono, H.; Okadome, H.; Sotome, I.; Nanayama, K.; Thanapase, W.; Isobe, S. Distribution of major xanthones in the pericarp, aril, and yellow gum of mangosteen (Garcinia mangostana linn.) fruit and their contribution to antioxidative activity. Biosci. Biotechnol. Biochem. 2013, 77, 984–987. [Google Scholar] [CrossRef] [Green Version]
- Husen, S.A.; Kalqutny, S.H.; Ansori, A.N.M.; Susilo, R.J.K.; Alymandy, A.D.; Winarni, D. Antioxidant and antidiabetic activity of Garcinia mangostana L. pericarp extract in streptozotocin-induced diabetic mice. Biosci. Res. 2017, 14, 1238–1245. [Google Scholar]
- Ovalle-Magallanes, B.; Eugenio-Pérez, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017, 109, 102–122. [Google Scholar] [CrossRef]
- Taher, M.; Zakaria, T.M.F.S.T.; Susanti, D.; Zakaria, Z.A. Hypoglycaemic activity of ethanolic extract of Garcinia mangostana Linn. in normoglycaemic and streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2016, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ryu, H.W.; Cho, J.K.; Curtis-Long, M.J.; Yuk, H.J.; Kim, Y.S.; Jung, S.; Kim, Y.S.; Lee, B.W.; Park, K.H. α-Glucosidase inhibition and antihyperglycemic activity of prenylated xanthones from Garcinia mangostana. Phytochemistry 2011, 72, 2148–2154. [Google Scholar] [CrossRef] [PubMed]
- Jariyapongskul, A.; Areebambud, C.; Suksamrarn, S.; Mekseepralard, C. Alpha-mangostin attenuation of hyperglycemia-induced ocular hypoperfusion and blood retinal barrier leakage in the early stage of type 2 diabetes rats. BioMed Res. Int. 2015, 2015, 785826. [Google Scholar] [CrossRef]
- Bugianesi, E.; McCullough, A.J.; Marchesini, G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology 2005, 42, 987–1000. [Google Scholar] [CrossRef]
- Lee, D.; Kim, Y.-M.; Jung, K.; Chin, Y.-W.; Kang, K.S. Alpha-mangostin improves insulin secretion and protects INS-1 cells from streptozotocin-induced damage. Int. J. Mol. Sci. 2018, 19, 1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- International Diabetes Federation. Diabetes in South-East Asia. 2019. Available online: https://idf.org/our-network/regions-members/south-east-asia/diabetes-in-sea.html (accessed on 3 March 2020).
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Davies, J.; Vollmer, S. Global economic burden of diabetes in adults: Projections from 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef] [Green Version]
- Cho, N.; Shaw, J.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Baghdadi, A.; Tayebi-Meigooni, A. Alpha-mangostin-rich extracts from mangosteen pericarp: Optimization of green extraction protocol and evaluation of biological activity. Molecules 2018, 23, 1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.-Q.; Feng, Y.-L.; Cao, G.; Zhao, Y.-Y. Natural products as a source for antifibrosis therapy. Trends Pharmacol. Sci. 2018, 39, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, M.; Fischhuber, K.; Heiss, E.H. Activation of Nrf2 signaling by natural products-can it alleviate diabetes? Biotechnol. Adv. 2018, 36, 1738–1767. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Park, J.; Jang, H.-J. Anti-diabetic effects of natural products an overview of therapeutic strategies. Mol. Cell. Toxicol. 2017, 13, 1–20. [Google Scholar] [CrossRef]
- Faisal, N.A.; Chatha, S.A.S.; Hussain, A.I.; Ikram, M.; Bukhari, S.A. Liaison of phenolic acids and biological activity of escalating cultivars of Daucus carota. Int. J. Food Prop. 2017, 20, 2782–2792. [Google Scholar] [CrossRef] [Green Version]
- Raskin, I.; Ribnicky, D.M.; Komarnytsky, S.; Ilic, N.; Poulev, A.; Borisjuk, N.; Brinker, A.; Moreno, D.A.; Ripoll, C.; Yakoby, N. Plants and human health in the twenty-first century. Trends Biotechnol. 2002, 20, 522–531. [Google Scholar] [CrossRef]
- Saraf, S. Applications of novel drug delivery system for herbal formulations. Fitoterapia 2010, 81, 680–689. [Google Scholar] [CrossRef]
- Daga, M.; de Graaf, I.A.; Argenziano, M.; Barranco, A.S.M.; Loeck, M.; Al-Adwi, Y.; Cucci, M.A.; Caldera, F.; Trotta, F.; Barrera, G. Glutathione-responsive cyclodextrin-nanosponges as drug delivery systems for doxorubicin: Evaluation of toxicity and transport mechanisms in the liver. Toxicol. In Vitro 2020, 65, 104800. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Srivastava, S.; Ghosh, S.; Khare, S.K. Phytochemical delivery through nanocarriers: A review. Colloids Surf. B. Biointerfaces 2020, 197, 111389. [Google Scholar] [CrossRef]
- Gafur, A.; Sukamdani, G.Y.; Kristi, N.; Maruf, A.; Xu, J.; Chen, X.; Wang, G.; Ye, Z. From bulk to nano-delivery of essential phytochemicals: Recent progress and strategies for antibacterial resistance. J. Mater. Chem. B 2020, 8, 9825–9835. [Google Scholar] [CrossRef]
- Pandey, P.J. Multifunctional nanosponges for the treatment of various diseases: A review. Asian J. Pharm. Pharmacol. 2019, 5, 235–248. [Google Scholar] [CrossRef]
- Almutairy, B.K.; Alshetaili, A.; Alali, A.S.; Ahmed, M.M.; Anwer, M.; Aboudzadeh, M.A. Design of olmesartan medoxomil-loaded nanosponges for hypertension and lung cancer treatments. Polymers 2021, 13, 2272. [Google Scholar] [CrossRef]
- Balwe, M.B. Nanosponge a novel drug delivery system. Res. J. Pharm. Dosage Forms Technol. 2020, 12, 261–266. [Google Scholar] [CrossRef]
- Pawar, S.; Shende, P.; Trotta, F. Diversity of β-cyclodextrin-based nanosponges for transformation of actives. Int. J. Pharm. 2019, 565, 333–350. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, A.; Kumar, S. Nanosponges: A promising nanocarrier systems for drug delivery. Curr. Res. Pharm. Sci. 2020, 01–05. [Google Scholar] [CrossRef]
- Jagtap, S.R.; Bhusnure, O.G.; Mujewar, I.N.; Gholve, S.B.; Panchabai, V. Nanosponges: A novel trend for targeted drug delivery. J. Drug Delivery Ther. 2019, 9, 931–938. [Google Scholar] [CrossRef]
- Silpa, G.; Manohar, R.D.; Mathan, S.; Dharan, S.S. Nanosponges: A potential nanocarrier: A review. J. Pharm. Sci. Res. 2020, 12, 1341–1344. [Google Scholar]
- Asasutjarit, R.; Meesomboon, T.; Adulheem, P.; Kittiwisut, S.; Sookdee, P.; Samosornsuk, W.; Fuongfuchat, A. Physicochemical properties of alpha-mangostin loaded nanomeulsions prepared by ultrasonication technique. Heliyon 2019, 5, e02465. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.-K.; Jiang, H.; Yang, K.; Wang, Y.-Q.; Zhang, Q.; Zuo, J. Development and in vivo evaluation of self-microemulsion as delivery system for α-mangostin. Kaohsiung J. Med. Sci. 2017, 33, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Moin, A.; Roohi, N.F.; Rizvi, S.M.D.; Ashraf, S.A.; Siddiqui, A.J.; Patel, M.; Ahmed, S.; Gowda, D.; Adnan, M. Design and formulation of polymeric nanosponge tablets with enhanced solubility for combination therapy. RSC Adv. 2020, 10, 34869–34884. [Google Scholar] [CrossRef]
- Deb, T.K.; Ramireddy, B.; Moin, A.; Shivakumar, H. In vitro-in vivo evaluation of xanthan gum and eudragit inter polyelectrolyte complex based sustained release tablets. Intl. J. Pharm. Invest. 2015, 5, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Li, H.; Lang, B.; O’Donnell, K.; Zhang, H.; Wang, Z.; Dong, Y.; Wu, C.; Williams III, R.O. Formulation and delivery of improved amorphous fenofibrate solid dispersions prepared by thin film freezing. Eur. J. Pharm. Biopharm. 2012, 82, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Ansari, M.J.; Das, S.S.; Alshahrani, S.M. Development and characterization of ethyl cellulose nanosponges for sustained release of brigatinib for the treatment of non-small cell lung cancer. J. Polym. Eng. 2020, 40, 823–832. [Google Scholar] [CrossRef]
- Pawar, S.; Shende, P. Design and optimization of cyclodextrin-based nanosponges of antimalarials using central composite design for dry suspension. J. Incl. Phenom. Macrocycl. Chem. 2021, 1–15. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Badr-Eldin, S.M.; Labib, G.S.; El-Kamel, A.H. Design and formulation of a topical hydrogel integrating lemongrass-loaded nanosponges with an enhanced antifungal effect: In vitro/in vivo evaluation. Int. J. Nanomed. 2015, 10, 893. [Google Scholar] [CrossRef] [Green Version]
- Penjuri, S.C.B.; Ravouru, N.; Damineni, S.; Bns, S.; Poreddy, S.R. Formulation and evaluation of lansoprazole loaded Nanosponges. Turk. J. Pharm. Sci. 2016, 13, 304–310. [Google Scholar] [CrossRef]
- Abass, M.M.; Rajab, N.A. Preparation and characterization of etodolac as a topical nanosponges hydrogel. Iraqi J. Pharm. Sci. 2019, 28, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Larsson, M.; Hill, A.; Duffy, J. Suspension stability; why particle size, zeta potential and rheology are important. Annu. Trans. Nord. Rheol. Soc. 2012, 20, 209–214. [Google Scholar]
- Xu, R. Progress in nanoparticles characterization: Sizing and zeta potential measurement. Particuology 2008, 6, 112–115. [Google Scholar] [CrossRef]
- Bachir, Y.N.; Bachir, R.N.; Hadj-Ziane-Zafour, A. Nanodispersions stabilized by β-cyclodextrin nanosponges: Application for simultaneous enhancement of bioactivity and stability of sage essential oil. Drug Dev. Ind. Pharm. 2019, 45, 333–347. [Google Scholar] [CrossRef]
- Shah, H.; Nair, A.B.; Shah, J.; Jacob, S.; Bharadia, P.; Haroun, M. Proniosomal vesicles as an effective strategy to optimize naproxen transdermal delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102479. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torne, S.J.; Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded nanosponges. Drug Deliv. 2010, 17, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Amer, R.I.; El-Osaily, G.H.; Gad, S.S. Design and optimization of topical terbinafine hydrochloride nanosponges: Application of full factorial design, in vitro and in vivo evaluation. J. Adv. Pharm. Technol. Res. 2020, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, P.; Reddy, A.J. Formulation and evaluation of isoniazid loaded nanosponges for topical delivery. Pharm. Nanotechnol. 2015, 3, 68–76. [Google Scholar] [CrossRef]
- Nelli, G.B.; Kilari, E.K. Antidiabetic effect of α-mangostin and its protective role in sexual dysfunction of streptozotocin induced diabetic male rats. Syst. Biol. Reprod. Med. 2013, 59, 319–328. [Google Scholar] [CrossRef]
- Stepensky, D.; Friedman, M.; Raz, I.; Hoffman, A. Pharmacokinetic-pharmacodynamic analysis of the glucose-lowering effect of metformin in diabetic rats reveals first-pass pharmacodynamic effect. Drug Metab. Dispos. 2002, 30, 861–868. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Mediani, A.; Nur Ashikin, A.H.; Azliana, A.B.S.; Abas, F. Antioxidant and α-glucosidase inhibitory activities of the leaf and stem of selected traditional medicinal plants. Intl. Food Res. J. 2014, 21, 379–386. [Google Scholar]
- Vongsak, B.; Kongkiatpaiboon, S.; Jaisamut, S.; Machana, S.; Pattarapanich, C. In vitro alpha glucosidase inhibition and free-radical scavenging activity of propolis from Thai stingless bees in mangosteen orchard. Rev. Bras. Farmacogn. 2015, 25, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.; Javaid, K.; Zafar, H.; Khan, K.M.; Khalil, R.; Ul-Haq, Z.; Perveen, S.; Choudhary, M.I. Synthesis, and In vitro and in silico α-glucosidase inhibitory studies of 5-chloro-2-aryl benzo[d]thiazoles. Bioorg. Chem. 2018, 78, 269–279. [Google Scholar] [CrossRef]
- Jagannathan, V.; Venkatesan, A.; Viswanathan, P. Kinetics and computational evaluation of eugenol and vanillic acid on inhibition of a potential enzyme of a nosocomial pathogen that promotes struvite formation. Curr. Enzym. Inhib. 2020, 16, 162–171. [Google Scholar] [CrossRef]
- Kerru, N.; Singh-Pillay, A.; Awolade, P.; Singh, P. Current anti-diabetic agents and their molecular targets: A review. Eur. J. Med. Chem. 2018, 152, 436–488. [Google Scholar] [CrossRef] [PubMed]
- Darandale, S.; Vavia, P. Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical characterization. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 315–322. [Google Scholar] [CrossRef]
- Omar, S.M.; Ibrahim, F.; Ismail, A. Formulation and evaluation of cyclodextrin-based nanosponges of griseofulvin as pediatric oral liquid dosage form for enhancing bioavailability and masking bitter taste. Saudi Pharm. J. 2020, 28, 349–361. [Google Scholar] [CrossRef]
- Varan, C.; Anceschi, A.; Sevli, S.; Bruni, N.; Giraudo, L.; Bilgiç, E.; Korkusuz, P.; İskit, A.B.; Trotta, F.; Bilensoy, E. Preparation and characterization of cyclodextrin nanosponges for organic toxic molecule removal. Int. J. Pharm. 2020, 585, 119485. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.S.; Usman, F.; Khan, M.A.; Khalil, R.; Ul-Haq, Z.; Mushtaq, A.; Qaiser, R.; Iqbal, J. Preparation and characterization of anticancer niosomal withaferin–A formulation for improved delivery to cancer cells: An in vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 101863. [Google Scholar] [CrossRef]
- Swaminathan, S.; Pastero, L.; Serpe, L.; Trotta, F.; Vavia, P.; Aquilano, D.; Trotta, M.; Zara, G.; Cavalli, R. Cyclodextrin-based nanosponges encapsulating camptothecin: Physicochemical characterization, stability and cytotoxicity. Eur. J. Pharm. Biopharm. 2010, 74, 193–201. [Google Scholar] [CrossRef]
- Kim, K.Y.; Nguyen, T.H.; Kurihara, H.; Kim, S.M. α-Glucosidase inhibitory activity of bromophenol purified from the red alga Polyopes lancifolia. J. Food Sci. 2010, 75, H145–H150. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Tuzcu, M.; Orhan, C.; Sahin, N.; Kucuk, O.; Ozercan, I.H.; Juturu, V.; Komorowski, J.R. Anti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocin. Br. J. Nutr. 2013, 110, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef]
- Asgary, S.; Parkhideh, S.; Solhpour, A.; Madani, H.; Mahzouni, P.; Rahimi, P. Effect of ethanolic extract of Juglans regia L. on blood sugar in diabetes-induced rats. J. Med. Food 2008, 11, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Properties/Models | Outcomes |
|---|---|
| Zeta Potential | −35.06 ± 4.91 mV |
| PDI | 0.3890 ± 0.0943 |
| Entrapment Efficiency | 89 ± 5 (%) |
| Production Yield | 75 ± 11 (%) |
| Hydrodynamic Diameter | 113 ± 8 nm |
| Zero-Order | 0.7935 |
| First-Order | 0.9959 |
| Higuchi Model | 0.9121 |
| Korse–Meyer Peppas, n Value | 0.9304, 0.4970 |
| Parameters of Activity | |||||||
|---|---|---|---|---|---|---|---|
| Formulation | (mg.h/dL) ± SEM | Max. Hypoglycemic Response (mg/dL) ± SEM | |||||
| Pure MGN Dispersion | 233.8 ± 15.31 | 67.13 ± 4.925 | 1 | ||||
| MGN Nanosponges | 235.1 ± 17.62 | 78.42 ± 11.52 | 8 | ||||
| Glucose Concentration (mg/dL) ± SEM | Plasma MGN Concentration (µg/mL) ± SEM | ||||||
| Sr.No | Groups Description | Pure MGN Dispersion | MGN Nanosponges | p-value | Pure MGN Dispersion | MGN Nanosponges | p-value |
| 1 | Normal Control | 85.64 ± 9.356 | 87.11 ± 6.579 | 0.8149 | --- | --- | --- |
| 2 | Diabetic Control | 233.8 ± 15.31 | 235.1 ± 17.62 | 0.9736 | --- | --- | --- |
| 3 | After 1 h | 67.13 ± 4.925 | 192.8 ± 20.71 | 0.0032 | 8.551 ± 2.689 | 5.307 ± 2.851 | 0.0384 |
| 4 | After 2 h | 156.8 ± 18.61 | 148.7 ± 24.91 | 0.4271 | 8.201 ± 1.662 | 6.568 ± 1.897 | 0.1254 |
| 5 | After 4 h | 172.4 ± 15.84 | 136.6 ± 15.74 | 0.1845 | 6.679 ± 3.415 | 7.462 ± 3.644 | 0.4918 |
| 6 | After 6 h | 184.7 ± 19.84 | 103.1 ± 15.32 | 0.0391 | 5.162 ± 1.204 | 7.108 ± 1.927 | 0.7612 |
| 7 | After 8 h | 201.5 ± 18.69 | 78.42 ± 11.52 | 0.0028 | 4.508 ± 1.691 | 8.824 ± 2.607 | 0.0064 |
| 8 | After 10 h | 223.1 ± 17.96 | 148.5 ± 16.71 | 0.0414 | 3.117 ± 1.141 | 4.971 ± 1.845 | 0.0217 |
| 9 | After 11 h | 242.6 ± 26.53 | 229.1 ± 18.24 | 0.4628 | Not detected | 1.035 ± 0.360 | 0.0138 |
| 10 | After 12 h | 296.2 ± 27.38 | 283.7 ± 31.10 | 0.4773 | Not detected | 0.352 ± 0.028 | 0.0413 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, F.; Shah, H.S.; Zaib, S.; Manee, S.; Mudassir, J.; Khan, A.; Batiha, G.E.-S.; Abualnaja, K.M.; Alhashmialameer, D.; Khan, I. Fabrication and Biological Assessment of Antidiabetic ?-Mangostin Loaded Nanosponges: In Vitro, In Vivo, and In Silico Studies. Molecules 2021, 26, 6633. https://doi.org/10.3390/molecules26216633
Usman F, Shah HS, Zaib S, Manee S, Mudassir J, Khan A, Batiha GE-S, Abualnaja KM, Alhashmialameer D, Khan I. Fabrication and Biological Assessment of Antidiabetic ?-Mangostin Loaded Nanosponges: In Vitro, In Vivo, and In Silico Studies. Molecules. 2021; 26(21):6633. https://doi.org/10.3390/molecules26216633
Chicago/Turabian StyleUsman, Faisal, Hamid Saeed Shah, Sumera Zaib, Sirikhwan Manee, Jahanzeb Mudassir, Ajmal Khan, Gaber El-Saber Batiha, Khamael M. Abualnaja, Dalal Alhashmialameer, and Imtiaz Khan. 2021. "Fabrication and Biological Assessment of Antidiabetic ?-Mangostin Loaded Nanosponges: In Vitro, In Vivo, and In Silico Studies" Molecules 26, no. 21: 6633. https://doi.org/10.3390/molecules26216633
APA StyleUsman, F., Shah, H. S., Zaib, S., Manee, S., Mudassir, J., Khan, A., Batiha, G. E.-S., Abualnaja, K. M., Alhashmialameer, D., & Khan, I. (2021). Fabrication and Biological Assessment of Antidiabetic ?-Mangostin Loaded Nanosponges: In Vitro, In Vivo, and In Silico Studies. Molecules, 26(21), 6633. https://doi.org/10.3390/molecules26216633