Dihydrofolate Reductase Inhibitors: The Pharmacophore as a Guide for Co-Crystal Screening
Abstract
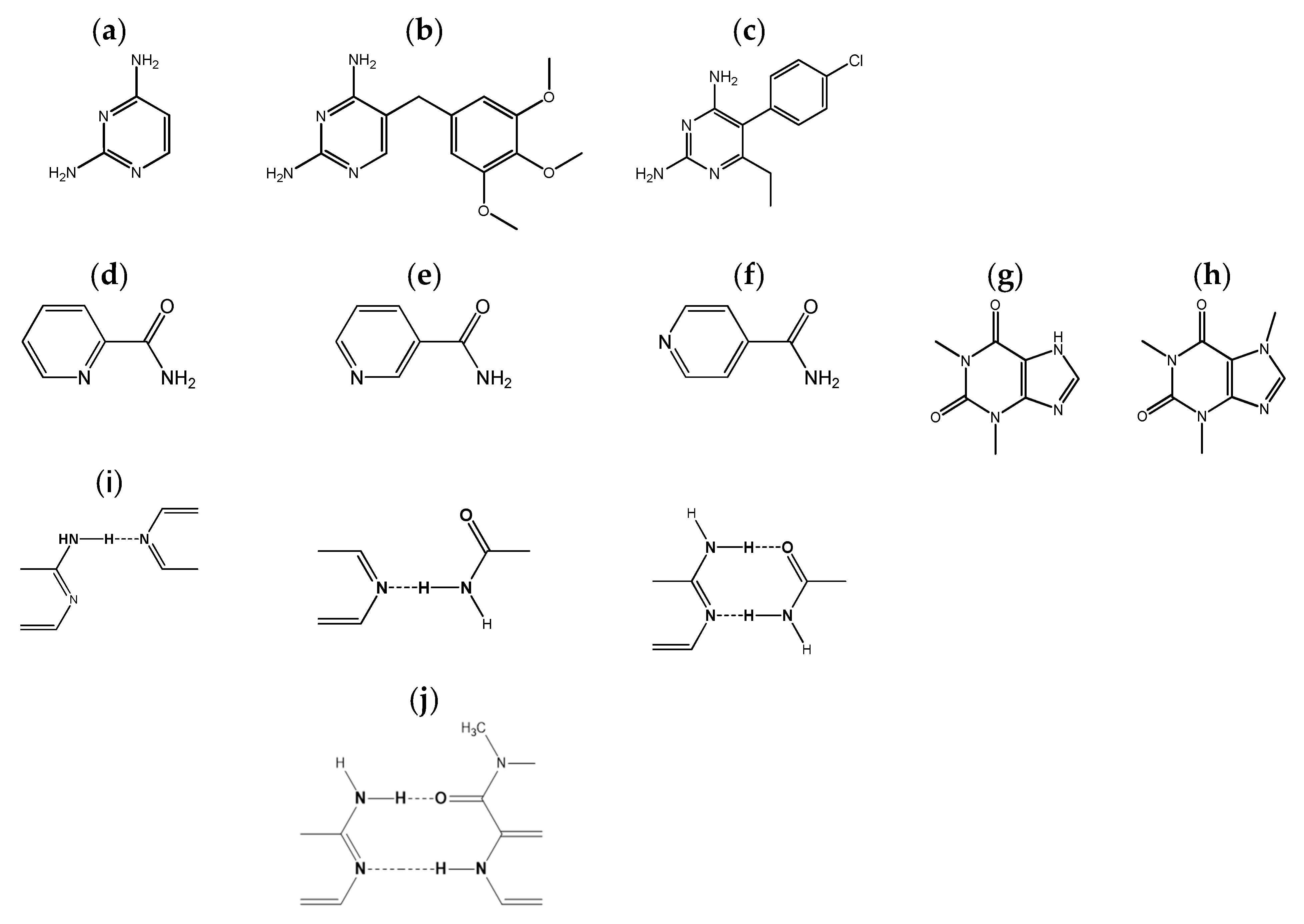
:1. Introduction
2. Results and Discussion
2.1. (DAP/TMP/PMA) + Pyridinecarboxamides Binary Systems
General Remarks
2.2. (DAP/TMP/PMA) + (THEO/CAF) Binary Systems
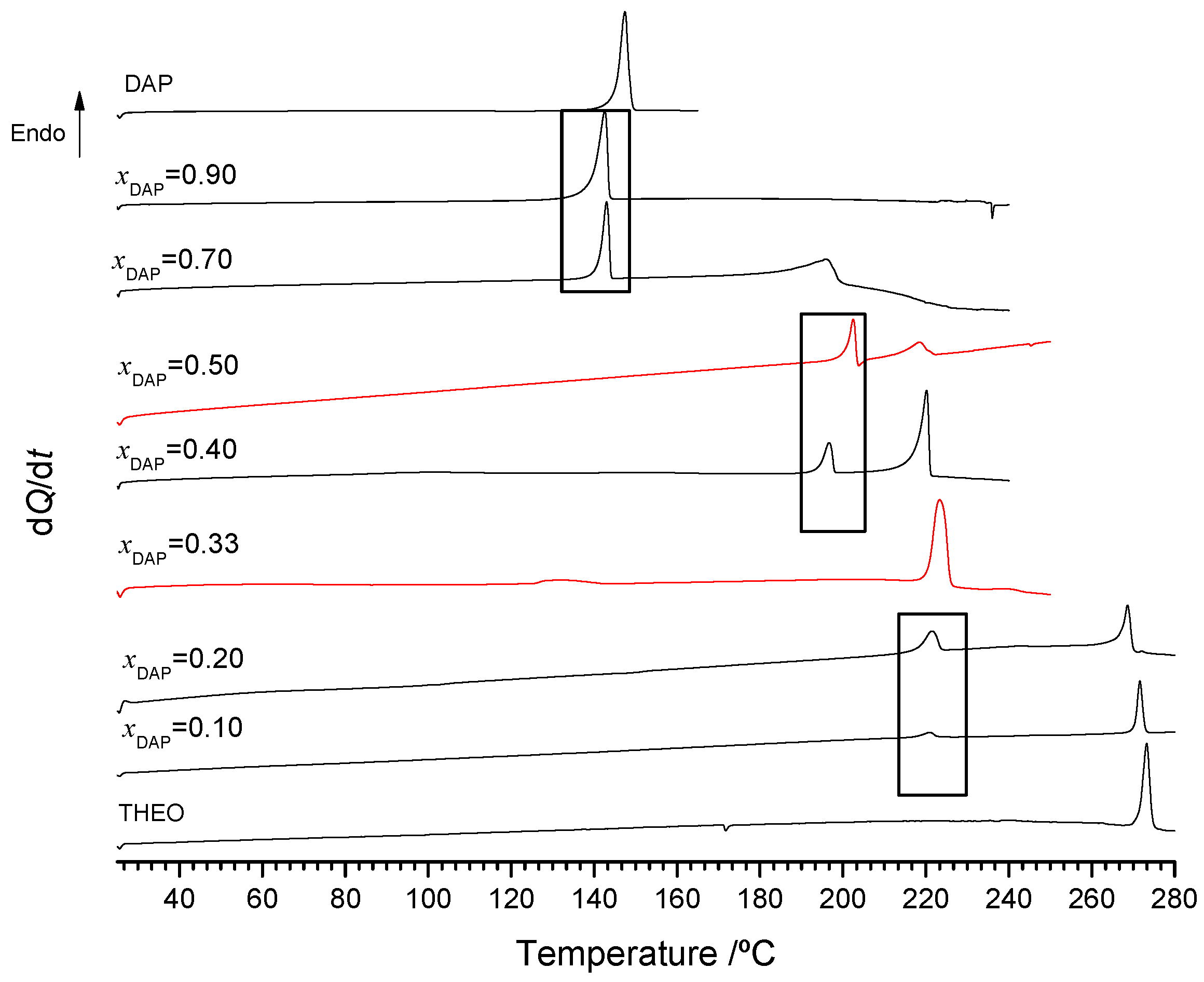
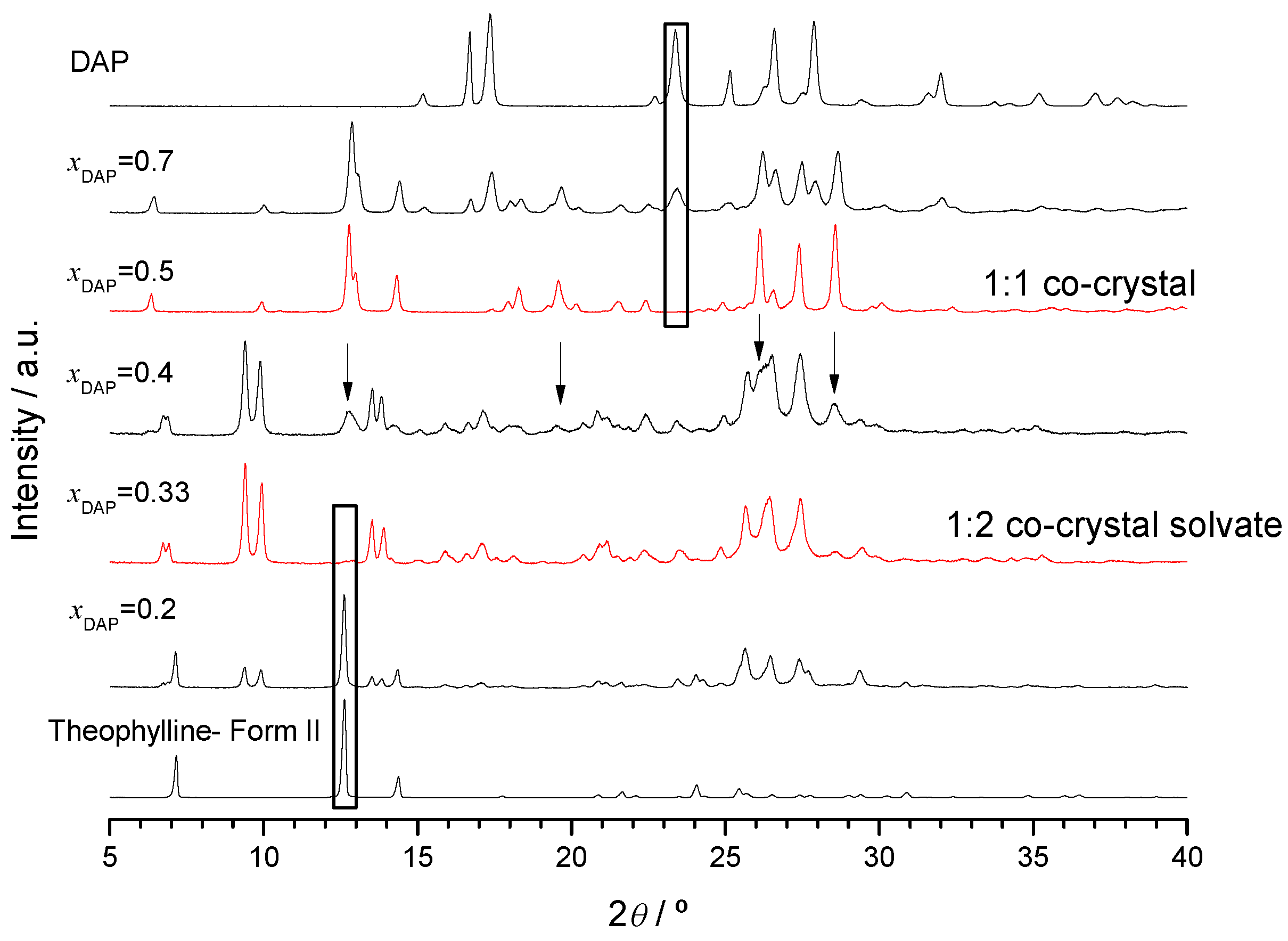
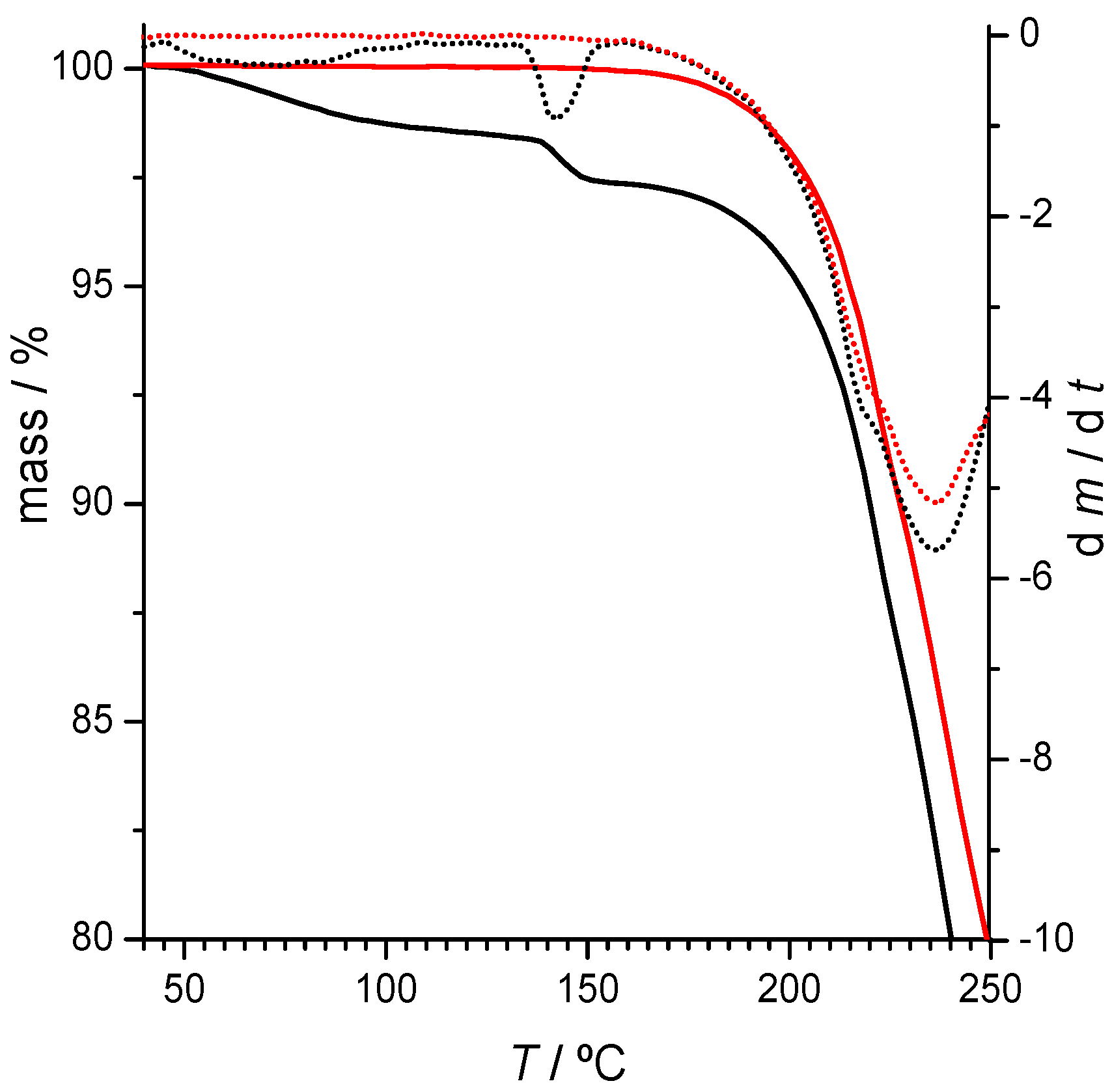
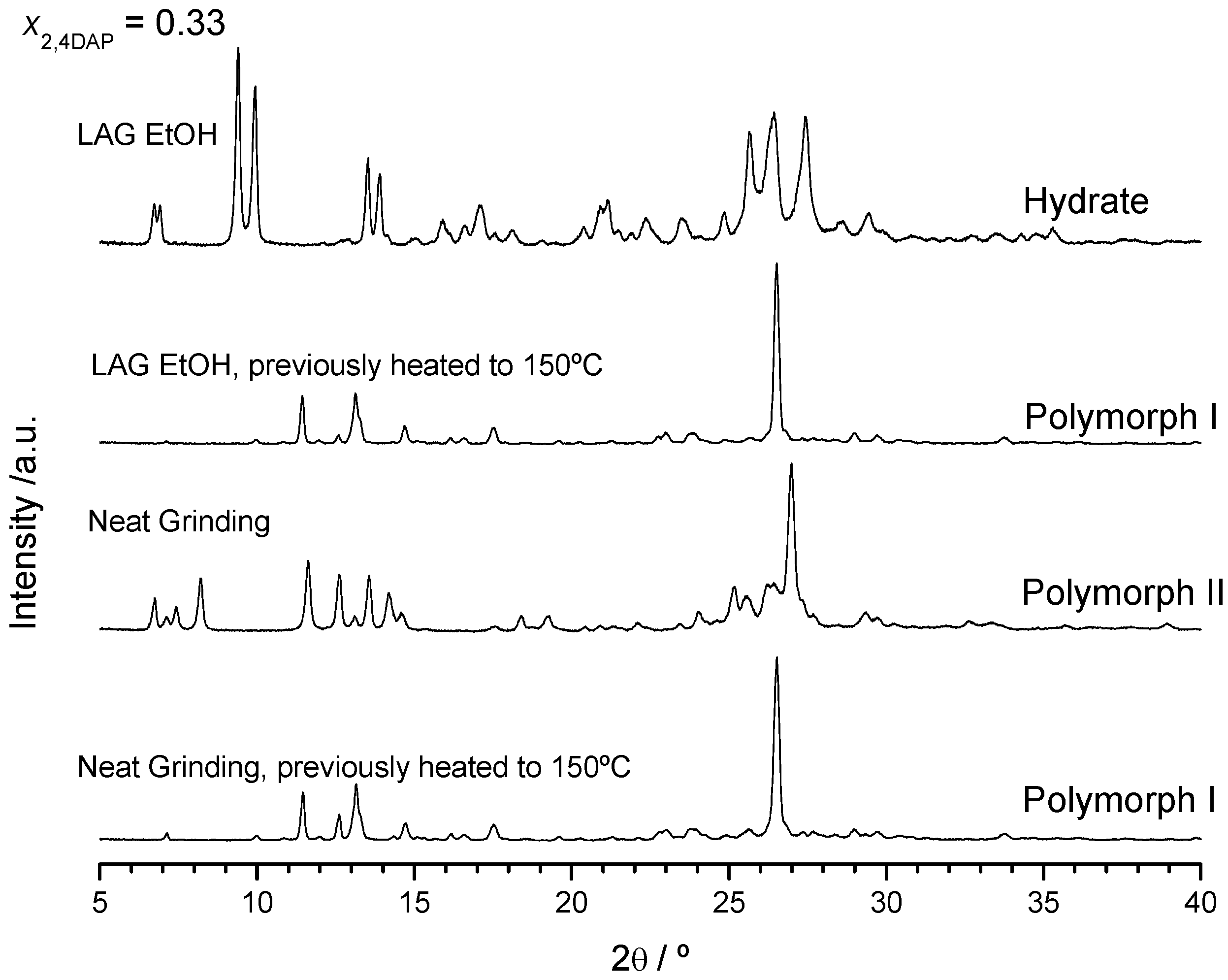
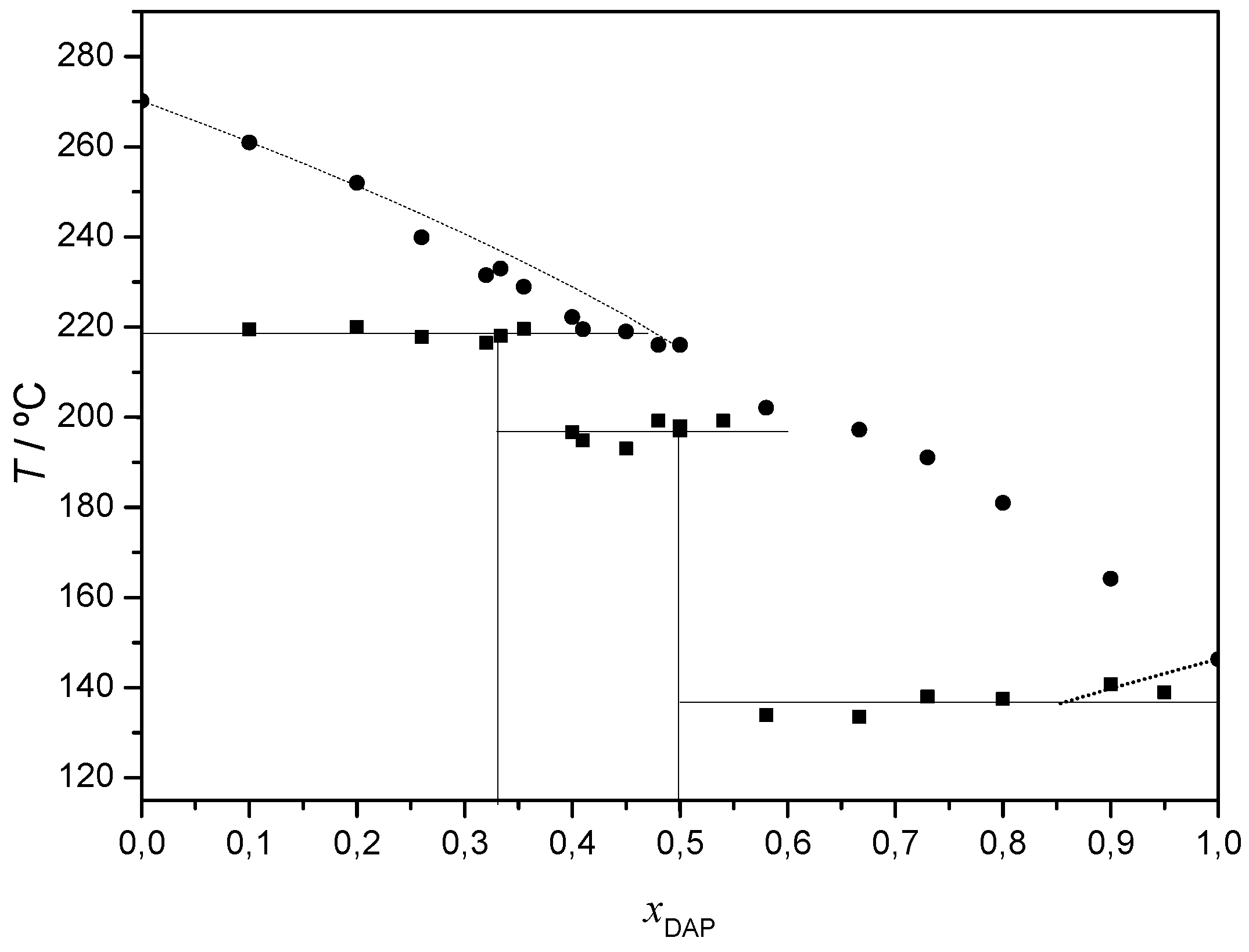
2.2.1. 2,4-Diaminopyrimidine + theophylline and 2,4-Diaminopyrimidine + caffeine
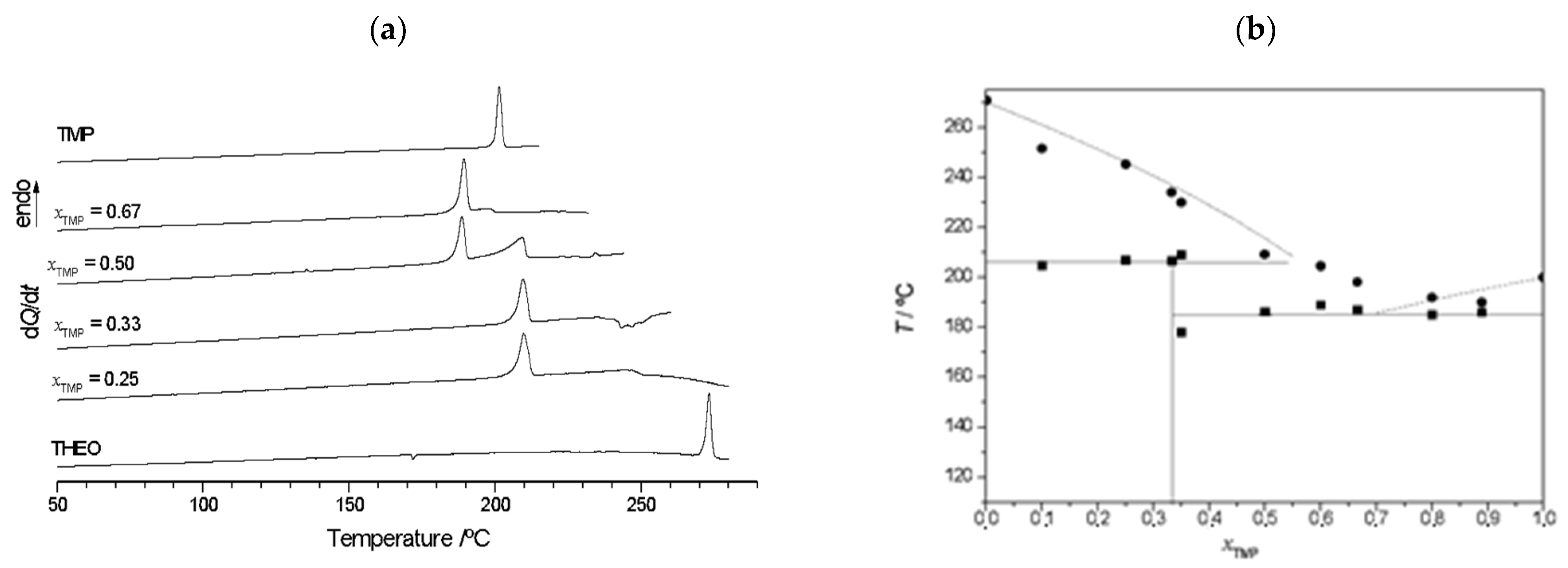
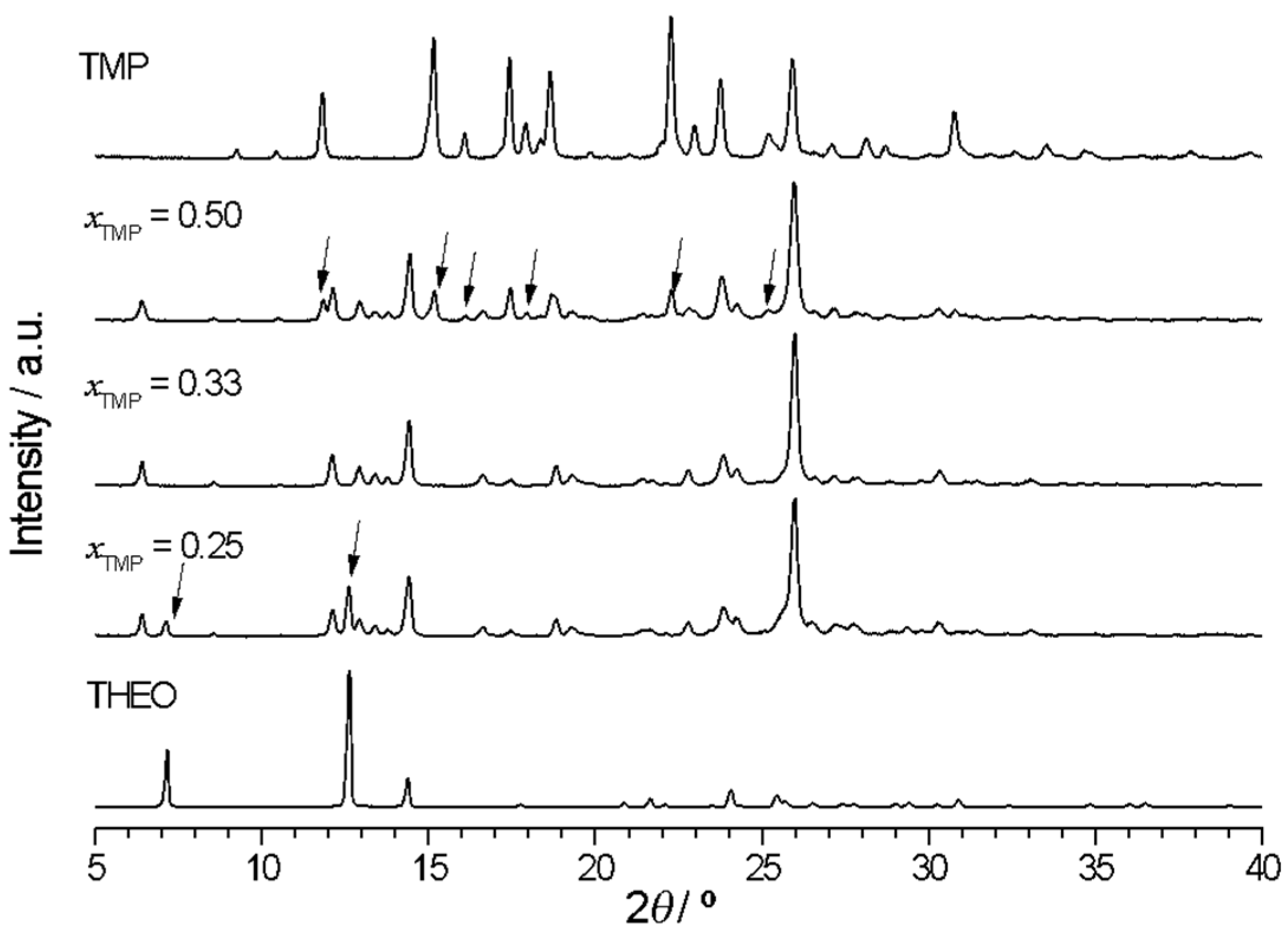
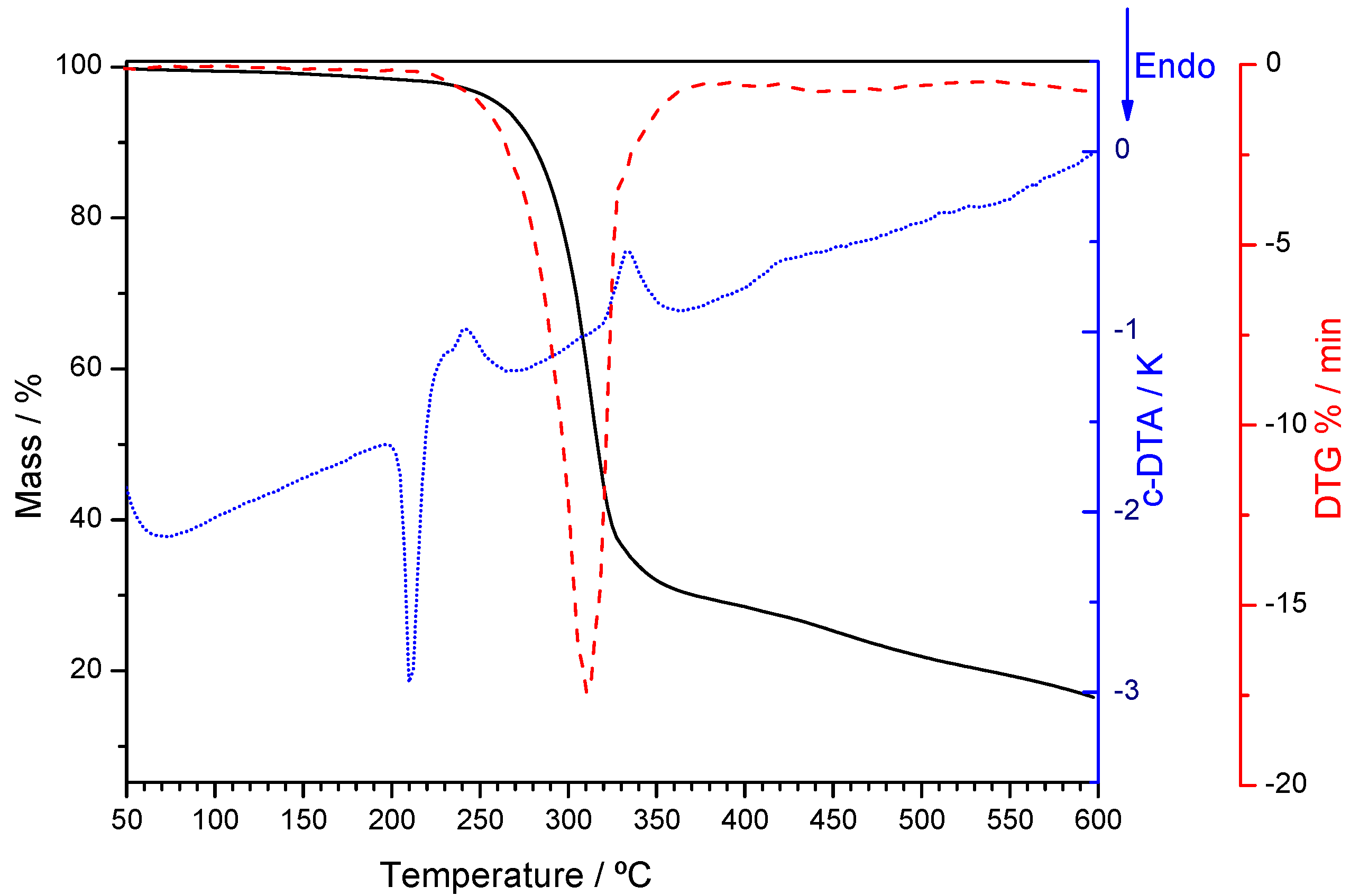
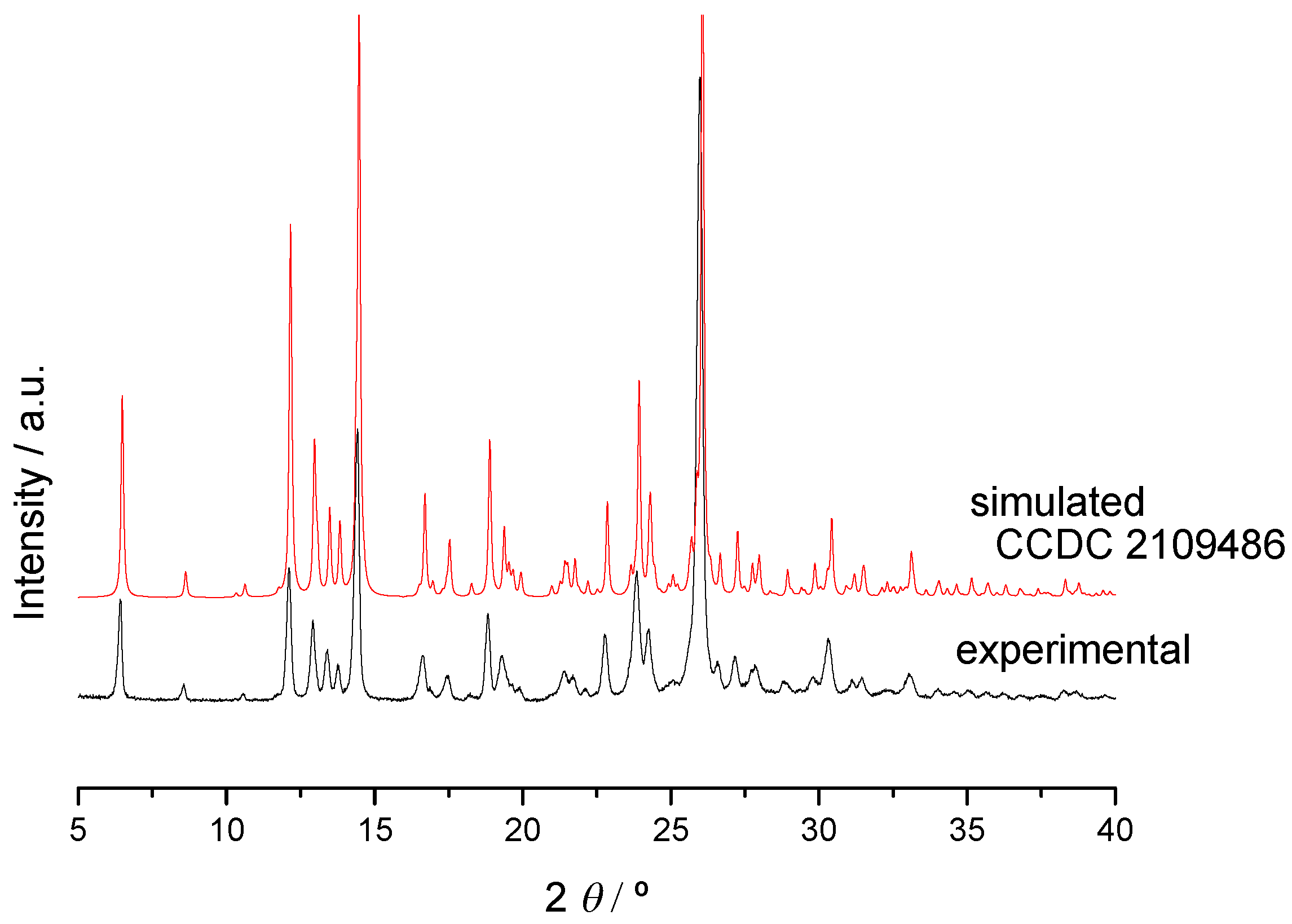
2.2.2. Trimethoprim + theophylline and Trimethoprim + caffeine
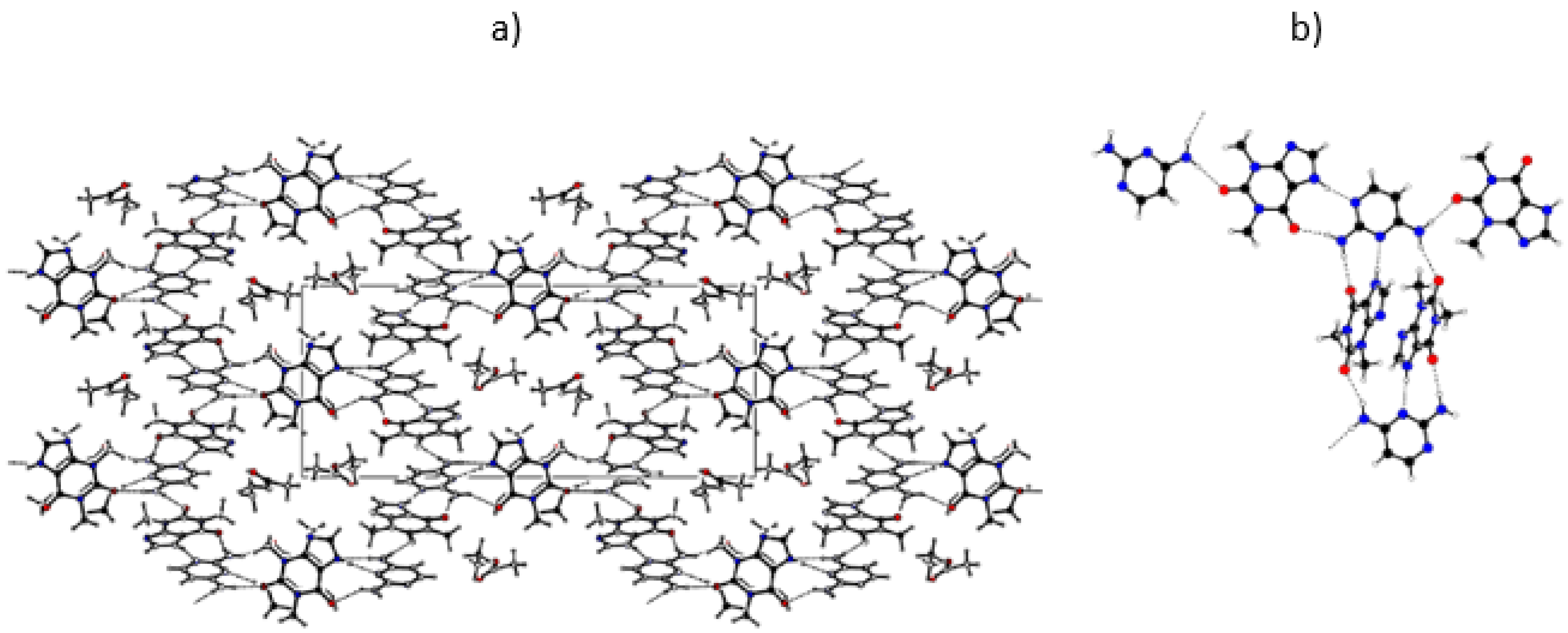
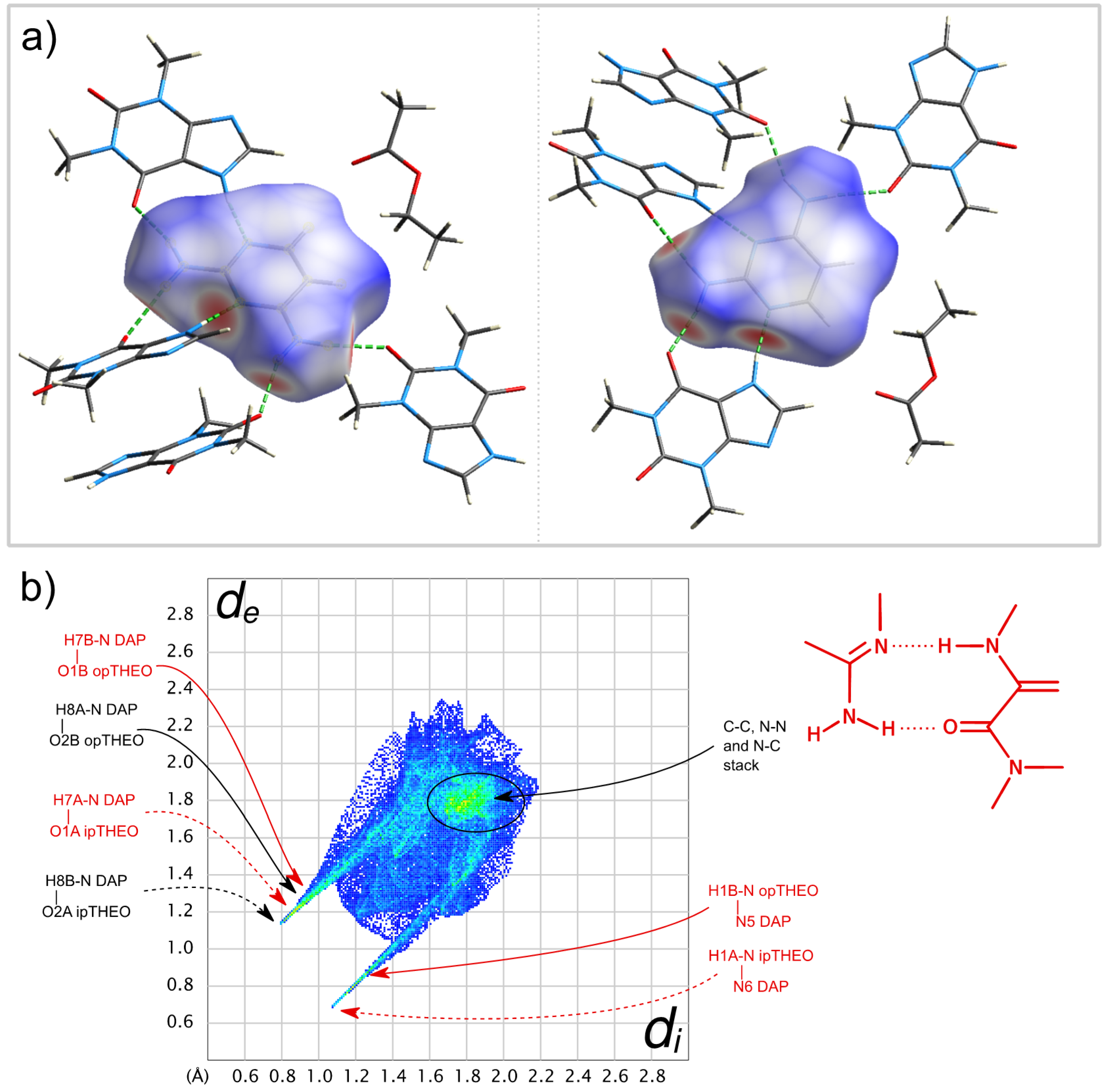
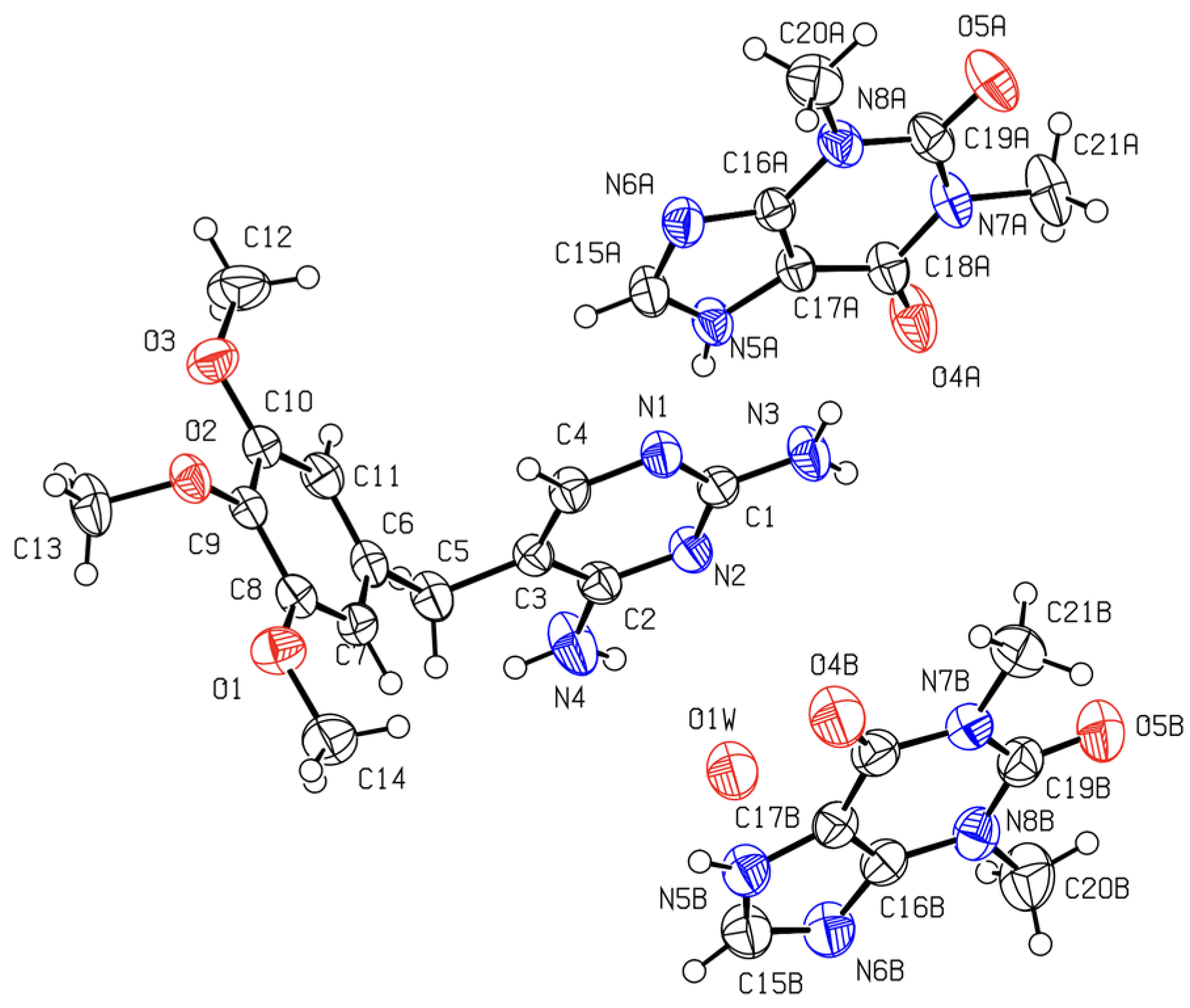
2.2.3. Crystalline Structures and Hirshfeld Surfaces Analysis
2.2.4. General Remarks
3. Experimental Procedures
3.1. Materials
3.2. Co-Crystals Screening
3.3. Differential Scanning Calorimetry (DSC)
3.4. Thermogravimetric Analysis (TGA)
3.5. Infrared Spectroscopy (FTIR)
3.6. X-ray Powder Diffraction (XRPD)
3.7. Single-Crystal X-ray Diffraction (SXD)
3.8. Hirshfeld Surfaces Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Then, R.L. History and future of antimicrobial diaminopyrimidines. J. Chemother. 1993, 5, 361–368. [Google Scholar] [CrossRef]
- Anderson, A.C. Targeting DHFR in parasitic protozoa. Drug Discov. Today 2005, 10, 121–128. [Google Scholar] [CrossRef]
- Rao, A.S.; Tapale, S.R. A study on dihydrofolate reductase and its inhibitors: A review. Int. J. Pharm. Sci. Res. 2013, 4, 2535–2547. [Google Scholar]
- Tropak, M.B.; Zhang, J.M.; Yonekawa, S.; Rigat, B.A.; Aulakh, V.S.; Smith, M.R.; Hwang, H.J.; Ciufolini, M.A.; Mahuran, D.J. Pyrimethamine Derivatives: Insight into Binding Mechanism and Improved Enhancement of Mutant beta-N-acetylhexosaminidase Activity. J. Med. Chem. 2015, 58, 4483–4493. [Google Scholar] [CrossRef] [PubMed]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, 33. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Qiao, W.L.; An, Q.; Yang, T.; Luo, Y.F. Dihydrofolate reductase inhibitors for use as antimicrobial agents. Eur. J. Med. Chem. 2020, 195, 112268. [Google Scholar] [CrossRef]
- Hopper, A.T.; Brockman, A.; Wise, A.; Gould, J.; Barks, J.; Radke, J.B.; Sibley, L.D.; Zou, Y.M.; Thomas, S. Discovery of Selective Toxoplasma gondii Dihydrofolate Reductase Inhibitors for the Treatment of Toxoplasmosis. J. Med. Chem. 2019, 62, 1562–1576. [Google Scholar] [CrossRef] [Green Version]
- Nelson, R.G.; Rosowsky, A. Dicyclic and tricyclic diaminopyrimidine derivatives as potent inhibitors of Cryptosporidium parvum dihydrofolate reductase: Structure-activity and structure-selectivity correlations. Antimicrob. Agents Chemother. 2001, 45, 3293–3303. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.Q.; Guo, T.L.; Li, X.T.; Dong, Y.; Galatsis, P.; Johnson, D.S.; Pan, Z.Y. Discovery of selective 2,4-diaminopyrimidine-based photoaffinity probes for glyoxalase I. MedChemComm 2014, 5, 352–357. [Google Scholar] [CrossRef]
- Raimondi, M.V.; Randazzo, O.; La Franca, M.; Barone, G.; Vignoni, E.; Rossi, D.; Collina, S. DHFR Inhibitors: Reading the Past for Discovering Novel Anticancer Agents. Molecules 2019, 24, 1140. [Google Scholar] [CrossRef] [Green Version]
- Nammalwar, B.; Bourne, C.R.; Wakeham, N.; Bourne, P.C.; Barrow, E.W.; Muddala, N.P.; Bunce, R.A.; Berlin, K.D.; Barrow, W.W. Modified 2,4-diaminopyrimidine-based dihydrofolate reductase inhibitors as potential drug scaffolds against Bacillus anthracis. Bioorg. Med. Chem. 2015, 23, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical cocrystals: Along the path to improved medicines. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Grothe, E.; Meekes, H.; Vlieg, E.; ter Horst, J.H.; de Gelder, R. Solvates, Salts, and Cocrystals: A Proposal for a Feasible Classification System. Cryst. Growth Des. 2016, 16, 3237–3243. [Google Scholar] [CrossRef] [Green Version]
- Desiraju, G.R. Supramolecular synthons in crystal engineering—A new organic synthesis. Angew. Chem. Int. Ed. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Weyna, D.R.; Shattock, T.; Vishweshwar, P.; Zaworotko, M.J. Synthesis and Structural Characterization of Cocrystals and Pharmaceutical Cocrystals: Mechanochemistry vs Slow Evaporation from Solution. Cryst. Growth Des. 2009, 9, 1106–1123. [Google Scholar] [CrossRef]
- Zheng, Q.X.; Unruh, D.K.; Hutchins, K.M. Cocrystallization of Trimethoprim and Solubility Enhancement via Salt Formation. Cryst. Growth Des. 2021, 21, 1507–1517. [Google Scholar] [CrossRef]
- Maity, D.K.; Paul, R.K.; Desiraju, G.R. Drug-Drug Binary Solids of Nitrofurantoin and Trimethoprim: Crystal Engineering and Pharmaceutical Properties. Mol. Pharm. 2020, 17, 4435–4442. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, B.; Das, S.; Lal, G.; Soni, S.R.; Ghosh, A.; Reddy, C.M.; Ghosh, S. Screening, crystal structures and solubility studies of a series of multidrug salt hydrates and cocrystals of fenamic acids with trimethoprim and sulfamethazine. J. Mol. Struct. 2020, 1199, 127028. [Google Scholar] [CrossRef]
- Darious, R.S.; Muthiah, P.T.; Perdih, F. Supramolecular hydrogen-bonding patterns in salts of the antifolate drugs trimethoprim and pyrimethamine. Acta Crystallogr. C Struct. 2018, 74, 487–503. [Google Scholar] [CrossRef]
- Giuseppetti, G.; Tadini, C.; Bettinetti, G.P. 1/1-Molecular-complex of trimethoprim and sulfametrole. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1994, 50, 1289–1291. [Google Scholar] [CrossRef]
- Muthiah, P.T.; Hemamalini, M.; Bocelli, G.; Cantoni, A. Hydrogen-bonded supramolecular motifs in crystal structures of trimethoprim barbiturate monohydrate (I) and 2-amino-4,6-dimethylpyrimidinium barbiturate trihydrate (II). Struct. Chem. 2007, 18, 171–180. [Google Scholar] [CrossRef]
- Raj, S.B.; Sethuraman, V.; Francis, S.; Hemamalini, M.; Muthiah, P.T.; Bocelli, G.; Cantoni, A.; Rychlewska, U.; Warzajtis, B. Supramolecular organization via hydrogen bonding in trimethoprim sulfonate salts. CrystEngComm 2003, 15, 70–76. [Google Scholar]
- Sardone, N.; Bettinetti, G.; Sorrenti, M. Trimethoprim-sulfadimidine 1:2 molecular complex monohydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1997, 53, 1295–1299. [Google Scholar] [CrossRef]
- Ton, Q.C.; Egert, E. Cocrystals of the antibiotic trimethoprim with glutarimide and 3,3-dimethylglutarimide held together by three hydrogen bonds. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2015, 71, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Tilborg, A.; Carletta, A.; Wouters, J. Structural and energy insights on solid-state complexes with trimethoprim: A combined theoretical and experimental investigation. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2015, 71, 406–415. [Google Scholar] [CrossRef]
- Stanley, N.; Sethuraman, V.; Muthiah, P.T.; Luger, P.; Weber, M. Crystal engineering of organic salts: Hydrogen-bonded supramolecular motifs in pyrimethamine hydrogen glutarate and pyrimethamine formate. Cryst. Growth Des. 2002, 2, 631–635. [Google Scholar] [CrossRef]
- Baptista, J.A.; Castro, R.A.E.; Rosado, M.T.S.; Maria, T.M.R.; Silva, M.R.; Canotilho, J.; Eusébio, M.E.S. Polymorphic Cocrystals of the Antimalarial Drug Pyrimethamine: Two Case Studies. Cryst. Growth Des. 2021, 21, 3699–3713. [Google Scholar] [CrossRef]
- Delori, A.; Galek, P.T.A.; Pidcock, E.; Patni, M.; Jones, W. Knowledge-based hydrogen bond prediction and the synthesis of salts and cocrystals of the anti-malarial drug pyrimethamine with various drug and GRAS molecules. CrystEngComm 2013, 15, 2916–2928. [Google Scholar] [CrossRef]
- Balasubramani, K.; Muthiah, P.T.; Bocelli, G.; Cantoni, A. Pyrimethaminium nicotinate monohydrate. Acta Crystallogr. Sect. E 2007, 63, O4452–O4683. [Google Scholar] [CrossRef]
- Balasubramani, K.; Muthiah, P.T.; Rychlewska, U.; Plutecka, A. Hydrogen-bonding patterns in pyrimethaminium dinitrate. Acta Crystallogr. Sect. C Cryst. Struct. 2005, 61, O586–O588. [Google Scholar] [CrossRef]
- Devi, P.; Muthiah, P.T.; Rychlewska, U.; Plutecka, A. Hydrogen-bonding patterns in pyrimethaminium 3-chlorobenzoate. Acta Crystallogr. Sect. E 2006, 62, O3704–O3706. [Google Scholar] [CrossRef] [Green Version]
- Hemamalini, M.; Muthiah, P.T.; Sridhar, B.; Rajaram, R.K. Pyrimethaminium sulfosalicylate monohydrate. Acta Crystallogr. Sect. E. 2005, 61, O1480–O1482. [Google Scholar] [CrossRef]
- Sethuraman, V.; Stanley, N.; Muthiah, P.T.; Sheldrick, W.S.; Winter, M.; Luger, P.; Weber, M. Isomorphism and crystal engineering: Organic ionic ladders formed by supramolecular motifs in pyrimethamine salts. Cryst. Growth Des. 2003, 3, 823–828. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wang, X.Y.; Hong, M.H.; Yi, D.X.; Qi, M.H.; Ren, G.B. Structure properties of scoparone: Polymorphs and cocrystals. J. Mol. Struct. 2019, 1191, 323–336. [Google Scholar] [CrossRef]
- Hutzler, W.M.; Bolte, M. Sulfur as hydrogen-bond acceptor in cocrystals of 2-thio-modified thymine. Acta Crystallogr. Sect. C 2018, 74, 21–30. [Google Scholar] [CrossRef]
- Hutzler, W.M.; Egert, E.; Bolte, M. 6-Propyl-2-thiouracil versus 6-methoxymethyl-2-thiouracil: Enhancing the hydrogen-bonded synthon motif by replacement of a methylene group with an O atom. Acta Crystallogr. Sect. C 2016, 72, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Hutzler, W.M.; Egert, E.; Bolte, M. One barbiturate and two solvated thiobarbiturates containing the triply hydrogen-bonded ADA/DAD synthon, plus one ansolvate and three solvates of their coformer 2,4-diaminopyrimidine. Acta Crystallogr. Sect. C 2016, 72, 705–715. [Google Scholar] [CrossRef]
- Matulkova, I.; Mathauserova, J.; Cisarova, I.; Nemec, I.; Fabry, J. The study of crystal structures and vibrational spectra of inorganic salts of 2,4-diaminopyrimidine. J. Mol. Struct. 2016, 1103, 82–93. [Google Scholar] [CrossRef]
- Hutzler, W.M.; Egert, E. Cocrystals of 6-methyl-2-thiouracil: Presence of the acceptor-donor-acceptor/donor-acceptor-donor synthon. Acta Crystallogr. Sect. C 2015, 71, 229–238. [Google Scholar] [CrossRef]
- Abramov, Y.A.; Loschen, C.; Klamt, A. Rational coformer or solvent selection for pharmaceutical cocrystallization or desolvation. J. Pharm. Sci. 2012, 101, 3687–3697. [Google Scholar] [CrossRef] [Green Version]
- Loschen, C.; Klamt, A. Solubility prediction, solvate and cocrystal screening as tools for rational crystal engineering. J. Pharm. Pharmacol. 2015, 67, 803–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prigogine, I.; Defay, R. Chemical Thermodynamics; Longmans, Green and Co: Glasgow, UK, 1954. [Google Scholar]
- Hohne, G.W.H.; Cammenga, H.K.; Eysel, W.; Gmelin, E.; Hemminger, W. The temperature calibration of scanning calorimeters. Thermochim. Acta 1990, 160, 1–12. [Google Scholar] [CrossRef]
- Gallis, H.E.; vanMiltenburg, J.C. Mixtures of d- and l-carvone 2. Adiabatic calorimetry on the equimolar mixture. Thermochim. Acta 1996, 274, 223–230. [Google Scholar] [CrossRef]
- Chan, H.C.S.; Woollam, G.R.; Wagner, T.; Schmidt, M.U.; Lewis, R.A. Can picolinamide be a promising cocrystal former? CrystEngComm 2014, 16, 4365–4368. [Google Scholar] [CrossRef] [Green Version]
- Habgood, M.; Deij, M.A.; Mazurek, J.; Price, S.L.; ter Horst, J.H. Carbamazepine Co-crystallization with Pyridine Carboxamides: Rationalization by Complementary Phase Diagrams and Crystal Energy Landscapes. Cryst. Growth Des. 2010, 10, 903–912. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond, IUCr Monographs on Crystallography; Oxford University Press: Oxford, UK, 2001; Volume 9. [Google Scholar]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Ebisuzaki, Y.; Boyle, P.D.; Smith, J.A. Methylxanthines 1. Anhydrous theophylline. Acta Crystallogr. Sect. C 1997, 53, 777–779. [Google Scholar] [CrossRef]
- Raauf, A.M.R.; Al-Smaism, R.F.; Thejeel, K.A.; Rasheed, H.A.M. Synthesis, characterization and antimicrobial study via new heterocyclic derivatives of trimethoprim. Nat. Prod. Res. 2019, 33, 1277–1283. [Google Scholar] [CrossRef]
- Sethuraman, V.; Muthiah, P.T. Hydrogen-bonded supramolecular ribbons in the antifolate drug pyrimethamine. Acta Crystallogr. Sect. E 2002, 58, o817–o819. [Google Scholar] [CrossRef]
- Rauf, K. (Department of Chemistry, Quaid-i-Azam University, Islamabad Pakistan); Bolte, M. (Institut für Anorganische Chemie, J.W. Goethe-Universität Frankfurt Germany). 2,4-Diamino-5-(3,4,5-trimethoxybenzyl)pyrimidine. CSD Communication (Private Communication), 2006. [CrossRef]
- Miwa, Y.; Mizuno, T.; Tsuchida, K.; Taga, T.; Iwata, Y. Experimental charge density and electrostatic potential in nicotinamide. Acta Crystallogr. Sect. B 1999, 55, 78–84. [Google Scholar] [CrossRef]
- Takano, T.; Sasada, Y.; Kakudo, M. Crystal and molecular structure of picolinamide. Acta Crystallogr. 1966, 21, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Evora, A.O.L.; Castro, R.A.E.; Maria, T.M.R.; Rosado, M.T.S.; Silva, M.R.; Canotilho, J.; Eusébio, M.E.S. Resolved structures of two picolinamide polymorphs. Investigation of the dimorphic system behaviour under conditions relevant to co-crystal synthesis. CrystEngComm 2012, 14, 8649–8657. [Google Scholar]
- Aakeroy, C.B.; Beatty, A.M.; Helfrich, B.A.; Nieuwenhuyzen, M. Do polymorphic compounds make good cocrystallizing agents? A structural case study that demonstrates the importance of synthon flexibility. Cryst. Growth Des. 2003, 3, 159–165. [Google Scholar]
- Lehmann, C.W.; Stowasser, F. The crystal structure of anhydrous beta-caffeine as determined from X-ray powder-diffraction data. Chem. Eur. J. 2007, 13, 2908–2911. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Klamt, A. COSMOfrag: A novel tool for high-throughput ADME property prediction and similarity screening based on quantum chemistry. J. Chem. Inf. Comput. Sci. 2005, 45, 1169–1177. [Google Scholar] [CrossRef] [Green Version]
- Evora, A.O.L.; Castro, R.A.E.; Maria, T.M.R.; Silva, M.R.; Canotilho, J.; Eusébio, M.E.S. Lamotrigine: Design and synthesis of new multicomponent solid forms. Eur. J. Pharm. Sci. 2019, 129, 148–162. [Google Scholar] [CrossRef]
- Choquesillo-Lazarte, D.; Garcia-Ruiz, J.M. Poly(ethylene) oxide for small-molecule crystal growth in gelled organic solvents. J. Appl. Crystallogr. 2011, 44, 172–176. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97, Program for Solution of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
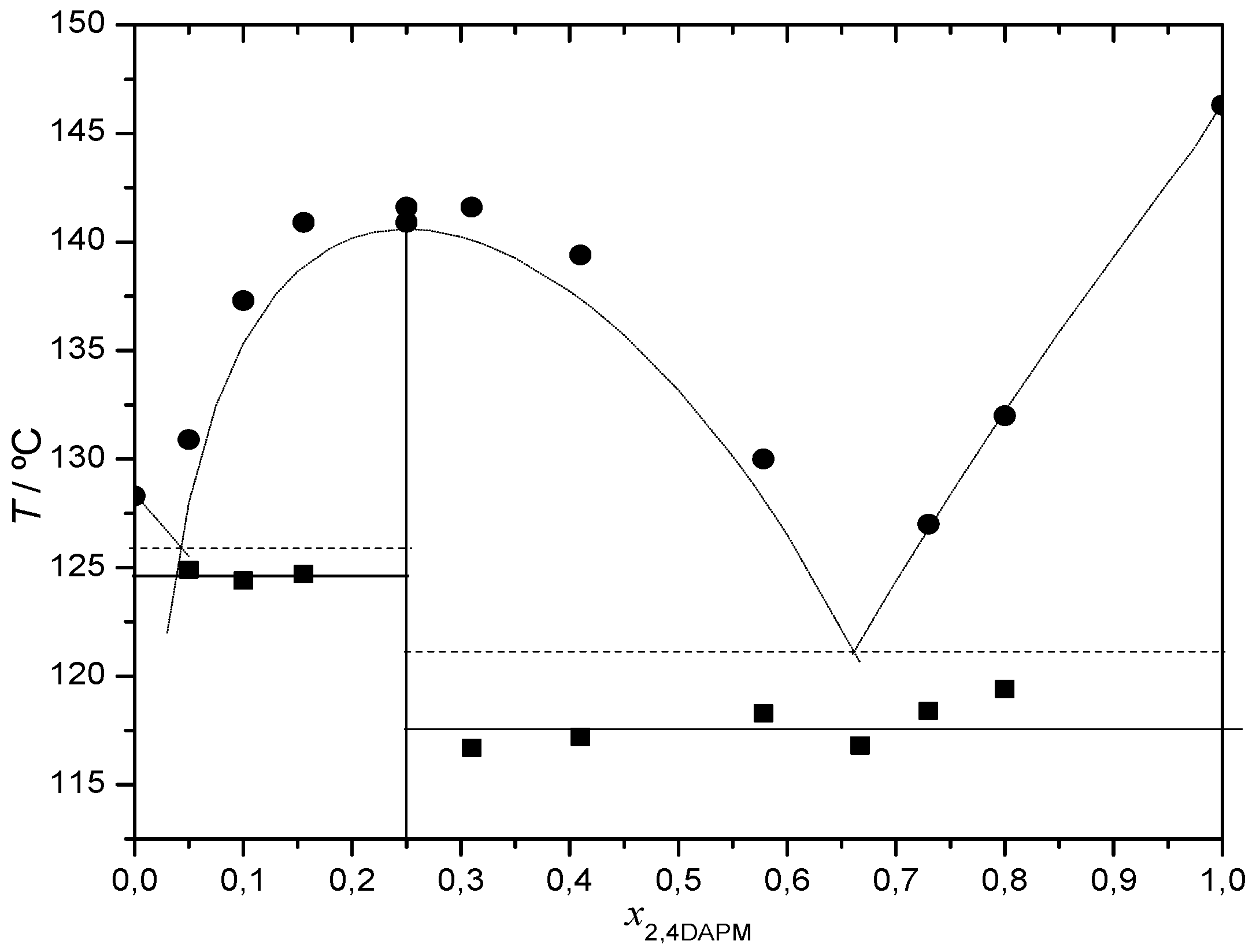




| ffit x = 0.50 | ΔHex x = 0.33 | ΔHex x = 0.50 | ΔHex x = 0.67 | ffit x = 0.50 | ΔHex x = 0.33 | ΔHex x = 0.5 | ΔHex x = 0.67 | ffit x = 0.50 | ΔHex x = 0.33 | ΔHex x = 0.5 | ΔHex x = 0.67 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Picolinamide | Isonicotinamide | Nicotinamide | ||||||||||
| DAP | 1.9 | −0.14 | −0.15 | −0.12 | 1.9 | −0.15 | −0.16 | −0.14 | 1.9 | −0.13 | −0.14 | −0.12 |
| TMP | 4.6 | 0.02 | 0.02 | 0.02 | 4.6 | 0.04 | 0.04 | 0.03 | 4.6 | 0.05 | 0.04 | 0.03 |
| PMA | 3.0 | −0.14 | −0.15 | −0.12 | 3.0 | −0.06 | −0.06 | −0.05 | 3.0 | −0.04 | −0.04 | −0.03 |
| Theophylline | Caffeine | |||||||||||
| DAP | 1.1 | −0.46 | −0.54 | −0.49 | 1.0 | −0.50 | −0.62 | −0.60 | ||||
| TMP | 3.9 | −0.26 | −0.28 | −0.25 | 4.0 | −0.14 | −0.16 | −0.14 | ||||
| PMA | 2.2 | −0.44 | −0.50 | −0.44 | 2.0 | −0.60 | −0.70 | −0.60 | ||||
| D–H···A | D–H/Å | H···A/Å | D···A/Å | D–H···A/° |
|---|---|---|---|---|
| (1:2:1) DAP:THEO:EtAc | ||||
| N1A–H1A···N6 | 0.86 | 1.91 | 2.760(3) | 171 |
| N1B–H1B···N5 | 0.86 | 2.05 | 2.907(3) | 178 |
| N7–H7A···O1A | 0.86 | 2.11 | 2.957(3) | 167 |
| N7–H7B···O1B | 0.86 | 2.20 | 2.992(3) | 152 |
| N8–H8A···O2Ba | 0.86 | 2.16 | 2.970(3) | 156 |
| N8–H8B···O2Ab | 0.86 | 2.08 | 2.932(3) | 174 |
| a: −1 − x, −y, 1 − z; b: 2 + x, 1/2 − y, 1/2 + z | ||||
| D–H···A | D–H/Å | H···A/Å | D∙∙∙A/Å | D–H∙∙∙A/° |
|---|---|---|---|---|
| N3–H3A∙∙∙N6Ai | 0.86 | 2.21 | 3.064(2) | 171 |
| N3–H3B∙∙∙O4A | 0.86 | 2.09 | 2.933(2) | 166 |
| N4–H4B∙∙∙O2i | 0.86 | 2.23 | 2.927(2) | 139 |
| N5A–H5A∙∙∙N1 | 0.86 | 1.97 | 2.820(2) | 170 |
| N5B–H5B∙∙∙N6Bv | 0.86 | 1.90 | 2.762(2) | 175 |
| i: x, −1 + y, z; ii: −x, 1/2 + y, 1/2 − z. | ||||
| (1:2:1) DAP:THEO:EtAc CCDC2108308 | (1:2) TMP:THEO (0.17 H2O) CCDC2109486 | |
|---|---|---|
| Temperature/K | 293 | 293 |
| Empirical formula | C22 H30 N12 O6 | C28H34N12O7.17 |
| Formula weight | 558.58 | 653.46 |
| Wavelength/Å | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c |
| a/Å | 7.4376(10) | 12.5845(6) |
| b/Å | 29.309(4) | 9.0152(4) |
| c/Å | 13.4200(14) | 27.8422(13) |
| β/º | 112.968(6) | 101.9030(10) |
| Volume/Å3 | 2693.5(6) | 3090.8(2) |
| Z | 4 | 4 |
| Calculated density/(g/cm3) | 1.377 | 1.404 |
| Absorption coefficient/mm–1 | 0.104 | 0.105 |
| F(000) | 1176 | 1374 |
| θ range for data collection/deg. | 2.15–25.49 | 1.49–25.72 |
| Index ranges | –8 < h < 9,–35 < k < 35, –16 < l < 16 | –15 < h < 15,–10 < k < 10,–33 < l < 33 |
| Reflections collected/unique | 28210/4963 | 57612/5861 |
| Completeness to θmax | 99.9% | 99.6 % |
| Data/restraints/parameters | 4963/0/367 | 5861/0/454 [R(int) = 0.0293] |
| Goodness-of-fit on F2 | 1.030 | 1.025 |
| Final R index [I > 2σ(I)] | R1 = 0.0599 wR2 = 0.1428 | R1 = 0.0388 wR2 = 0.1052 |
| R index (all data) | R1 = 0.1140 wR2 = 0.1744 | R1 = 0.0484 wR2 = 0.1144 |
| Largest diff. peak and hole (e Å –3) | –0.296 and 0.488 | –0.175 and 0.252 |
| Co-Former | Target | ||
|---|---|---|---|
| DAP | TMP | PMA | |
| PA |  |  |  |
| INA |  |  |  |
| NA |  |  |  |
| THEO |  |  |  (27,28) (27,28) |
| CAF |  |  |  (27) (27) |
 —no co-crystal;
—no co-crystal;  —co-crystal formed.
—co-crystal formed.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baptista, J.A.; Rosado, M.T.S.; Castro, R.A.E.; Évora, A.O.L.; Maria, T.M.R.; Ramos Silva, M.; Canotilho, J.; Eusébio, M.E.S. Dihydrofolate Reductase Inhibitors: The Pharmacophore as a Guide for Co-Crystal Screening. Molecules 2021, 26, 6721. https://doi.org/10.3390/molecules26216721
Baptista JA, Rosado MTS, Castro RAE, Évora AOL, Maria TMR, Ramos Silva M, Canotilho J, Eusébio MES. Dihydrofolate Reductase Inhibitors: The Pharmacophore as a Guide for Co-Crystal Screening. Molecules. 2021; 26(21):6721. https://doi.org/10.3390/molecules26216721
Chicago/Turabian StyleBaptista, João A., Mário T. S. Rosado, Ricardo A. E. Castro, António O. L. Évora, Teresa M. R. Maria, Manuela Ramos Silva, João Canotilho, and M. Ermelinda S. Eusébio. 2021. "Dihydrofolate Reductase Inhibitors: The Pharmacophore as a Guide for Co-Crystal Screening" Molecules 26, no. 21: 6721. https://doi.org/10.3390/molecules26216721






