HPLC Analysis of the Urinary Iodine Concentration in Pregnant Women
Abstract
:1. Introduction
2. Results
2.1. Development of the Ion-Pair HPLC–UV Method
2.2. Validation Results
2.2.1. Linearity, LOD and LOQ
2.2.2. Accuracy and Precision
2.2.3. Stability
2.2.4. In Vivo Study
3. Discussion
Limitations and Strength of the Study
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Ion-Pair HPLC–UV Conditions and Apparatus
4.3. Preparation of Standard Solutions and Quality Control Samples
4.4. Sample Preparation
4.5. Method Validation
4.6. In Vivo Application
4.7. Data Processing and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersson, M.; Karumbunathan, V.; Zimmermann, M.B. Global Iodine Status in 2011 and Trends over the Past Decade. J. Nutr. 2012, 142, 744–750. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-Deficiency Disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef]
- O’Kane, S.M.; Mulhern, M.S.; Pourshahidi, L.K.; Strain, J.J.; Yeates, A.J. Micronutrients, Iodine Status and Concentrations of Thyroid Hormones: A Systematic Review. Nutr. Rev. 2018, 76, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, D.; Majewska, K.; Kalantarova, A.; Szczepanek-Parulska, E.; Ruchała, M. The Rationale for Selenium Supplementation in Patients with Autoimmune Thyroiditis, According to the Current State of Knowledge. Endokrynol. Pol. 2021, 72, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Biban, B.; Lichiardopol, C. Iodine Deficiency, Still a Global Problem? Curr. Health Sci. J. 2017, 43, 103–111. [Google Scholar] [CrossRef]
- Ha, S.K. Dietary Salt Intake and Hypertension. Electrolyte Blood Press. 2014, 12, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Musso, N.; Conte, L.; Carloni, B.; Campana, C.; Chiusano, M.C.; Giusti, M. Low-Salt Intake Suggestions in Hypertensive Patients Do Not Jeopardize Urinary Iodine Excretion. Nutrients 2018, 10, 1548. [Google Scholar] [CrossRef] [Green Version]
- Sant’Ana Leone de Souza, L.; de Oliveira Campos, R.; Dos Santos Alves, V.; Cerqueira, T.L.O.; da Silva, T.M.; Teixeira, L.S.G.; Feitosa, A.C.R.; de Aragão Dantas Alves, C.; Ramos, H.E. Hypertension and Salt-Restrictive Diet Promotes Low Urinary Iodine Concentration in High-Risk Pregnant Women: Results from a Cross-Sectional Study Conducted After Salt Iodination Reduction in Brazil. Biol. Trace Elem. Res. 2020, 197, 445–453. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iodine Global Network (IGN)—Global Iodine Nutrition Scorecard and Map. Available online: https://www.ign.org/scorecard.htm (accessed on 19 April 2021).
- Hubalewska-Dydejczyk, A.; Lewiński, A.; Milewicz, A.; Radowicki, S.; Poręba, R.; Karbownik-Lewińska, M.; Kostecka-Matyja, M.; Trofimiuk-Müldner, M.; Pach, D.; Zygmunt, A.; et al. Management of Thyroid Diseases during Pregnancy. Endokrynol. Pol. 2011, 62, 362–381. [Google Scholar] [PubMed]
- Zimmermann, M.B.; Gizak, M.; Abbott, K.; Andersson, M.; Lazarus, J.H. Iodine Deficiency in Pregnant Women in Europe. Lancet Diabetes Endocrinol. 2015, 3, 672–674. [Google Scholar] [CrossRef]
- Zimmer, M.; Sieroszewski, P.; Oszukowski, P.; Huras, H.; Fuchs, T.; Pawlosek, A. Polish Society of Gynecologists and Obstetricians Recommendations on Supplementation during Pregnancy. Ginekol. Pol. 2020, 91, 644–653. [Google Scholar] [CrossRef]
- Pearce, E.N.; Lazarus, J.H.; Moreno-Reyes, R.; Zimmermann, M.B. Consequences of Iodine Deficiency and Excess in Pregnant Women: An Overview of Current Knowns and Unknowns. Am. J. Clin. Nutr. 2016, 104 (Suppl. 3), 918S–923S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toloza, F.J.K.; Motahari, H.; Maraka, S. Consequences of Severe Iodine Deficiency in Pregnancy: Evidence in Humans. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef]
- Monaghan, A.M.; Mulhern, M.S.; McSorley, E.M.; Strain, J.J.; Dyer, M.; van Wijngaarden, E.; Yeates, A.J. Associations between Maternal Urinary Iodine Assessment, Dietary Iodine Intakes and Neurodevelopmental Outcomes in the Child: A Systematic Review. Thyroid. Res. 2021, 14, 14. [Google Scholar] [CrossRef]
- Abel, M.H.; Caspersen, I.H.; Meltzer, H.M.; Haugen, M.; Brandlistuen, R.E.; Aase, H.; Alexander, J.; Torheim, L.E.; Brantsæter, A.-L. Suboptimal Maternal Iodine Intake Is Associated with Impaired Child Neurodevelopment at 3 Years of Age in the Norwegian Mother and Child Cohort Study. J. Nutr. 2017, 147, 1314–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bath, S.C.; Steer, C.D.; Golding, J.; Emmett, P.; Rayman, M.P. Effect of Inadequate Iodine Status in UK Pregnant Women on Cognitive Outcomes in Their Children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013, 382, 331–337. [Google Scholar] [CrossRef]
- Levie, D.; Korevaar, T.I.M.; Bath, S.C.; Murcia, M.; Dineva, M.; Llop, S.; Espada, M.; van Herwaarden, A.E.; de Rijke, Y.B.; Ibarluzea, J.M.; et al. Association of Maternal Iodine Status With Child IQ: A Meta-Analysis of Individual Participant Data. J. Clin. Endocrinol. Metab. 2019, 104, 5957–5967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hynes, K.L.; Otahal, P.; Hay, I.; Burgess, J.R. Mild Iodine Deficiency during Pregnancy Is Associated with Reduced Educational Outcomes in the Offspring: 9-Year Follow-up of the Gestational Iodine Cohort. J. Clin. Endocrinol. Metab. 2013, 98, 1954–1962. [Google Scholar] [CrossRef] [Green Version]
- Reaching Optimal Iodine Nutrition in Pregnant and Lactating Women and Young Children. Available online: https://www.who.int/publications/m/item/WHO-statement-IDD-pregnantwomen-children (accessed on 14 April 2021).
- World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers; World Health Organization: Geneva, Switzerland, 2007; ISBN 978-92-4-159582-7. [Google Scholar]
- WHO. Urinary Iodine Concentrations for Determining Iodine Status in Populations. Available online: http://www.who.int/vmnis/indicators/urinaryiodine/en/ (accessed on 14 April 2021).
- Harding, K.B.; Peña-Rosas, J.P.; Webster, A.C.; Yap, C.M.; Payne, B.A.; Ota, E.; De-Regil, L.M. Iodine Supplementation for Women during the Preconception, Pregnancy and Postpartum Period. Cochrane Database Syst. Rev. 2017, 3, CD011761. [Google Scholar] [CrossRef]
- Jiang, H.; Powers, H.J.; Rossetto, G.S. A Systematic Review of Iodine Deficiency among Women in the UK. Public Health Nutr. 2019, 22, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, P.; Cook, P. The Assessment of Iodine Status—Populations, Individuals and Limitations. Ann. Clin. Biochem. 2019, 56, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. Methods to Assess Iron and Iodine Status. Br. J. Nutr. 2008, 99 (Suppl. 3), S2–S9. [Google Scholar] [CrossRef]
- König, F.; Andersson, M.; Hotz, K.; Aeberli, I.; Zimmermann, M.B. Ten Repeat Collections for Urinary Iodine from Spot Samples or 24-Hour Samples Are Needed to Reliably Estimate Individual Iodine Status in Women. J. Nutr. 2011, 141, 2049–2054. [Google Scholar] [CrossRef]
- Perrine, C.G.; Cogswell, M.E.; Swanson, C.A.; Sullivan, K.M.; Chen, T.-C.; Carriquiry, A.L.; Dodd, K.W.; Caldwell, K.L.; Wang, C.-Y. Comparison of Population Iodine Estimates from 24-Hour Urine and Timed-Spot Urine Samples. Thyroid 2014, 24, 748–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelor, C.P.; Dasgupta, P.K. Review of Analytical Methods for the Quantification of Iodine in Complex Matrices. Anal. Chim. Acta 2011, 702, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Haap, M.; Roth, H.J.; Huber, T.; Dittmann, H.; Wahl, R. Urinary Iodine: Comparison of a Simple Method for Its Determination in Microplates with Measurement by Inductively-Coupled Plasma Mass Spectrometry. Sci. Rep. 2017, 7, 39835. [Google Scholar] [CrossRef] [Green Version]
- Eastman, C.J.; Ma, G.; Li, M. Optimal Assessment and Quantification of Iodine Nutrition in Pregnancy and Lactation: Laboratory and Clinical Methods, Controversies and Future Directions. Nutrients 2019, 11, 2378. [Google Scholar] [CrossRef] [Green Version]
- Khazan, M.; Azizi, F.; Hedayati, M.; Hedayati, M. A Review on Iodine Determination Methods in Salt and Biological Samples. Scimetr 2013, 1. [Google Scholar] [CrossRef]
- Yu, S.; Yin, Y.; Cheng, Q.; Han, J.; Cheng, X.; Guo, Y.; Sun, D.; Xie, S.; Qiu, L. Validation of a Simple Inductively Coupled Plasma Mass Spectrometry Method for Detecting Urine and Serum Iodine and Evaluation of Iodine Status of Pregnant Women in Beijing. Scand. J. Clin. Lab. Investig. 2018, 78, 501–507. [Google Scholar] [CrossRef]
- Li, Y.; Ding, S.; Han, C.; Liu, A.; Shan, Z.; Teng, W.; Mao, J. Concentration-Dependent Differences in Urinary Iodine Measurements Between Inductively Coupled Plasma Mass Spectrometry and the Sandell-Kolthoff Method. Biol. Trace Elem. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Macours, P.; Aubry, J.C.; Hauquier, B.; Boeynaems, J.M.; Goldman, S.; Moreno-Reyes, R. Determination of Urinary Iodine by Inductively Coupled Plasma Mass Spectrometry. J. Trace Elem. Med. Biol. 2008, 22, 162–165. [Google Scholar] [CrossRef]
- Cui, L.; Wen, J.; Zhou, T.; Wang, S.; Fan, G. Optimization and Validation of an Ion-Pair RP-HPLC-UV Method for the Determination of Total Free Iodine in Rabbit Plasma: Application to a Pharmacokinetic Study. Biomed. Chromatogr. 2009, 23, 1151–1159. [Google Scholar] [CrossRef]
- Ion Pair Chromatography—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/chemistry/ion-pair-chromatography (accessed on 15 September 2021).
- Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/bioanalytical-method-validation (accessed on 14 April 2021).
- Mizéhoun-Adissoda, C.; Desport, J.-C.; Houinato, D.; Bigot, A.; Dalmay, F.; Preux, P.-M.; Bovet, P.; Moesch, C. Evaluation of Iodine Intake and Status Using Inductively Coupled Plasma Mass Spectrometry in Urban and Rural Areas in Benin, West Africa. Nutrition 2016, 5, 560–565. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, W.; Mo, Z.; Wang, Y.; Mao, G.; Wang, X.; Lou, X. An Increase in Consuming Adequately Iodized Salt May Not Be Enough to Rectify Iodine Deficiency in Pregnancy in an Iodine-Sufficient Area of China. Int. J. Environ. Res. Public Health 2017, 14, 206. [Google Scholar] [CrossRef] [Green Version]
- Mioto, V.C.B.; Monteiro, A.C.D.C.N.; de Camargo, R.Y.A.; Borel, A.R.; Catarino, R.M.; Kobayashi, S.; Chammas, M.C.; Marui, S. High Prevalence of Iodine Deficiency in Pregnant Women Living in Adequate Iodine Area. Endocr. Connect. 2018, 7, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Snart, C.J.P.; Keeble, C.; Taylor, E.; Cade, J.E.; Stewart, P.M.; Zimmermann, M.; Reid, S.; Threapleton, D.E.; Poston, L.; Myers, J.E.; et al. Maternal Iodine Status and Associations with Birth Outcomes in Three Major Cities in the United Kingdom. Nutrients 2019, 11, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, B.A.; Shields, B.M.; He, X.; Pearce, E.N.; Braverman, L.E.; Sturley, R.; Vaidya, B. Iodine Deficiency amongst Pregnant Women in South-West England. Clin. Endocrinol. 2017, 86, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Dahl, L.; Wik Markhus, M.; Sanchez, P.V.R.; Moe, V.; Smith, L.; Meltzer, H.M.; Kjellevold, M. Iodine Deficiency in a Study Population of Norwegian Pregnant Women—Results from the Little in Norway Study (LiN). Nutrients 2018, 10, 513. [Google Scholar] [CrossRef] [Green Version]
- Manousou, S.; Andersson, M.; Eggertsen, R.; Hunziker, S.; Hulthén, L.; Nyström, H.F. Iodine Deficiency in Pregnant Women in Sweden: A National Cross-Sectional Study. Eur. J. Nutr. 2020, 59, 2535–2545. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.-T.; Vila, L.; Manresa, J.-M.; Casamitjana, R.; Prieto, G.; Toran, P.; Falguera, G.; Francés, L. The IODEGEST Study Group Impact of Dietary Habit, Iodine Supplementation and Smoking Habit on Urinary Iodine Concentration During Pregnancy in a Catalonia Population. Nutrients 2020, 12, 2656. [Google Scholar] [CrossRef] [PubMed]
- Manousou, S.; Eggertsen, R.; Hulthén, L.; Filipsson Nyström, H. A Randomized, Double-Blind Study of Iodine Supplementation during Pregnancy in Sweden: Pilot Evaluation of Maternal Iodine Status and Thyroid Function. Eur. J. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ittermann, T.; Albrecht, D.; Arohonka, P.; Bilek, R.; de Castro, J.J.; Dahl, L.; Filipsson Nystrom, H.; Gaberscek, S.; Garcia-Fuentes, E.; Gheorghiu, M.L.; et al. Standardized Map of Iodine Status in Europe. Thyroid 2020, 30, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Iodine Deficiency in Europe: A Continuing Public Health Problem. Available online: https://www.who.int/publications/i/item/9789241593960 (accessed on 22 June 2021).
- World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects|Global Health|JAMA|JAMA Network. Available online: https://jamanetwork.com/journals/jama/fullarticle/1760318 (accessed on 14 April 2021).
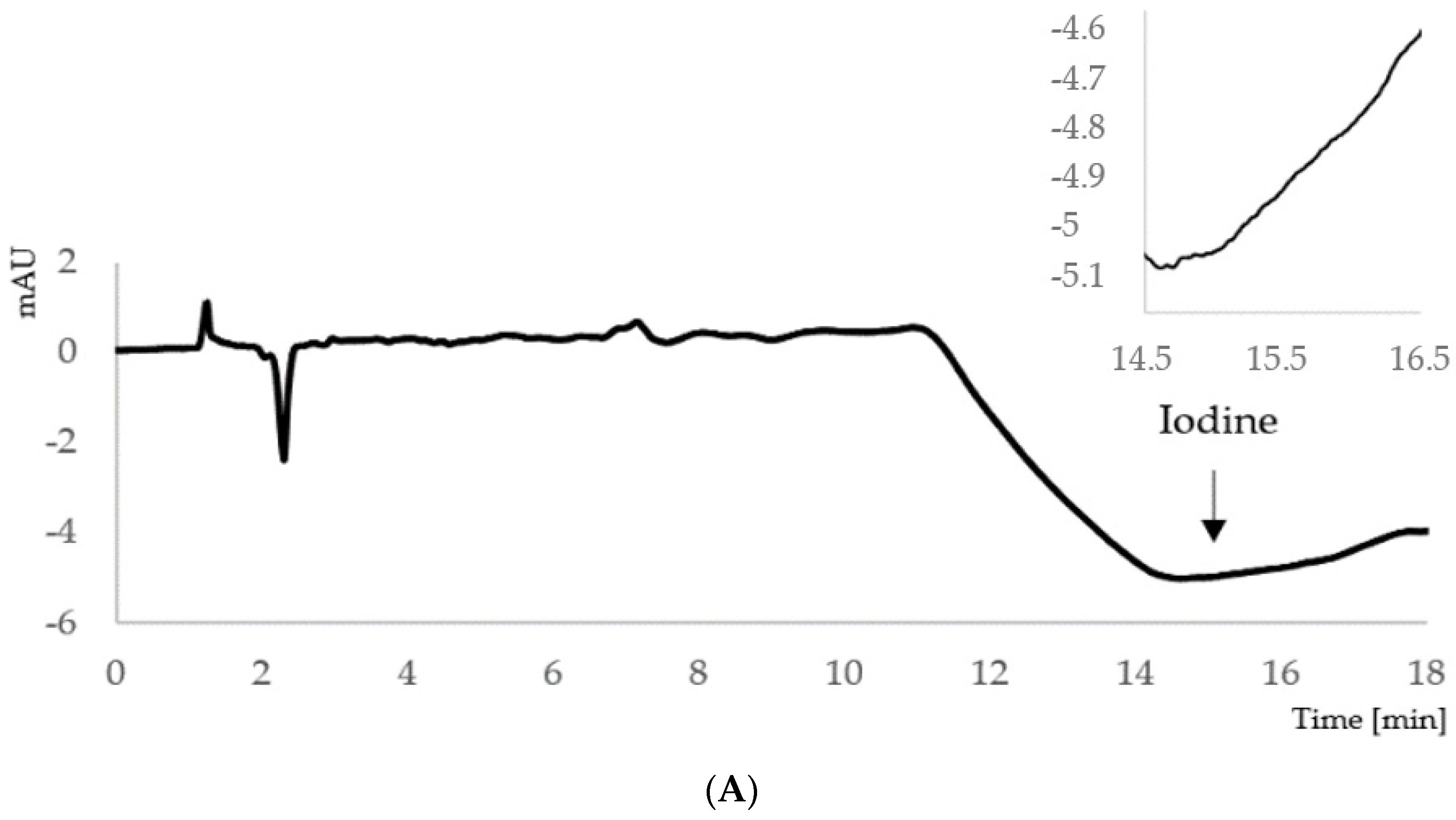

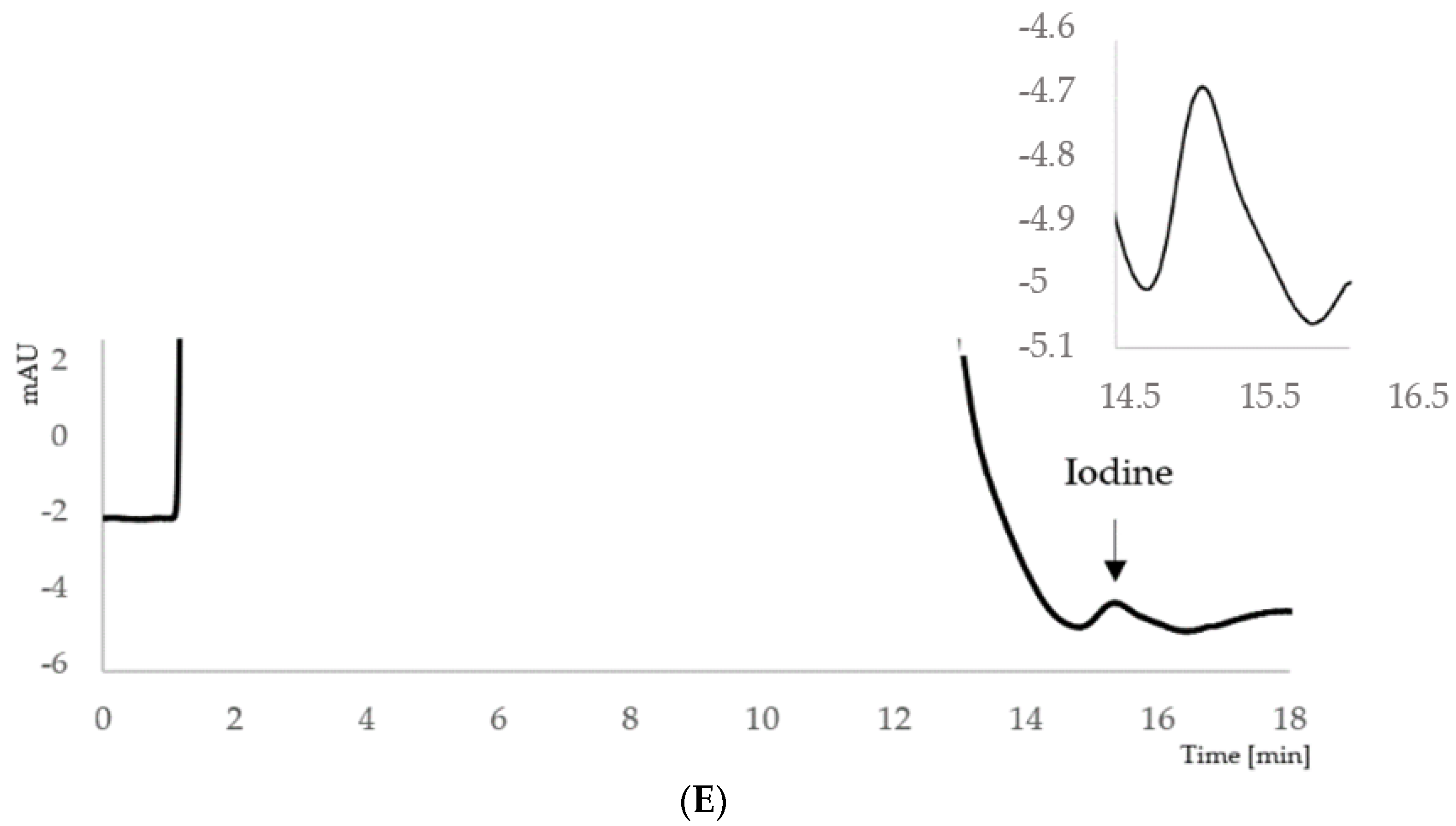


| Category of Iodine Supply in Pregnant Women | Median Urinary Iodine Concentration [μg/L] |
|---|---|
| Insufficient | <150 |
| Adequate | 150–249 |
| Above requirements | 250–499 |
| Excessive | ≥500 |
| QCs Concentration | Precision (RSD, %) | Accuracy (%RE) | ||
|---|---|---|---|---|
| Within-Run n = 5 | Between-Run n = 5 | Within-Run n = 5 | Between-Run n = 5 | |
| LOQ (50 μg/L) | 1.07 | 9.72 | 20.00 | 9.43 |
| Low (75 μg/L) | 8.44 | 9.12 | 0.06 | 2.16 |
| Medium (150 μg/L) | 8.57 | 9.71 | 4.88 | 4.77 |
| High (250 μg/L) | 2.92 | 4.69 | 0.38 | 0.99 |
| Concentration | High (n = 3) | Medium (n = 3) | Low (n = 3) |
|---|---|---|---|
| Long-term stability (−20 °C for 2 months) | |||
| Initial concentration (mean ± SD, µg/L) | 273 ± 2 | 145 ± 15 | 68.2 ± 6.0 |
| Concentration determined after stability study (mean ± SD, µg/L) | 263 ± 10 | 154 ± 6 | 73.3 ± 2.5 |
| Accuracy (RE, %) | 3.79 | 5.77 | 7.54 |
| Short-term stability (25 °C for 3 h) | |||
| Initial concentration (mean ± SD, µg/L) | 278 ± 22 | 196 ± 9 | 96.2 ± 7.9 |
| Concentration determined after stability study (mean ± SD, µg/L) | 263 ± 21 | 188 ± 8 | 108 ± 10 |
| Accuracy (RE, %) | 5.24 | 3.72 | 12.43 |
| Freeze-thaw stability (After three freeze-thaw cycles) | |||
| Initial concentration (mean ± SD, µg/L) | 273 ± 2 | 145 ± 15 | 68.2 ± 6.0 |
| Concentration determined after stability study (mean ± SD, µg/L) | 260 ± 18 | 127 ± 3 | 61.2 ± 4.6 |
| Accuracy (RE, %) | 4.76 | 12.75 | 10.27 |
| Stability in autosampler (25 °C for 24 h) | |||
| Initial concentration (mean ± SD, µg/L) | 306 ± 6 | 164 ± 30 | 107 ± 3 |
| Concentration determined after stability study (mean ± SD, µg/L) | 307 ± 5 | 173 ± 9 | 117 ± 3 |
| Accuracy (RE, %) | 0.29 | 5.17 | 9.28 |
| Method | Sample Preparation | Urine Volume | LOD [μg/L] | LOQ [μg/L] | Precision [%] | Ref. |
|---|---|---|---|---|---|---|
| Ion-pair HPLC–UV | 200 μL of sodium thiosulfate solution and 50 μL of acetonitrile | 200 μL | 18 | 50 | <10 | - |
| ICP–MS | dilution (1:20) in an aqueous solution of 0.5 mL/L Triton X-100 and suprapure concentrated HCl | 500 μL | 4 | 20 | n.a. | [36] |
| ICP–MS | dilution (1:9) in 0.1% EDTA and 0.1% ammonia | 1 mL | 3.3 | 10 | 2.2 | [31] |
| ICP–MS | dilution (1:20) by adding an aqueous solution containing 0.1 mg/L NH4OH, 0.1 g/L EDTA, 5 mg/L n-butanol and 0.1% Triton X-100 | 200 μL | 0.1 | n.a. | n.a. | [40] |
| ICP–MS | dilution (1:9) using water with 1.5% isopropanol and 7 mmol hydrous ammonium | 200 μL | 0.87 | 8.1 | <8 | [34] |
| S–K method | ammonium persulfate as the digestion regent | n.a. | 2.0 | n.a. | <4 | [34] |
| S–K method | dilution with water (1:1), perchloric acid digestion, incubation 25 min at 200 °C | 200 μL | n.a. | 13 | <19.2 | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikulska, A.A.; Filipowicz, D.; Główka, F.K.; Szczepanek-Parulska, E.; Ruchała, M.; Bartecki, M.; Karaźniewicz-Łada, M. HPLC Analysis of the Urinary Iodine Concentration in Pregnant Women. Molecules 2021, 26, 6797. https://doi.org/10.3390/molecules26226797
Mikulska AA, Filipowicz D, Główka FK, Szczepanek-Parulska E, Ruchała M, Bartecki M, Karaźniewicz-Łada M. HPLC Analysis of the Urinary Iodine Concentration in Pregnant Women. Molecules. 2021; 26(22):6797. https://doi.org/10.3390/molecules26226797
Chicago/Turabian StyleMikulska, Aniceta A., Dorota Filipowicz, Franciszek K. Główka, Ewelina Szczepanek-Parulska, Marek Ruchała, Michał Bartecki, and Marta Karaźniewicz-Łada. 2021. "HPLC Analysis of the Urinary Iodine Concentration in Pregnant Women" Molecules 26, no. 22: 6797. https://doi.org/10.3390/molecules26226797
APA StyleMikulska, A. A., Filipowicz, D., Główka, F. K., Szczepanek-Parulska, E., Ruchała, M., Bartecki, M., & Karaźniewicz-Łada, M. (2021). HPLC Analysis of the Urinary Iodine Concentration in Pregnant Women. Molecules, 26(22), 6797. https://doi.org/10.3390/molecules26226797








