Evaluation of the Membrane Damage Mechanism of Chlorogenic Acid against Yersinia enterocolitica and Enterobacter sakazakii and Its Application in the Preservation of Raw Pork and Skim Milk
Abstract
:1. Introduction
2. Results and Discussion
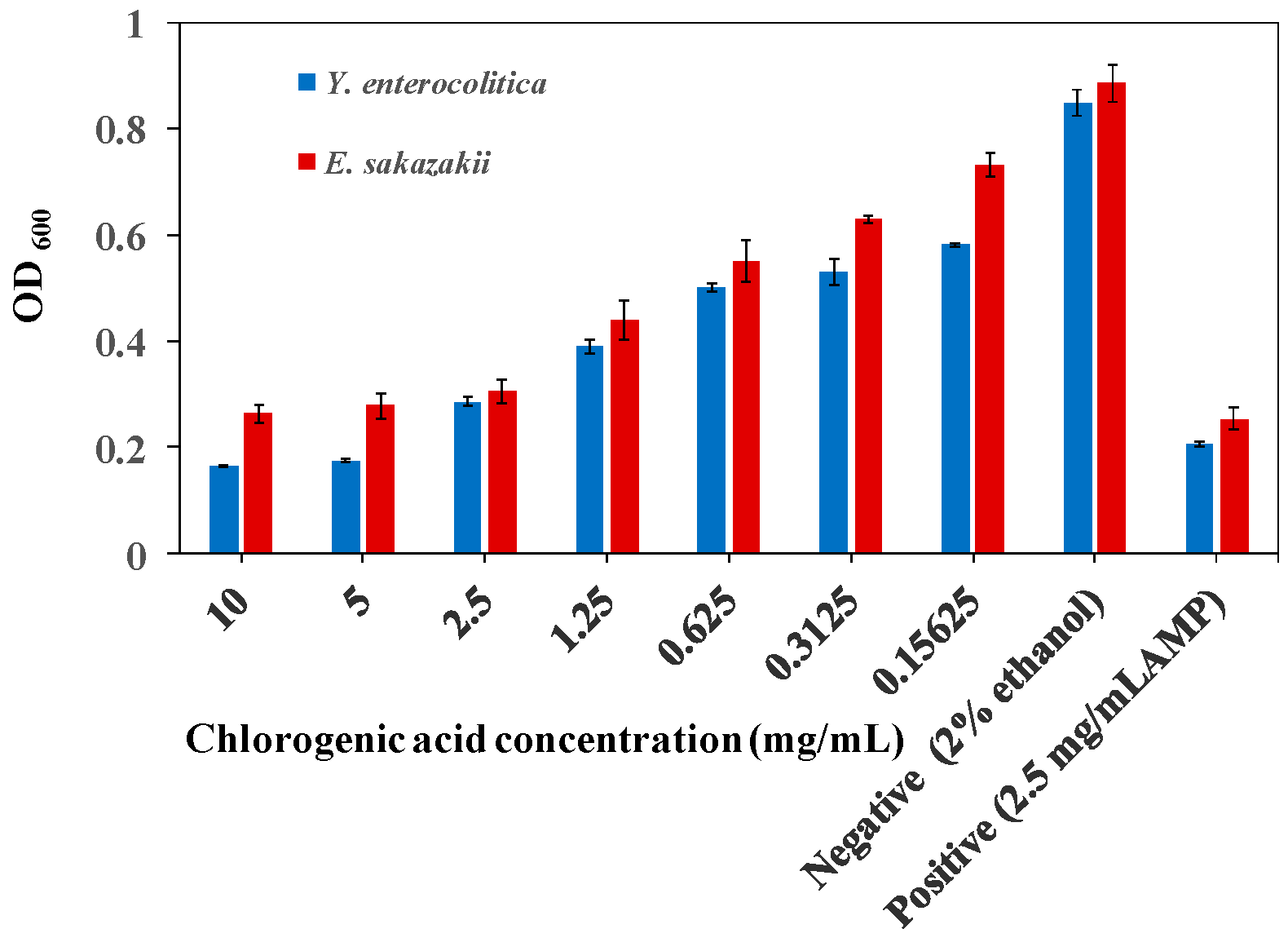
2.1. MIC of CA on Y. enterocolitica and E. sakazakii
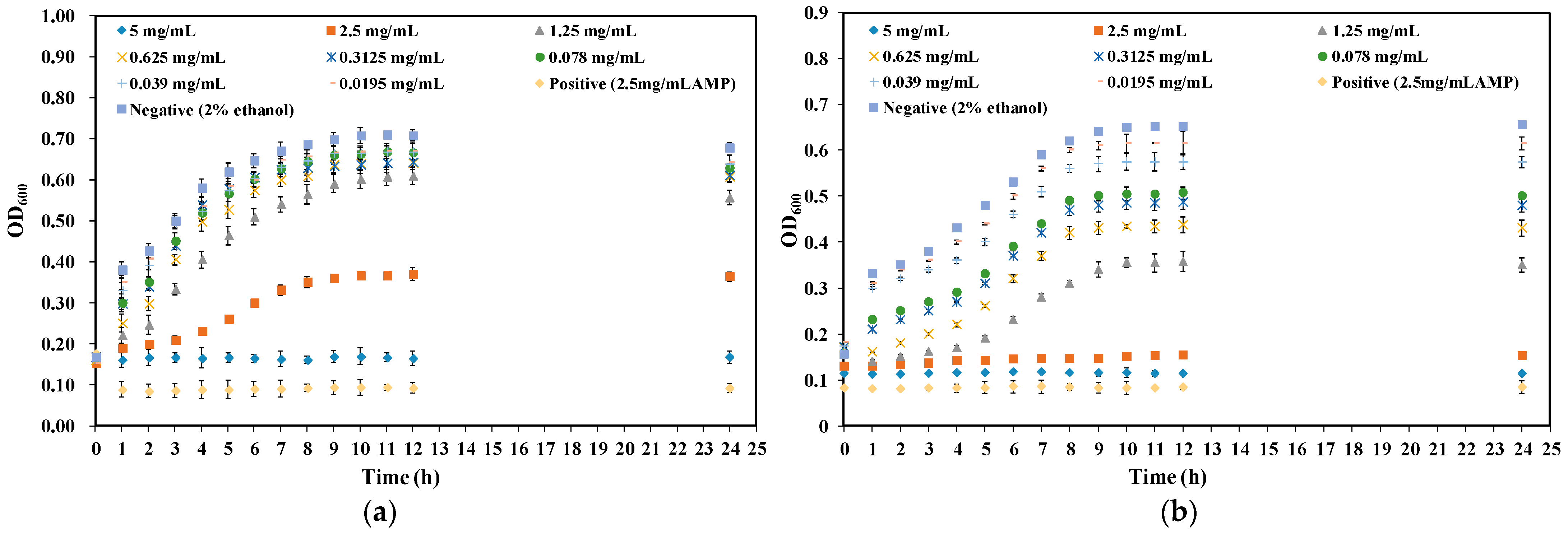
2.2. Effect of CA on Growth Curve of Y. enterocolitica and E. sakazakii
2.3. Effect of CA on Membrane Potential of Y. enterocolitica and E. sakazakii
2.4. Effect of CA on Intracellular ATP of Y. enterocolitica and E. sakazakii
2.5. Effect of CA on Intracellular pH of Y. enterocolitica and E. sakazakii
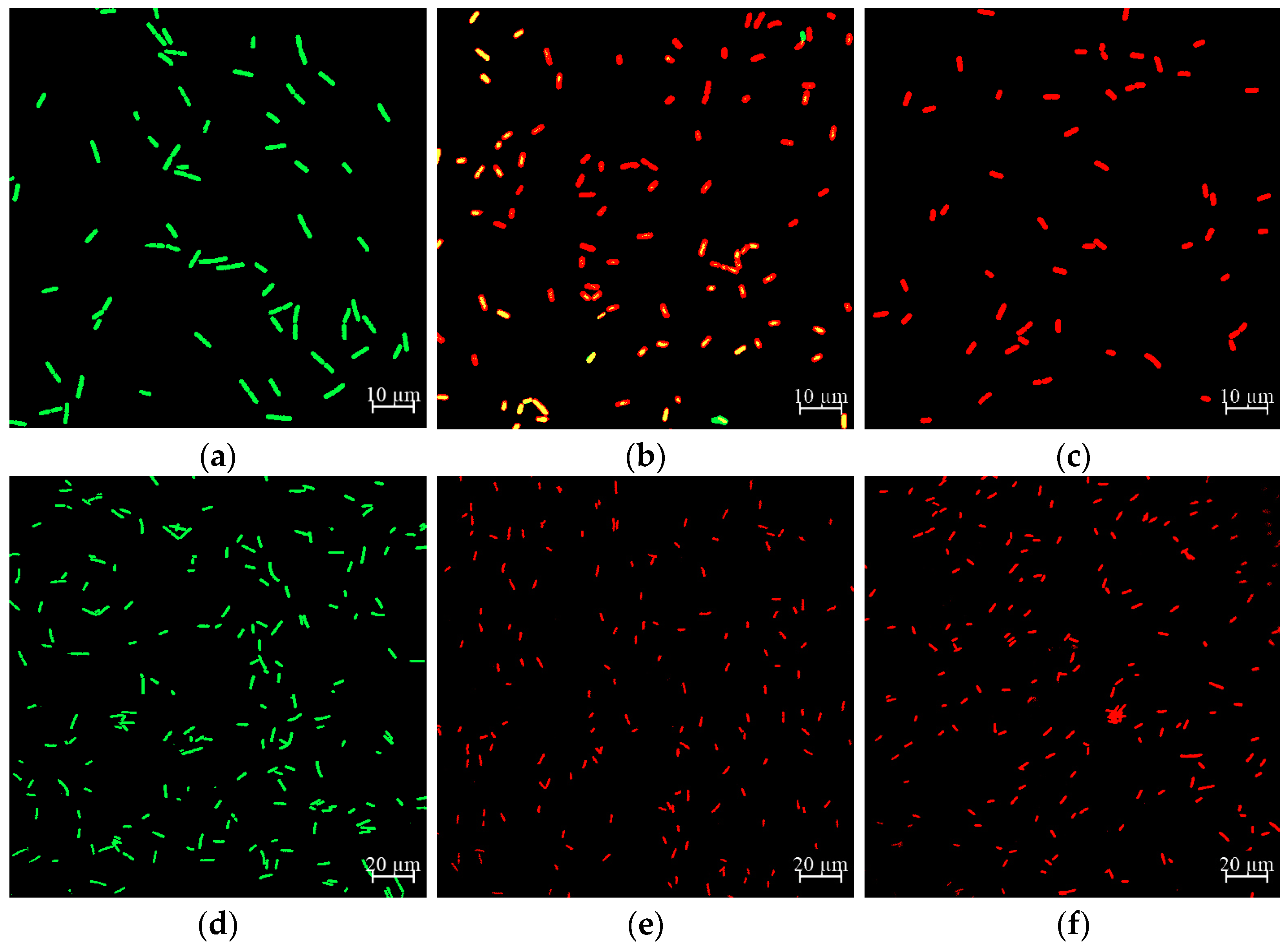
2.6. Effect of CA on Cell Membrane Damage of Y. enterocolitica and E. sakazakii
2.7. Effect of CA on Cell Morphology of Y. enterocolitica and E. sakazakii
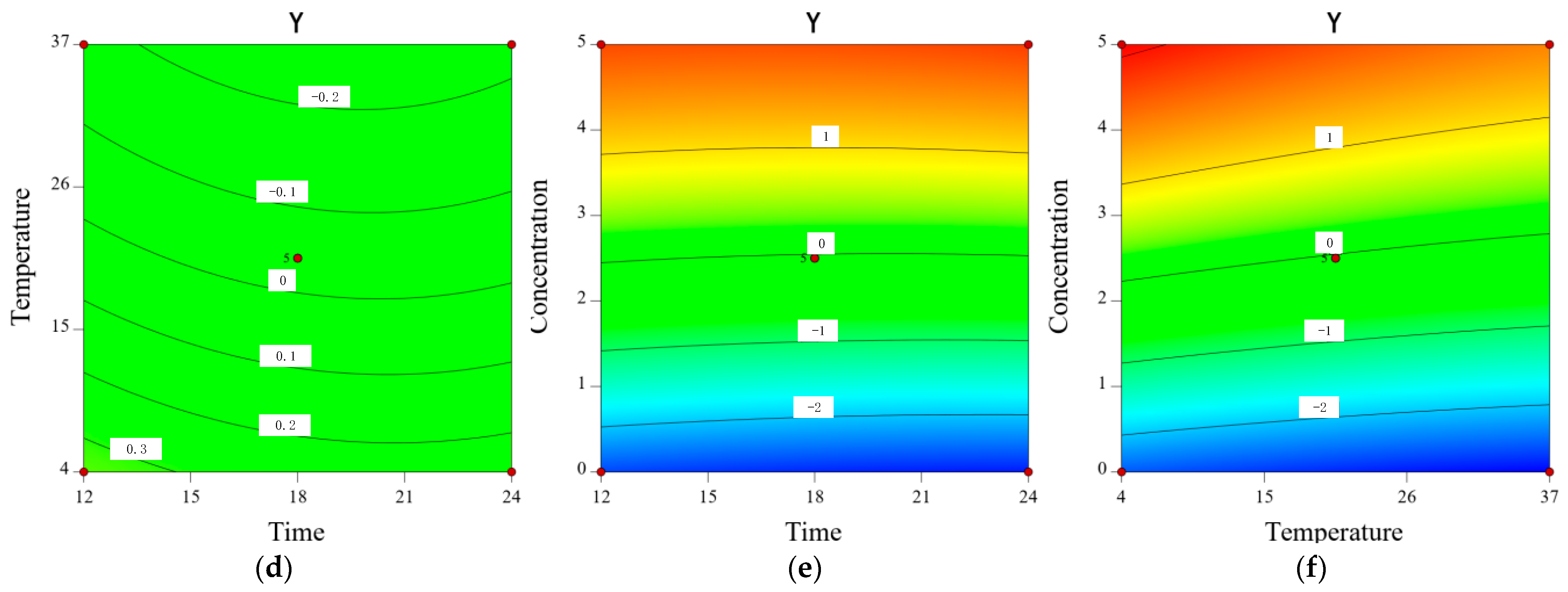
2.8. Inhibitory Effect of CA on Y. enterocolitica in Raw Pork and E. sakazakii in Skim Milk
3. Materials and Methods
3.1. Reagents
3.2. Bacterial Strains and Culture Conditions
3.3. Determination of MIC
3.4. Bacterial Growth Curve
3.5. Determination of Membrane Potential
3.6. Measurement of Intracellular ATP Concentrations
3.7. Measurement of Intracellular pH Level
3.8. Confocal Laser Scanning Microscopy Analysis
3.9. Field Emission Gun Scanning Electron Microscope Analysis
3.10. Modeling the Inhibitory Effect of Chlorogenic Acid on the Growth of Y. enterocolitica in Raw Pork and E. sakazalii in Skim Milk
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bosch, A.; Gkogka, E.; Le Guyader, F.S.; Loisy-Hamon, F.; Lee, A.; van Lieshout, L.; Marthi, B.; Myrmel, M.; Sansom, A.; Schultz, A.C.; et al. Foodborne viruses: Detection, risk assessment, and control options in food processing. Int. J. Food Microbiol. 2018, 285, 110–128. [Google Scholar] [CrossRef]
- Effland, T.; Lawson, A.; Balter, S.; Devinney, K.; Reddy, V.; Waechter, H.; Gravano, L.; Hsu, D. Discovering foodborne illness in online restaurant reviews. J. Am. Med. Inform. Assoc. 2018, 25, 1586–1592. [Google Scholar] [CrossRef]
- Liang, J.; Kou, Z.; Qin, S.; Chen, Y.; Li, Z.; Li, C.; Duan, R.; Hao, H.; Zha, T.; Gu, W.; et al. Novel Yersinia enterocolitica Prophages and a Comparative Analysis of Genomic Diversity. Front. Microbiol. 2019, 10, 1184. [Google Scholar] [CrossRef]
- Leon-Velarde, C.G.; Jun, J.W.; Skurnik, M. Yersinia Phages and Food Safety. Viruses 2019, 11, 1105. [Google Scholar] [CrossRef] [Green Version]
- Luciani, M.; Schirone, M.; Portanti, O.; Visciano, P.; Armillotta, G.; Tofalo, R.; Suzzi, G.; Sonsini, L.; Di Febo, T. Development of a rapid method for the detection of Yersinia enterocolitica serotype O:8 from food. Food Microbiol. 2018, 73, 85–92. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Cheng, N.; Xu, Y.; Huang, K.; Luo, Y.; Wang, P.; Duan, D.; Xu, W. Ultrasensitive Detection of Viable Enterobacter sakazakii by a Continual Cascade Nanozyme Biosensor. Anal. Chem. 2017, 89, 10194–10200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, D. Rapid Detection of Enterobacter Sakazakii in milk Powder using amino modified chitosan immunomagnetic beads. Int. J. Biol. Macromol. 2016, 93, 615–622. [Google Scholar] [CrossRef]
- Abbring, S.; Xiong, L.; Diks, M.A.P.; Baars, T.; Garssen, J.; Hettinga, K.; van Esch, B.C.A.M. Loss of allergy-protective capacity of raw cow’s milk after heat treatment coincides with loss of immunologically active whey proteins. Food Funct. 2020, 11, 4982–4993. [Google Scholar] [CrossRef] [PubMed]
- Skariyachan, S.; Govindarajan, S. Biopreservation potential of antimicrobial protein producing Pediococcus spp. towards selected food samples in comparison with chemical preservatives. Int. J. Food Microbiol. 2019, 291, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Ritota, M.; Manzi, P. Natural Preservatives from Plant in Cheese Making. Animals 2020, 10, 749. [Google Scholar] [CrossRef]
- Mei, J.; Ma, X.; Xie, J. Review on Natural Preservatives for Extending Fish Shelf Life. Foods 2019, 8, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, M.U.; Wali, A.F.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective Strategies for Neurological Disorders by Natural Products: An update. Curr. Neuropharmacol. 2019, 17, 247–267. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Iqbal, M.S.; Srivastava, J.K. Therapeutic Promises of Chlorogenic Acid with Special Emphasis on its Anti-Obesity Property. Curr. Mol. Pharmac. 2020, 13, 7–16. [Google Scholar] [CrossRef]
- Chen, X.D.; Tang, J.J.; Feng, S.; Huang, H.; Lu, F.N.; Lu, X.M.; Wang, Y.T. Chlorogenic Acid Improves PTSD-like Symptoms and Associated Mechanisms. Curr. Neuropharmacol. 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Liang, S.; Zhang, M.; Wang, Z.; Wang, Z.; Ren, X. The Effect of Chlorogenic Acid on Bacillus subtilis Based on Metabolomics. Molecules 2020, 25, 4038. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Xu, Y.; Zhang, B.; Xia, X. Antimicrobial effect and mode of action of chlorogenic acid on Staphylococcus aureus. Eur. Food Res. Technol. 2014, 238, 589–596. [Google Scholar] [CrossRef]
- Liu, P.; Miller, E.W. Electrophysiology, Unplugged: Imaging Membrane Potential with Fluorescent Indicators. Acc. Chem. Res. 2019, 53, 11–19. [Google Scholar] [CrossRef]
- Cho, J.; Choi, H.; Lee, J.; Kim, M.S.; Sohn, H.Y.; Lee, D.G. The antifungal activity and membrane-disruptive action of dioscin extracted from Dioscorea nipponica. Biochim. Biophys. Acta 2013, 1828, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Wang, X.; Liu, R.; Zhang, D.; Wang, X.; Sun, R.; Guo, W.; Yang, S.; Li, H.; Gong, G. Antibacterial mechanism of thymol against Enterobacter sakazakii. Food Control 2021, 123, 107716. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial Activity and Mechanism of Ginger Essential Oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Untiet, V.; Rose, C.R. Ionic signalling in astroglia beyond calcium. J. Physiol. 2020, 598, 1655–1670. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Guo, J.; Song, Y.; Chen, Z.; Lu, C.; Han, Y.; Li, H.; Hou, Y.; Zhao, R. Acceleration mechanism of bioavailable Fe(III) on Te(IV) bioreduction of Shewanella oneidensis MR-1: Promotion of electron generation, electron transfer and energy level. J. Hazard. Mater. 2021, 403, 123728. [Google Scholar] [CrossRef] [PubMed]
- Mallouk, Y.; Vayssier-Taussat, M.; Bonventre, J.V.; Polla, B.S. Heat shock protein 70 and ATP as partners in cell homeostasis (Review). Int. J. Mol. Med. 1999, 4, 463–474. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Zhang, Z.; Chen, M.; Zhang, D.; Tian, C.; Liu, M.; Jiang, G. The Antibacterial Activity and Mechanism of Action of Luteolin Against Trueperella pyogenes. Infect. Drug Resist. 2020, 13, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Kudo, H.; Sasaki, Y. Intracellular pH Determination for the Study of Acid Tolerance of Lactic Acid Bacteria. Methods Mol. Biol. 2019, 1887, 33–41. [Google Scholar] [PubMed]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Li, G.; Xu, Y.; Wang, X.; Zhang, B.; Shi, C.; Zhang, W.; Xia, X. Tannin-rich fraction from pomegranate rind damages membrane of Listeria monocytogenes. Foodborne Pathog. Dis. 2014, 11, 313–319. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Wang, L.; Chen, Y.; Long, Y. Optimization of staining with SYTO9/propidium iodide: Interplay, kinetics and impact on Brevibacillus brevis. Biotechniques 2020, 69, 88–98. [Google Scholar] [CrossRef]
- Su, M.; Liu, F.; Luo, Z.; Wu, H.; Zhang, X.; Wang, D.; Zhu, Y.; Sun, Z.; Xu, W.; Miao, Y. The Antibacterial Activity and Mechanism of Chlorogenic Acid Against Foodborne Pathogen Pseudomonas aeruginosa. Foodborne Pathog. Dis. 2019, 16, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, C.M.S.; Contreras-Castillo, C.J.; Da Gloria, E.M. In vitro mechanism of antibacterial action of a citrus essential oil on an enterotoxigenic Escherichia coli and Lactobacillus rhamnosus. J. Appl. Microbiol. 2020, 129, 541–553. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. Antibacterial and antioxidant effects of five spice and herb extracts as natural preservatives of raw pork. J. Sci. Food Agric. 2009, 89, 1879–1885. [Google Scholar] [CrossRef]
- Katawera, V.; Siedner, M.; Li, Y.B. Evaluation of the modified colorimetric resazurin microtiter plate-based antibacterial assay for rapid and reliable tuberculosis drug susceptibility testing. BMC Microbiol. 2014, 14, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Kong, F.; Shi, X.; Han, H.; Li, M.; Guan, B.; Yang, M.; Cao, X.; Tao, D.; Zheng, Y.; et al. Antibacterial activity and mechanism of lactobionic acid against Pseudomonas fluorescens and Methicillin-resistant Staphylococcus aureus and its application on whole milk. Food Control 2020, 108, 106876. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Qi, H.; Jiang, Y.; Gao, H. Antibacterial Activity and Membrane-Disruptive Mechanism of 3-p-trans-Coumaroyl-2-hydroxyquinic Acid, a Novel Phenolic Compound from Pine Needles of Cedrus deodara, against Staphylococcus aureus. Molecules 2016, 21, 1084. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene against Listeria monocytogenes. Molecules 2019, 25, 33. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Zhang, X.; Sun, Y.; Yang, M.; Song, K.; Zheng, Z.; Chen, Y.; Liu, X.; Jia, Z.; Dong, R.; et al. Antimicrobial activity of Ferulic Acid Against Cronobacter sakazakii and Possible Mechanism of Action. Foodborne Pathog. Dis. 2016, 13, 196–204. [Google Scholar] [CrossRef]
- Du, H.; Yang, J.; Lu, X.; Lu, Z.; Bie, X.; Zhao, H.; Zhang, C.; Lu, F. Purification, Characterization, and Mode of Action of Plantaricin GZ1-27, a Novel Bacteriocin against Bacillus cereus. J. Agric. Food Chem. 2018, 66, 4716–4724. [Google Scholar] [CrossRef]
- Xue, Y.; Yang, M.; Li, S.; Li, Z.; Liu, H.; Guo, Q.; Wang, C. The antibiotic activity and mechanisms of active metabolites (Streptomyces alboflavus TD-1) against Ralstonia solanacearum. Biotechnol. Lett. 2019, 41, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Li, X.; Zhang, D.; Hou, C.; Ijaz, M.; Bai, Y.; Xiao, X.; Zheng, X. Influence of adding cinnamon bark oil on meat quality of ground lamb during storage at 4 °C. Meat Sci. 2021, 171, 108269. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, J.; Aweya, J.J.; Yuan, Z.; Weng, W.; Zhang, Y.; Liu, G.M. Antimicrobial mechanism of Larimichthys crocea whey acidic protein-derived peptide (LCWAP) against Staphylococcus aureus and its application in milk. Int. J. Food Microbiol. 2020, 335, 108891. [Google Scholar] [CrossRef] [PubMed]



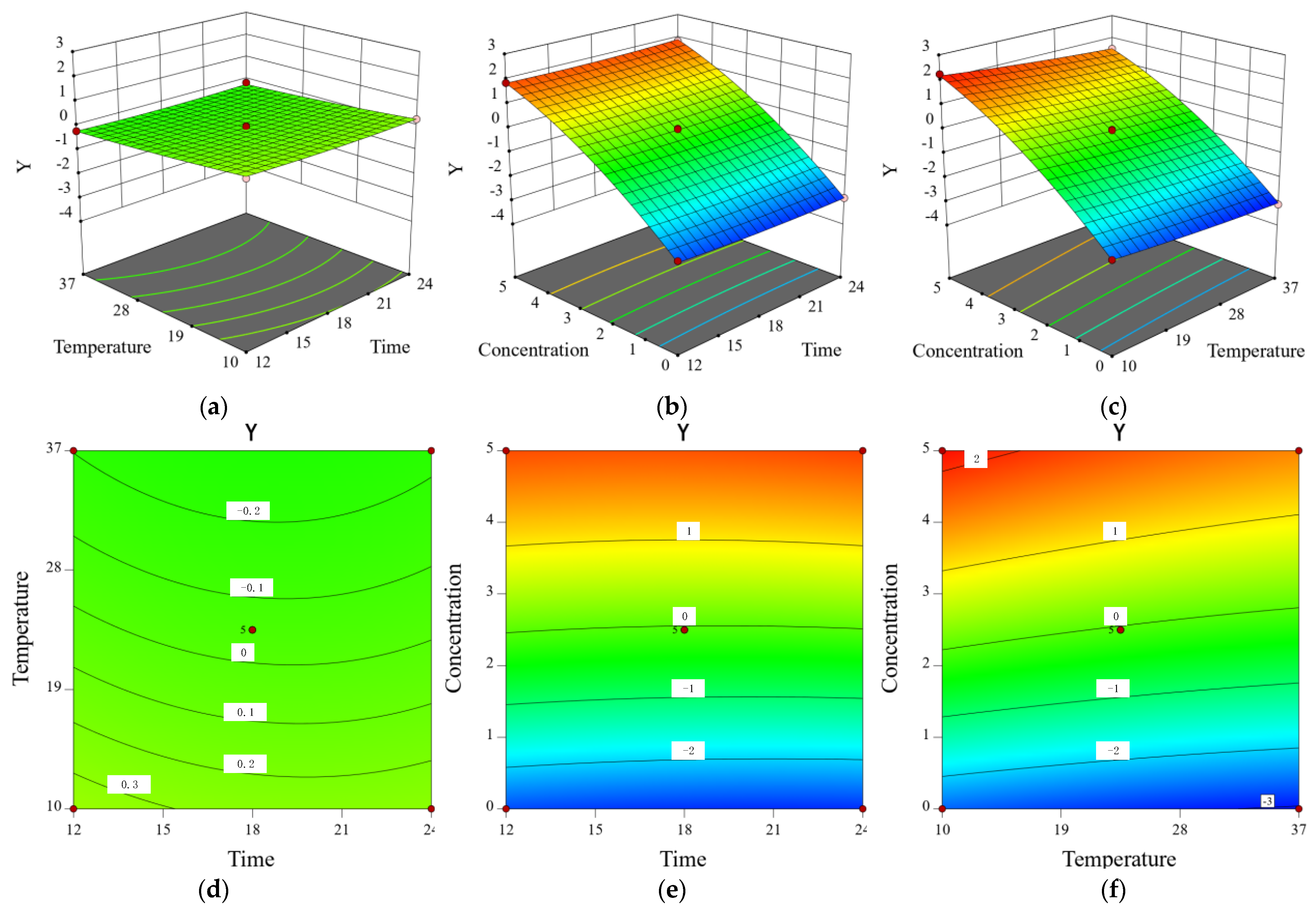

| Trial | X1 (h) | X2 (°C) | X3 (mg/mL) | Y (SD) |
|---|---|---|---|---|
| 1 | 12 | 37 | 2.5 | −0.17(0.02) |
| 2 | 18 | 20.5 | 2.5 | −0.07(0.06) |
| 3 | 12 | 4 | 2.5 | 0.29(0.03) |
| 4 | 18 | 20.5 | 2.5 | −0.08(0.05) |
| 5 | 18 | 20.5 | 2.5 | −0.04(0.02) |
| 6 | 24 | 4 | 2.5 | 0.26(0.04) |
| 7 | 12 | 20.5 | 5 | 1.77(0.03) |
| 8 | 12 | 20.5 | 0 | −2.6(0.06) |
| 9 | 18 | 37 | 0 | −3.01(0.05) |
| 10 | 24 | 20.5 | 0 | −2.87(0.02) |
| 11 | 18 | 4 | 0 | −2.54(0.04) |
| 12 | 18 | 20.5 | 2.5 | 0.03(0.03) |
| 13 | 18 | 37 | 5 | 1.46(0.05) |
| 14 | 18 | 4 | 5 | 2.13(0.06) |
| 15 | 24 | 20.5 | 5 | 1.76(0.03) |
| 16 | 24 | 37 | 2.5 | −0.16(0.05) |
| 17 | 18 | 20.5 | 2.5 | −0.05(0.02) |
| Trial | X1 (h) | X2 (°C) | X3 (mg/mL) | Y (SD) |
|---|---|---|---|---|
| 1 | 18 | 23.5 | 2.5 | −0.09(0.07) |
| 2 | 24 | 23.5 | 0 | −2.89(0.02) |
| 3 | 18 | 10 | 5 | 2.24(0.04) |
| 4 | 24 | 23.5 | 5 | 1.83(0.05) |
| 5 | 12 | 37 | 2.5 | −0.2(0.02) |
| 6 | 12 | 10 | 2.5 | 0.3(0.03) |
| 7 | 12 | 23.5 | 0 | −2.66(0.05) |
| 8 | 18 | 23.5 | 2.5 | −0.09(0.02) |
| 9 | 12 | 23.5 | 5 | 1.85(0.04) |
| 10 | 18 | 37 | 0 | −3.12(0.03) |
| 11 | 18 | 23.5 | 2.5 | −0.07(0.06) |
| 12 | 18 | 23.5 | 2.5 | 0.01(0.03) |
| 13 | 24 | 10 | 2.5 | 0.29(0.07) |
| 14 | 24 | 37 | 2.5 | −0.15(0.04) |
| 15 | 18 | 10 | 0 | −2.58(0.02) |
| 16 | 18 | 37 | 5 | 1.53(0.03) |
| 17 | 18 | 23.5 | 2.5 | −0.02(0.05) |
| Source | Sum of Squares | df | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 42.71 | 9 | 4.75 | 1238.22 | <0.0001 |
| X1 | 0.0113 | 1 | 0.0113 | 2.94 | 0.1304 |
| X2 | 0.5100 | 1 | 0.5100 | 133.07 | <0.0001 |
| X3 | 41.13 | 1 | 41.13 | 10,731.54 | <0.0001 |
| X1X2 | 0.0004 | 1 | 0.0004 | 0.1044 | 0.7561 |
| X1X3 | 0.0169 | 1 | 0.0169 | 4.41 | 0.0739 |
| X2X3 | 0.0100 | 1 | 0.0100 | 2.61 | 0.1503 |
| X12 | 0.0110 | 1 | 0.0110 | 2.86 | 0.1348 |
| X23 | 0.0089 | 1 | 0.0089 | 2.32 | 0.1712 |
| X32 | 1.03 | 1 | 1.03 | 268.08 | <0.0001 |
| Residual | 0.0268 | 7 | 0.0038 | ||
| Lack of Fit | 0.0194 | 3 | 0.0065 | 3.45 | 0.1314 |
| Pure Error | 0.0075 | 4 | 0.0019 | ||
| Cor Total | 42.74 | 16 |
| Source | Sum of Squares | df | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 45.31 | 9 | 5.03 | 932.19 | <0.0001 |
| X1 | 0.0055 | 1 | 0.0055 | 1.02 | 0.3460 |
| X2 | 0.5995 | 1 | 0.5995 | 111.01 | <0.0001 |
| X3 | 43.71 | 1 | 43.71 | 8093.61 | <0.0001 |
| X1X2 | 0.0009 | 1 | 0.0009 | 0.1666 | 0.6953 |
| X1X3 | 0.0110 | 1 | 0.0110 | 2.04 | 0.1961 |
| X2X3 | 0.0072 | 1 | 0.0072 | 1.34 | 0.2854 |
| X12 | 0.0170 | 1 | 0.0170 | 3.14 | 0.1195 |
| X23 | 0.0099 | 1 | 0.0099 | 1.83 | 0.2178 |
| X32 | 0.9661 | 1 | 0.9661 | 178.88 | <0.0001 |
| Residual | 0.0378 | 7 | 0.0054 | ||
| Lack of Fit | 0.0297 | 3 | 0.0099 | 4.91 | 0.0793 |
| Pure Error | 0.0081 | 4 | 0.0020 | ||
| Cor Total | 45.35 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Wu, M.; Guo, W.; Li, H.; Gai, Z.; Gong, G. Evaluation of the Membrane Damage Mechanism of Chlorogenic Acid against Yersinia enterocolitica and Enterobacter sakazakii and Its Application in the Preservation of Raw Pork and Skim Milk. Molecules 2021, 26, 6748. https://doi.org/10.3390/molecules26216748
Tian L, Wu M, Guo W, Li H, Gai Z, Gong G. Evaluation of the Membrane Damage Mechanism of Chlorogenic Acid against Yersinia enterocolitica and Enterobacter sakazakii and Its Application in the Preservation of Raw Pork and Skim Milk. Molecules. 2021; 26(21):6748. https://doi.org/10.3390/molecules26216748
Chicago/Turabian StyleTian, Lu, Mi Wu, Wenyao Guo, Hui Li, Zhongchao Gai, and Guoli Gong. 2021. "Evaluation of the Membrane Damage Mechanism of Chlorogenic Acid against Yersinia enterocolitica and Enterobacter sakazakii and Its Application in the Preservation of Raw Pork and Skim Milk" Molecules 26, no. 21: 6748. https://doi.org/10.3390/molecules26216748
APA StyleTian, L., Wu, M., Guo, W., Li, H., Gai, Z., & Gong, G. (2021). Evaluation of the Membrane Damage Mechanism of Chlorogenic Acid against Yersinia enterocolitica and Enterobacter sakazakii and Its Application in the Preservation of Raw Pork and Skim Milk. Molecules, 26(21), 6748. https://doi.org/10.3390/molecules26216748






