Application of Capillary Electrophoresis for Determination of Inorganic Analytes in Waters
Abstract
:1. Introduction
2. Basic Modes of Analytical Capillary Electrophoresis Measurements
3. Application of Different Detection Methods
3.1. Application of UV Detection
3.2. Luminescence-Based Detection Methods
3.3. Electrochemical Detection Methods
3.4. Mass Spectrometry Detection
3.5. Atomic Spectrometry Detection
4. Sample Processing in CE Systems for Water Analysis
4.1. Off-Line Sample Processing
4.2. On-Line (In-Capillary) Pretreatment Operations
5. Simultaneous Determination of Anions and Cations
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Viratanen, R. Zone electrophoresis in a narrow-bore tube employing potentiometric detection—Theoretical and experimental study. Acta Polyt. Scand. Chem. Incl. Metall. Ser. 1974, 123, 1–67. [Google Scholar]
- Jorgenson, J.W.; Lukacs, K.D. Zone electrophoresis in open-tubular glass capillaries. Anal. Chem. 1981, 53, 1298–1302. [Google Scholar] [CrossRef]
- Voeten, R.L.; Ventouri, I.K.; Haselberg, R.; Somsen, G.W. Capillary electrophoresis: Trends and recent advances. Anal. Chem. 2018, 90, 1464–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, O.-K.; Cho, J.-S. Analysis of inorganic anions in various drinking waters by capillary electrophoresis. Anal. Sci. Technol. 1995, 8, 835–841. [Google Scholar]
- Haddad, P.R. Comparison of ion chromatography and capillary electrophoresis for the determination of inorganic ions. J. Chromatogr. A 1997, 770, 281–290. [Google Scholar] [CrossRef]
- Pacakova, V.; Stulik, K. Capillary electrophoresis of inorganic anions and its comparison with ion chromatography. J. Chromatogr. A 1997, 789, 169–180. [Google Scholar] [CrossRef]
- Breadmore, M.C. Capillary and microchip electrophoresis: Challenging the common conceptions. J. Chromatogr. A 2012, 1221, 42–55. [Google Scholar] [CrossRef]
- Torres, N.T.; Hauser, P.C.; Furrer, G.; Brandl, H.; Muller, B. Sediment porewater extraction and analysis combining filter tube samplers and capillary electrophoresis. Environ. Sci. Process. Impacts 2013, 15, 715–720. [Google Scholar] [CrossRef] [Green Version]
- Timerbaev, A.R. Recent advances and trends in capillary electrophoresis of inorganic ions. Electrophoresis 2002, 23, 3884–3906. [Google Scholar] [CrossRef]
- Timerbaev, A.R. Capillary electrophoresis of inorganic ions: An update. Electrophoresis 2004, 25, 4008–4031. [Google Scholar] [CrossRef]
- Kubáň, P.; Timerbaev, A.R. Inorganic analysis using CE: Advanced methodologies to face old challenges. Electrophoresis 2014, 35, 225–233. [Google Scholar] [CrossRef]
- Mala, Z.; Gebauer, P. Recent progress in analytical capillary isotachophoresis. Electrophoresis 2019, 40, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Fukushi, K.; Takeda, S.; Chayama, K.; Walida, S. Application of capillary electrophoresis to the analysis of inorganic ions in environmental samples. J. Chromatogr. A 1999, 834, 349–362. [Google Scholar] [CrossRef]
- Valsecchi, S.M.; Polsello, S. Analysis of inorganic species in environmental samples by capillary electrophoresis. J. Chromatogr. A 1999, 834, 363–385. [Google Scholar] [CrossRef]
- Ali, I.; Aboul-Enein, H.Y. Determination of metal ions in water, soil and sediment by capillary electrophoresis. Anal. Lett. 2002, 35, 2053–2076. [Google Scholar] [CrossRef]
- Lewis, A.P.; Cranny, A.; Harris, N.R.; Green, N.G.; Wharton, J.A.; Wood, R.J.K.; Stokes, K.R. Review on the development of truly portable and in-situ capillary electrophoresis systems. Meas. Sci. Technol. 2013, 24, 042001. [Google Scholar] [CrossRef]
- El Fellah, S.; Duporté, G.; Sirén, H. Steroid hormones, inorganic ions and botrydial in drinking water. Determination with capillary electrophoresis and liquid chromatography-orbitrap high resolution mass spectrometry. Microchem. J. 2017, 133, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, P.; Olędzka, I.; Plenis, A.; Bączek, T. Dynamic double coating, electrophoretic method with indirect detection for the simultaneous quantification of mono- and divalent cations in various water samples. Electrophoresis 2017, 38, 477–485. [Google Scholar] [CrossRef]
- Varden, L.; Bou-Abdallah, F. Detection and separation of inorganic cations in natural, potable, and wastewater samples using capillary zone electrophoresis with indirect UV detection. Am. J. Analyt. Chem. 2017, 8, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Lancioni, C.; Aspromonte, J.; Tascon, M.; Gagliardi, L.G. Development of a background electrolyte for the determination of inorganic cations in high ionic strength samples by capillary electrophoresis with indirect UV-absorption detection. J. Chromatogr. A 2021, 1645, 462091. [Google Scholar] [CrossRef]
- Pappoe, M.; Bottaro, C.S. Systematic optimization of a pyromellitic acid background electrolyte for capillary electrophoresis with indirect UV-vis detection and online preconcentration analysis of thiosalt anions in treated mine tailings. Anal. Meth. 2014, 6, 9305–9312. [Google Scholar] [CrossRef]
- Donkor, K.K.; Guo, Z.C.; Soliman, L.C.; Law, Y.T.; Risley, J.M.; Schmidt, K.J.; Crabtree, H.J.; Warrender, N.A. Determination of sulfate and chloride ions in highly saline oilfield water by capillary electrophoresis using bilayer-coated capillaries and indirect absorption detection. Intern. J. Environ. Anal. Chem. 2015, 95, 175–186. [Google Scholar] [CrossRef]
- Wang, Q.-P.; Chen, Z.-L.; Chen, G.-N.; Lin, J.-M. Simultaneous determination of phosphate and calcium in river water samples by capillary zone electrophoresis with UV detection. Intern. J. Environ. Anal. Chem. 2011, 91, 255–262. [Google Scholar] [CrossRef]
- Marák, J.; Staňová, A.; Vaváková, V.; Hrenáková, M.; Kaniansky, D. On-line capillary isotachophoresis–capillary zone electrophoresis analysis of bromate in drinking waters in an automated analyzer with coupled columns and photometric detection. J. Chromatogr. A 2012, 1267, 252–258. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, F.; Meng, L.; Tang, W.; Xia, Y.; Wu, Y.; Zhang, S. Preparation and application of trimethylamine amination polychloromethyl styrene nanolatex coated capillary column for the determination of bromate by field-amplified sample stacking open-tubular capillary electrochromatography. Electrophoresis 2013, 34, 1312–1318. [Google Scholar] [CrossRef]
- Fukushi, K.; Fujita, Y.; Nonogaki, J.; Tsujimoto, J.; Hattori, T.; Inui, H.; Beškoski, V.P.; Hotta, H.; Hayashi, M.; Nakano, T. Capillary zone electrophoresis determination of fluoride in seawater using transient isotachophoresis. Anal. Bioanal. Chem. 2018, 410, 1825–1831. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Li, J.; Lu, W.; Wen, Y.; Cai, X.; You, J.; Ma, J.; Ding, Y.; Chen, L. Speciation analysis of mercury in water samples by dispersive liquid–liquid microextraction coupled to capillary electrophoresis. Electrophoresis 2014, 35, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; He, M.; Chen, B.; Hu, B. Automated dynamic hollow fiber liquid–liquid–liquid microextraction combined with capillary electrophoresis for speciation of mercury in biological and environmental samples. J. Chromatogr. A 2015, 1415, 48–56. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Hu, B. Phase transfer membrane supported liquid–liquid–liquid microextraction combined with large volume sample injection capillary electrophoresis–ultraviolet detection for the speciation of inorganic and organic mercury. J. Chromatogr. A 2011, 1218, 9414–9421. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Peng, M.; Hou, X.; Zheng, C.; Long, Z. Improved hollow fiber supported liquid–liquid–liquid membrane microextraction for speciation of inorganic and organic mercury by capillary electrophoresis. Anal. Methods 2013, 5, 1185–1191. [Google Scholar] [CrossRef]
- Duan, J.; Hu, B.; He, M. Nanometer-sized alumina packed microcolumn solid-phase extraction combined with field-amplified sample stacking-capillary electrophoresis for the speciation analysis of inorganic selenium in environmental water samples. Electrophoresis 2012, 33, 2953–2960. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Choi, K.; Kim, J.; Sung, I.-H.; Chung, D.S. Sensitive arsenic analysis by carrier-mediated counter-transport single drop microextraction coupled with capillary electrophoresis. Microchem. J. 2013, 106, 220–225. [Google Scholar] [CrossRef]
- Lee, H.G.; Kwon, J.Y.; Chung, D.S. Sensitive arsenic speciation by capillary electrophoresis using UV absorbance detection with on-line sample preconcentration techniques. Talanta 2018, 181, 366–372. [Google Scholar] [CrossRef]
- Saito, S.; Nakano, Y.; Hikichi, A.; Suzuki, R.; Yoshimoto, K.; Maeda, M.; Aoyamab, M.; Shibukawa, M. Ultrasensitive CE for heavy metal ions using the variations in the chemical structures formed from new octadentate fluorescent probes and cationic polymers. Analyst 2011, 136, 2697–2707. [Google Scholar] [CrossRef]
- Pei, L.; Schmidt, K.J.; Crabtree, H.J.; Lucy, C.A. Determination of inorganic anions in oilfield water using capillary electrophoresis with indirect fluorescence detection. Anal. Methods 2015, 7, 8689–8696. [Google Scholar] [CrossRef]
- Trojanowicz, M. Recent developments in electrochemical flow detections—A Review, Part I. Flow analysis and capillary electrophoresis. Anal. Chim. Acta 2009, 653, 36–58. [Google Scholar] [CrossRef]
- Zemann, A.J.; Schnell, E.; Volgger, D.; Bonn, G.K. Contactless conductivity detection for capillary electrophoresis. Anal. Chem. 1998, 70, 563–567. [Google Scholar] [CrossRef]
- Da Silva, J.A.F.; Do Lago, C.L. An oscillometric detector for capillary electrophoresis. Anal. Chem. 1998, 70, 4339–4343. [Google Scholar] [CrossRef]
- Kubáň, P.; Hauser, P.C. Contactless conductivity detection for analytical techniques—Developments from 2014 to 2016. Electrophoresis 2017, 38, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Kubáň, P.; Hauser, P.C. 20th anniversary of axial capacitively coupled contactless conductivity detection in capillary electrophoresis. Trends Anal. Chem. 2018, 102, 311–321. [Google Scholar] [CrossRef]
- Silva, M.; Mendiguchía, C.; Moreno, C.; Kubáň, P. Electromembrane extraction and capillary electrophoresis with capacitively coupled contactless conductivity detection: Multi-extraction capabilities to analyses trace metals from saline samples. Electrophoresis 2018, 39, 2152–2159. [Google Scholar] [CrossRef]
- Ferreira Santos, M.S.; Cordeiro, T.G.; Noell, A.C.; Garcia, C.D.; Mora, M.F. Analysis of inorganic cations and amino acids in high salinity samples by capillary electrophoresis and conductivity detection: Implications for in-situ exploration of ocean worlds. Electrophoresis 2018, 39, 2890–2897. [Google Scholar] [CrossRef]
- Kubáň, P.; Boček, P. Preconcentration in micro-electromembrane extraction across free liquid membranes. Anal. Chim. Acta 2014, 848, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Opekar, F.; Tůma, P. Dual-channel capillary electrophoresis for simultaneous determination of cations and anions. J. Chromatogr. A 2016, 1446, 158–163. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, L.; Zhang, D.; Ge, X.; Ye, J.; Chu, Q. Sensitive determination of bromate in water samples by capillary electrophoresis coupled with electromembrane extraction. Food Anal. Methods 2016, 9, 393–400. [Google Scholar] [CrossRef]
- Kuban, P.; Strieglerova, L.; Gebauer, P.; Bocek, P. Electromembrane extraction of heavy metal cations followed by capillary electrophoresis with capacitively coupled contactless conductivity detection. Electrophoresis 2011, 32, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.A.; Le, M.D.; Nguyen, K.D.M.; Hauser, P.C.; Pham, H.V.; Mai, T.D. In-house-made capillary electrophoresis instruments coupled with contactless conductivity detection as a simple and inexpensive solution for water analysis: A case study in Vietnam. Environ. Sci. Process. Impacts 2015, 17, 1941–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, T.D.; Schmid, S.; Muller, B.; Hauser, P.C. Capillary electrophoresis with contactless conductivity detection coupled to a sequential injection analysis manifold for extended automated monitoring applications. Anal. Chim. Acta 2010, 665, 1–6. [Google Scholar] [CrossRef]
- Mai, T.D.; Le, M.D.; Sáiz, J.; Duong, H.A.; Koenka, I.J.; Pham, H.V.; Hauser, P.C. Triple-channel portable capillary electrophoresis instrument with individual background electrolytes for the concurrent separations of anionic and cationic species. Anal. Chim. Acta 2016, 911, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, T.D.; Pham, T.T.T.; Pham, H.V.; Sáiz, J.; Ruiz, C.G.; Hauser, P.C. Portable capillary electrophoresis instrument with automated injector and contactless conductivity detection. Anal. Chem. 2013, 85, 2333–2339. [Google Scholar] [CrossRef]
- Sáiz, J.; Koenka, I.J.; Garćıa-Ruiz, C.; Müller, B.; Chwalek, T.; Hauser, P.C. Micro-injector for capillary electrophoresis. Electrophoresis 2015, 36, 1941–1944. [Google Scholar] [CrossRef]
- Torres, N.T.; Och, L.M.; Hauser, P.C.; Furrer, G.; Brandl, H.; Vologina, E.; Sturm, M.; Burgmanna, H.; Muller, B. Early diagenetic processes generate iron and manganese oxide layers in the sediments of Lake Baikal, Siberia. Environ. Sci. Process. Impacts 2014, 16, 879–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiplagat, I.K.; Doan, T.K.O.; Kubáň, P.; Boček, P. Trace determination of perchlorate using electromembrane extraction and capillary electrophoresis with capacitively coupled contactless conductivity detection. Electrophoresis 2011, 32, 3008–3015. [Google Scholar] [CrossRef]
- Gaudry, A.J.; Guijt, R.M.; Mack, M.; Hutchinson, J.P.; Johns, C.; Hilder, E.F.; Dicinoski, G.W.; Nesterenko, P.N.; Haddad, P.R.; Breadmore, M.C. On-line simultaneous and rapid separation of anions and cations from a single sample using dual-capillary sequential injection-capillary electrophoresis. Anal. Chim. Acta 2013, 781, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.T.; Mai, T.D.; Nguyen, T.D.; Sáiz, J.; Pham, H.V.; Hauser, P.C. Automated dual capillary electrophoresis system with hydrodynamic injection for the concurrent determination of cations and anions. Anal. Chim. Acta 2014, 841, 77–83. [Google Scholar] [CrossRef]
- Koenka, I.J.; Mai, T.D.; Hauser, P.C.; Sáiz, J. Simultaneous separation of cations and anions in capillary electrophoresis—Recent applications. Anal. Methods 2016, 8, 1452–1456. [Google Scholar] [CrossRef] [Green Version]
- Flanigan, P.M.; Ross, D.; Shackman, J.G. Determination of inorganic ions in mineral water by gradient elution moving boundary electrophoresis. Electrophoresis 2010, 31, 3466–3474. [Google Scholar] [CrossRef]
- Mai, T.D.; Hauser, P.C. Simultaneous separations of cations and anions by capillary electrophoresis with contactless conductivity detection employing a sequential injection analysis manifold for flexible manipulation of sample plugs. J. Chromatogr. A 2012, 1267, 266–272. [Google Scholar] [CrossRef]
- Liu, S.; Pan, Z.; Liang, Y.; Li, F.; Breadmore, M.C.; Zhang, M. An electrophoretic ion analyzer for on-site autonomous water monitoring. J. Chromatogr. A 2021, 1637, 461791. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, N.; Cabot, J.M.; Lam, S.C.; Rodriguez, E.S.; Paull, B. Selective capillary electrophoresis separation of mono and divalent cations within a high-surface area-to-volume ratio multi-lumen capillary. Anal. Chim. Acta 2019, 1051, 41–48. [Google Scholar] [CrossRef]
- Fuiko, R.; Saracevic, E.; Koenka, I.J.; Hauser, P.C.; Krampe, J. Capillary electrophoresis for continuous nitrogen quantification in wastewater treatment processes. Talanta 2019, 195, 366–371. [Google Scholar] [CrossRef]
- Neaga, I.-O.; Iacob, B.C.; Bodoki, E. The analysis of small ions with physiological implications using capillary electrophoresis with contactless conductivity detection. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2072–2090. [Google Scholar] [CrossRef]
- Koenka, I.J.; Kung, N.; Kuban, P.; Chwalek, T.; Furrer, G.; Wehrli, B.; Muller, B.; Hauser, P.C. Thermostated dual-channel portable capillary electrophoresis instrument. Electrophoresis 2016, 37, 2368–2375. [Google Scholar] [CrossRef]
- Tian, Z.; Qin, W. Capillary electrophoretic separation of anions in dimethylformamide–acetic acid medium. Anal. Methods 2014, 6, 5353–5359. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, X.; Mo, F.; Huang, L.; Wu, Z.; Wu, Y.; Xu, L.; Fu, F. Ultra-sensitive speciation analysis of mercury by CE-ICP-MS together with field-amplified sample stacking injection and dispersive solid-phase extraction. Electrophoresis 2016, 37, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-H. Rapid speciation analysis of mercury by short column capillary electrophoresis on-line coupled with inductively coupled plasma mass spectrometry. Anal. Methods 2011, 3, 116–121. [Google Scholar] [CrossRef]
- Liu, L.; He, B.; Yun, Z.; Sun, J.; Jiang, G. Speciation analysis of arsenic compounds by capillary electrophoresis on-line coupled with inductively coupled plasma mass spectrometry using a novel interface. J. Chromatogr. A 2013, 1304, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yun, Z.; He, B.; Jiang, G. Efficient interface for online coupling of capillary electrophoresis with inductively coupled plasma—Mass spectrometry and its application in simultaneous speciation analysis of arsenic and selenium. Anal. Chem. 2014, 86, 8167–8175. [Google Scholar] [CrossRef]
- Zhou, D.; Lin, Y.; Long, H.; Xu, Y.; Wang, B.; Xian, L.; Xia, C.H.; Hou, X.; Zheng, C.h. Simultaneous total and speciation analysis of rhenium by capillary electrophoresis-inductively coupled plasma mass spectrometry. Spectrochim. Acta Part B Atmo. Spectrosc. 2021, 180, 106211. [Google Scholar] [CrossRef]
- Dominguez-Alvarez, J. Capillary electrophoresis coupled to electrospray mass spectrometry for the determination of organic and inorganic arsenic compounds in water samples. Talanta 2020, 212, 120803. [Google Scholar] [CrossRef]
- Zhang, H.; Gavina, J.; Feng, Y.-L. Understanding mechanisms of pressure-assisted electrokinetic injection: Application to analysis of bromate, arsenic and selenium species in drinking water by capillary electrophoresis-mass spectrometry. J. Chromatogr. A 2011, 1218, 3095–3104. [Google Scholar] [CrossRef]
- Gaspar, A.; Pesti, A.; Szabo, M.; Kecskemeti, A. Determination of chlorine species by capillary electrophoresis—Mass spectrometry. Electrophoresis 2019, 40, 2637–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.; Deng, B.; Shen, C.; Long, C.; Deng, Q.; Tao, C. Selenium speciation using capillary electrophoresis coupled with modified electrothermal atomic absorption spectrometry after selective extraction with 5-sulfosalicylic acid functionalized magnetic nanoparticles. J. Chromatogr. A 2015, 1395, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Qin, X.; Xiao, Y.; Wang, Y.; Yin, H.; Xu, X.; Shen, C. Interface of on line coupling capillary electrophoresis with hydride generation electrothermal atomic absorption spectrometry and its application to arsenic speciation in sediment. Talanta 2013, 109, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Chen, Z.; Gjelstad, A.; Pedersen-Bjergaard, S.; Shen, X. Electromembrane extraction. Trends Anal. Chem. 2017, 95, 47–56. [Google Scholar] [CrossRef]
- Oedit, A.; Ramautar, R.; Hankemeier, T.; Lindenburg, P.W. Electroextraction and electromembrane extraction: Advances in hyphenation to analytical techniques. Electrophoresis 2016, 37, 1170–1186. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kim, S.J.; Jin, Y.G.; Jang, Y.O.; Kim, J.S.; Chung, D.S. Single drop microextraction using commercial capillary electrophoresis instruments. Anal. Chem. 2009, 81, 225–230. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Dawod, M.; Kim, J.; Chung, D.S. Single-drop microextraction as a powerful pretreatment tool for capillary electrophoresis: A review. Anal. Chim. Acta 2012, 739, 14–24. [Google Scholar] [CrossRef]
- Ranjbar, L.; Gaudry, A.J.; Breadmore, M.C.; Shellie, R.A. Online comprehensive two-dimensional ion chromatography × capillary electrophoresis. Anal. Chem. 2015, 87, 8673–8678. [Google Scholar] [CrossRef]
- Meng, H.-B.; Wang, T.-R.; Guo, B.-Y.; Hashi, Y.; Guo, C.-X.; Lin, J.-M. Simultaneous determination of inorganic anions and cations in explosive residues by ion chromatography. Talanta 2008, 76, 241–245. [Google Scholar] [CrossRef]
- Fa, Y.; Yu, Y.; Li, F.; Du, F.; Liang, X.; Liu, H. Simultaneous detection of anions and cations in mineral water by two dimensional ion chromatography. J. Chromatogr. A 2018, 1554, 123–127. [Google Scholar] [CrossRef]
- Rodriguez, E.S.; Plummer, C.; Nation, M.; Moy, A.; Curran, M.; Haddad, P.R.; Paull, B. Sub- 1 mL sample requirement for simultaneous determination of 17 organic and inorganic anions and cations in Antarctic ice core samples by dual capillary ion chromatography. Anal. Chim. Acta 2019, 1063, 167–177. [Google Scholar] [CrossRef]
- Sáiz, J.; Koenka, I.J.; Mai, T.D.; Hauser, P.C.; García-Ruiz, C. Simultaneous separation of cations and anions in capillary electrophoresis. Trends Anal. Chem. 2014, 62, 162–172. [Google Scholar] [CrossRef]
- Clavijo, S.; Avivar, J.; Suárez, R.; Cerdá, V. Analytical strategies for coupling separation and flow-injection techniques. Trends Anal. Chem. 2015, 67, 26–33. [Google Scholar] [CrossRef]
- Yamamoto, S.; Fujiwara, H.; Maruyama, K.; Tanaka, Y.; Kinoshita, M.; Suzuki, S. Simultaneous determination of inorganic anions and cations in water and biological samples by capillary electrophoresis with a capacitive coupled contactless conductivity detector using capillary filling method. Anal. Sci. 2019, 35, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Hens, A.; Fernandez-Romero, J.M. Microfluidic systems in analytical chemistry. In Encyclopedia of Analytical Chemistry; Wiley Online Library: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Ragab, M.A.A.; El-Kimary, E.I. Recent advances and applications of microfluidic capillary electrophoresis: A comprehensive review (2017–Mid 2019). Crit. Rev. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Renaudot, R.; Agache, V.; Fouillet, Y.; Laffite, G.; Bisceglia, E.; Jalabert, L.; Kumemura, M.; Collard, D.; Fujita, H. A programmable and reconfigurable microfluidic chip. Lab Chip 2013, 13, 4517–4524. [Google Scholar] [CrossRef] [PubMed]
- Sriram, G.; Bhat, M.P.; Patil, P.; Uthappa, U.T.; Jung, H.-Y.; Altalhi, T.; Kumeria, T.; Aminabhavi, T.M.; Pai, R.K.; Kurkuri, M.D. Paper-based microfluidic analytical devices for colorimetric detection of toxic ions: A review. Trends Anal. Chem. 2017, 93, 212–227. [Google Scholar] [CrossRef]
- Konry, T.; Bale, S.S.; Bhushan, A.; Shen, K.; Seker, E.; Polyak, B.; Yarmush, M. Particles and microfluidics merged: Perspectives of highly sensitive diagnostic detection. Microchim. Acta 2012, 176, 251–269. [Google Scholar] [CrossRef]
- Marle, L.; Greenway, G.M. Microfluidic devices for environmental monitoring. Trends Anal. Chem. 2005, 24, 795–802. [Google Scholar] [CrossRef]
- Kudr, J.; Zitka, O.; Klimanek, M.; Vrba, R.; Adam, V. Microfluidic electrochemical devices for pollution analysis—A review. Sens. Actuat. B Chem. 2017, 246, 578–590. [Google Scholar] [CrossRef]
- Bridle, H.; Miller, B.; Desmulliez, M.P.Y. Application of microfluidics in waterborne pathogen monitoring: A review. Water Res. 2014, 55, 256–271. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Beaton, A.D.; Mowlen, M.C. Trends in microfluidic systems for in situ chemical analysis of natural waters. Sens. Actuat. B: Chem. 2015, 221, 1398–1405. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Phung, S.C.; Smejkal, P.; Guijt, R.M.; Breadmore, M.C. Recent trends in capillary and micro-chip electrophoretic instrumentation for field-analysis. Trends Environ. Anal. Chem. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Gertsch, J.C.; Noblitt, S.D.; Cropek, D.M.; Henry, C.S. Rapid analysis of perchlorate in drinking water at parts per billion levels using microchip electrophoresis. Anal. Chem. 2010, 82, 3426–3429. [Google Scholar] [CrossRef] [PubMed]
- Masár, M.; Bomastyk, B.; Bodor, R.; Horčičiak, M.; Danč, L.; Troška, P.; Kuss, H.-M. Determination of chloride, sulfate and nitrate in drinking water by microchip electrophoresis. Microchim Acta 2012, 177, 309–316. [Google Scholar] [CrossRef]
- Yan, X.; Liu, W.; Yuan, Y.; Chen, C. Indium tin oxide coated PET film contactless conductivity detector for microchip capillary electrophoresis. Anal. Methods 2015, 7, 5295–5302. [Google Scholar] [CrossRef]
- Luc, M.; Kruk, P.; Masar, M. Determination of ammonium in wastewaters by capillary electrophoresis on a column-coupling chip with conductivity detection. J. Sep. Sci. 2011, 34, 1561–1567. [Google Scholar] [CrossRef]
- Koczka, P.I.; Bodoki, E.; Gaspár, A. Application of capacitively coupled contactless conductivity as an external detector for zone electrophoresis in poly(dimethylsiloxane) chips. Electrophoresis 2016, 37, 398–405. [Google Scholar] [CrossRef]
- Mahabadi, K.A.; Rodriguez, I.; Lim, C.Y.; Maurya, D.K.; Hauser, P.; De Rooij, N.F. Capacitively coupled contactless conductivity detection with dual top–bottom cell configuration for microchip electrophoresis. Electrophoresis 2010, 31, 1063–1070. [Google Scholar] [CrossRef]
- Freitas, C.B.; Moreira, R.C.; De Oliveira Tavares, M.G.; Coltro, W.K.T. Monitoring of nitrite, nitrate, chloride and sulfate in environmental samples using electrophoresis microchips coupled with contactless conductivity detection. Talanta 2016, 147, 335–341. [Google Scholar] [CrossRef]
- Da Silva, E.N.T.; Petroni, J.M.; Lucca, B.G.; Ferreira, V.S. Pencil graphite leads as simple amperometric sensors for microchip electrophoresis. Electrophoresis 2017, 38, 2733–2740. [Google Scholar] [CrossRef]
- Petroni, J.M.; Lucca, B.G.; Ferreira, V.S. Simple approach for the fabrication of screen-printed carbon-based electrode for amperometric detection on microchip electrophoresis. Anal. Chem. Acta 2017, 954, 88–96. [Google Scholar] [CrossRef]
- Chen, X.; Hong, F.; Zhang, W.; Wu, D.; Li, T.; Hu, F.; Gan, N.; Lin, J.; Wang, Q. Microchip electrophoresis based multiplexerd assay for silver and mercury ions simultaneous detection in complex samples using a stirring bar modified with encoded hairpin probes for specific extraction. J. Chromatogr. A 2019, 1589, 173–181. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Namieśnik, J. Moving your laboratories to the field—Advantages and limitations of the use of field portable instruments in environmental sample analysis. Environ. Res. 2015, 140, 593–603. [Google Scholar] [CrossRef]
- Van Schepdael, A. Recent advances in portable analytical electromigration devices. Separations 2016, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Zamuruyev, K.; Ferreira Santos, M.S.; Mora, M.F.; Kurfman, E.A.; Noell, A.C.; Willis, P.A. Automated capillary electrophoresis system compatible with multiple detectors for potential in situ spaceflight missions. Anal. Chem. 2021, 93, 9647–9655. [Google Scholar] [CrossRef]
- Beutner, A.; Piendl, S.K.; Wet, S.; Matysik, F.-M. Methodical studies of the simultaneous determinations of anions and cations by ICxCE-MS using arsenic species as model analytes. Anal. Bioanal. Chem. 2018, 410, 6321–6330. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, L.; Foley, J.P.; Breadmore, M.C. Multidimensional liquid-phase separations combining both chromatography and electrophoresis—A review. Anal. Chim. Acta 2017, 959, 7–31. [Google Scholar] [CrossRef]
- Chen, N.; Meng, X.; Ding, P.; Su, Y.; Wang, H.; He, Y. Biomimetic preparation of core-shell structured surface-enhanced Raman scattering substrate with antifouling ability, good stability, and reliable quantitative capability. Electrophoresis 2019, 40, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Nakamura, K.; Timerbaev, A.R.; Hirokawa, T. Another approach toward over 100,000-fold sensitivity increase in capillary electrophoresis: Electrokinetic supercharging with optimized sample injection. Anal. Chem. 2011, 83, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, K.; Hirokawa, T.; Timerbaev, A.R. Recent developments of capillary electrophoresis in seawater analysis. J. Chromatogr. A 2019, 1606, 360240. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Alharbi, O.M.L.; Sanagi, M.M. Nano-capillary electrophoresis for environmental analysis. Environ. Chem. Lett. 2016, 14, 79–98. [Google Scholar] [CrossRef] [PubMed]












| Analyte(s) | Sample | Sample Pretreatment | Separation Conditions | Detection, LOD | First Author, Year of Publication | Ref. |
|---|---|---|---|---|---|---|
| Anions (Cl−, SO42−) | Highly saline oilfield waters | Dilution | Coated capillary: 60 cm, 50 μm I.D. BGE: 50 mM TRIS, 30 mM SDS, 5% MeOH and 26 mM CrO3 (pH 6.7) V: −10 kV | Indirect UV 280 nm Cl− 2,61 mg⋅L−1, SO42− 2,98 mg⋅L−1 | Donkor, 2015 | [22] |
| Anions (Br−,Cl−, NO3−, SO42−) | Oilfield waters | Dilution | Coated capillary: 60.2 (50) * cm, 50 μm I.D. BGE: 10 mM HPTS, 0.4 M formic acid (pH 2) V: −22.5 kV | Indirect FL—LED 405/520 nm 0.4 mg·L−1 for SO42−, 1.4 mg·L−1 for Cl− | Pei, 2015 | [35] |
| Anions (ClO4−, Cl−, NO3−, SO42−) | Tap and well water | μ-EME with FLM | Capillary: 46 (13) cm, 25 μm I.D. BGE: 10% AcOH (pH 2.2) V: −20 kV | C4D EF 30 | Kuban, 2014 | [43] |
| Anions (ClO4−, Cl−, NO3−, SO42−, NO2−) | Surface, rain tap, snow, potable waters | EME | Capillary: 60 (45) cm, 50 μm I.D. BGE: 7.5 mM His and 40 mM AcOH (pH 4.1) V: −30 kV | C4D 1 mg⋅L−1 tap water 0.25–0.35 mg·L−1 for environmental and potable waters | Kiplagat, 2011 | [53] |
| Anions (BF4−, ClO4−, PF6−, I−, NO3−, Br−, Cl−) | Tap water | Capillary: 60 (50) cm, 75 μm I.D. BGE: DMF–AcOH V: −14 kV | C4D 0.83–3.83 μM | Tian, 2014 | [62] | |
| Anions (total Re, Re(IV), and Re (VII)) | Groundwater | Reduction of Re(VII) to Re(IV) | Capillary: 104 cm, 75 μm I.D. BGE: 10 mM K2CO3 (pH 11) V: 27 kV | ICP-MS 0.02 μg·L−1 for TRe, 0.01 μg·L−1 for Re(VII) | Zhou, 2021 | [69] |
| Anions, chlorine containing (ClO3−,ClO2−, ClO4−, Cl−) | Drinking and swimming pool waters | Capillary: 90 cm, 50 μm I.D. BGE: 100 mM ammonium formate (pH 6.5) V: −20 kV | MS 0.25–2.1 mg⋅L−1 for ClO3− 6 μg·L−1 (for large sample volume) | Gaspar, 2019 | [72] | |
| Anions, sulfur containing (SO42−, S2O32−, S3O62−,vS4O62−, S5O62−) | Tailings pond water | FASS | Capillary: 48.5 (40.0) cm, 50 μm I.D. BGE: 2.00 mM PMA, 0.80 mM HMOH (pH 8.0) V: −20 kV | Indirect UV 350 nm 0.02–0.12 mg·L−1 | Pappoe, 2014 | [21] |
| Anions (Cl−, NO3−, SO42−) Cations (Ca2+, K+, Mg2+, Na+, NH4+) | Cold and hot tap waters, well water | Capillary 60 (51.5) cm, 50 µm I.D. BGE1: 9 mM pyridine, 12 mM glycolic acid, and 5 mM 18-crown-6 ether (pH 3.6) BGE2: PMA (pH 7.7) V: ±20 kV | Indirect UV (DAD) 220, 254 nm 0.05–0.10 mg·L−1 (cations), 0.10 mg·L−1 (anions) | Fellah, 2017 | [17] | |
| Anions (Cl−, NO3−, NO2−, SO42−) Cations (K+, Na+, Ca2+, Mg2+, NH4+) Phosphate As(III) | Ground and surface waters | Capillary: 65 (49) cm, 25 μm I.D. BGE1: 12 mM His,2 mM 18-crown-6 (pH 3.7) V: 15 kV; Capillary: 52 (36) cm, 25 μm I.D. BGE2: 12 mM His (pH 4.0 AcOH), V: −15 kV; Capillary: 52 (36) cm, 25 μm I.D. BGE3: 1 mM His (pH 3.5 AcOH) V: −15 kV; Capillary: 60 (52) cm, 25 μm I.D. BGE4: 12 mM MES, 21 mM Arg, 30 mM CTAB (pH 8.9) V: −20 kV | C4D 2.5–10 μM | Duong, 2015 | [47] | |
| Anions (Cl−, NO3−, SO42−, NO2−, F−, H2PO4−) Cations (NH4+, K+, Ca2+, Na+, Mg2+, Li+) | Tap water sewage | Portable | Capillary: 50 (36) cm, 50 μm I.D. BGE: 12 mM His, 2 mM 18-crown-6 (pH 4 AcOH) V: ±15 kV | C4D 1.5–17 μM | Mai, 2013 | [50] |
| Anions (Cl−, NO3−, SO42−) Cations (Na+, K+, NH4+, Ca2+, Mg2+, Mn2+, Fe2+) | Sediment porewater | Portable | Capillary: 55 cm, 50 μm I.D. BGE: 11 mM His, 50 mM AcOH, 1.5 mM 18-crown-6, 0.1 mM citric acid V:±15 kV | C4D Cations 0.46–1.55 μM Anions 0.28–0.98 μM | Torres, 2013, 2014 | [8,52] |
| Anions (Cl−, NO3−, SO42−, NO2−, F−, PO43−) Cations (NH4+, Na+, K+, Ca2+, Mg2+) | Tap water Lake water | On-site ion analyzer | Coated capillary: 60 (50) cm, 25 μm I.D. BGE: 400 mM Bis-Tris, 400 mM MOPS and 2 mM 18-crown-6 (pH 6.8) V: ±15 kV | C4D 2.1 μM (K+) 6.8 μM (PO43−) | Li, 2021 | [58] |
| Arsenic speciation (As (III), As (V), DMA, MMA, AsB, AsC, 3-NHPAA, 4-NPAA, o-ASA and p-UPAA) | Groundwater | Capillary: 100 cm, 50 μm I.D. BGE: 12 mM NaH2PO4 and 8 mM HBO3 (pH 9.20) V: 30 kV | ICP-MS 19–65 fg As | Liu, 2013 | [67] | |
| Arsenic speciation (As(III), As(V), MMA, DMA) | Tap water | SDME | Capillary: 60 (50) cm, 25 μm I.D. BGE: 15 mM phosphate buffer (pH 10.6) V: 25 kV | UV 200 nm As(III)–0.2 μM, DMA–0.7 μM, MMA–0.1 μM, As(V)–0.2 μM | Cheng, 2013 | [32] |
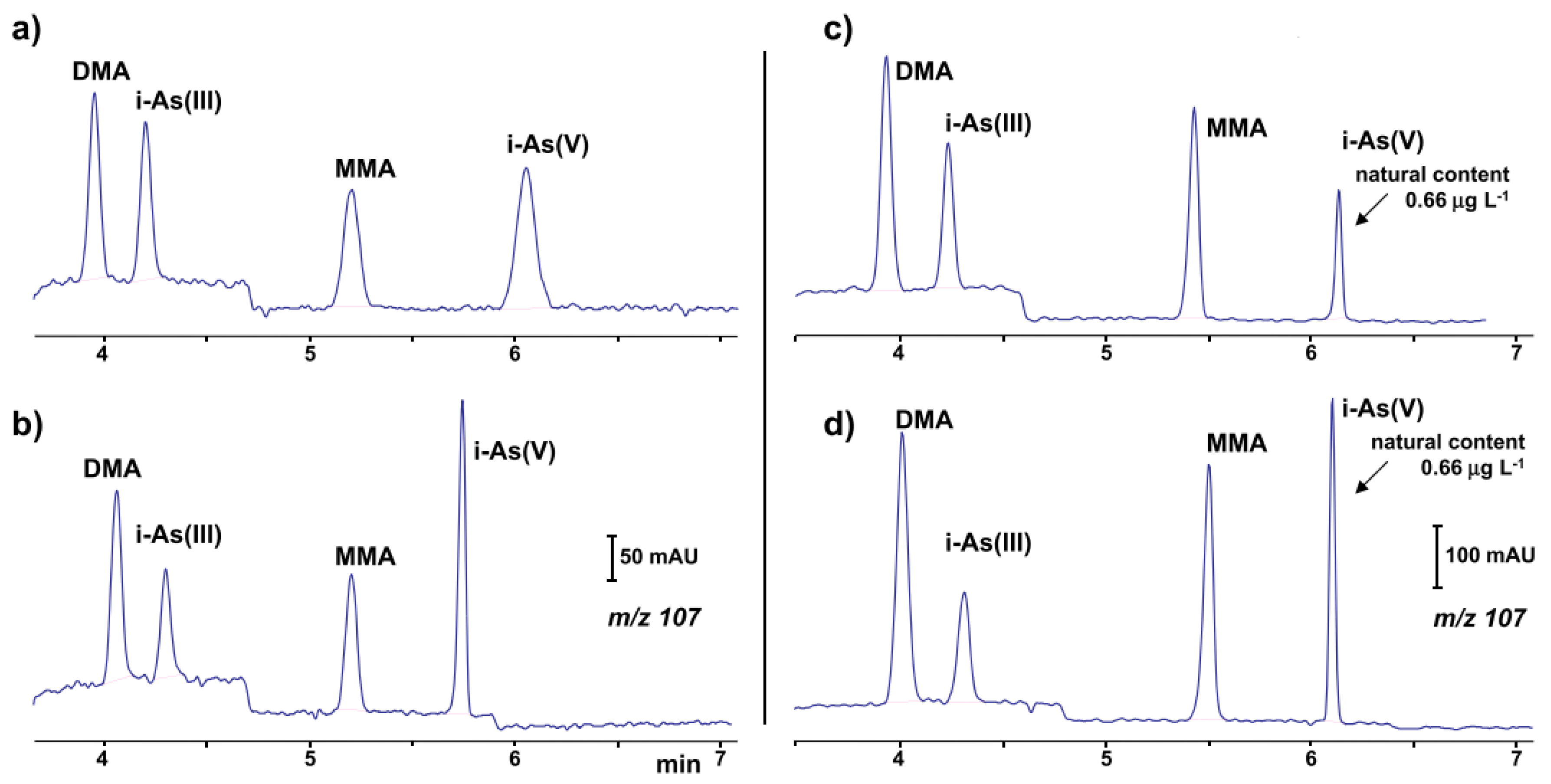
| Arsenic speciation (As(III), As(V), MMA, DMA) | Spring water | CF-EKS | Capillary: 60 (50) cm, 50 μm I.D., μSiL-FC coated BGE: phosphate buffer (pH 9.6) LE and CHES (pH 9.6) TL V: −20 kV | UV 200 nm 0.08–0.3 μg·L−1 As EFs 6300–45,000-fold | Lee, 2018 | [33] |
| Arsenic speciation (DMA, MMA, As(III), As(V)) | Grandwater, well water, bottled water | Partial evaporation of the sample (50:1) | Capillary: 72 cm, 50 μm I.D. BGE: 57 mM HFIP (pH 10.3) V: 30 kV | ESI-MS 0.02–0.04 μg·L−1 (As) | Dominguez-Alvarez, 2020 | [70] |
| Arsenic speciation (As(III), As(V)) | River sediment | Microwave extraction | Capillary: 60 cm, 100 μm I.D. BGE: 25 mM NaH2PO4—Na2HPO4 (pH 6.6) V: 25 kV | HG-ETAAS As(III)–135 ng·g−1 As(V)–160 ng·g−1 | Deng, 2013 | [74] |
| Arsenic speciation (As(III), As(V), MMA, DMA, AsB) Selenium speciation (Se(IV), Se(VI), SeCys, SeMet, MeSeCys) | Groundwater, tap water | Capillary: 60 cm, 75 μm I.D. BGE: 6 mM NaH2PO4, 9 mM H3BO3 (pH 9.0) V: 25 kV | ICP-MS 0.11−0.37 μg·L−1 for arsenic compounds 1.33−2.31 μg·L−1 for selenium species | Liu, 2014 | [68] | |
| Aresenic speciation (As(III) and As(V)]) Selenium speciation (Se(IV) and Se(VI)) Bromate | Drinking water | PAEKI | Capillary: 110 cm, 50 μm I.D. BGE: 20 mM ammonium carbonate (pH 9.2) V: 30 kV + 50 mbar | ESI-MS/MS 1–3 μg·L−1 | Zhang, 2011 | [71] |
| Bromate | Drinking water | On-line ITP | Capillary: 24 (18) cm, 300 μm I.D. BGE: 50 mM phosphate, 20 mM glycine (pH 2.0), 0.1% MHEC, constant current mode 50 μA | UV 200 nm 0.6 μg·L−1 | Marak, 2012 | [24] |
| Bromate | Tap water | FASS | OT-CEC (coated with TMAPL) Capillary: 50 (41.5) cm 50 μm I.D. BGE: 20 mM Tris–14 mM HClO4 (pH 7.80) V: −20kV | DAD 235 8 μg·L−1 | Guo, 2013 | [25] |
| Bromate | Tap and bottled water | EME | Capillary: 80 (73) cm, 25 μm I.D. BGE: 300 mM AcOH, V: −18 kV | C4D 0.12 μg·L−1 | Zhang, 2016 | [45] |
| Cations (NH4+, K+, Ca2+, Na+, Sr2+, Cd2+, Pb2+; Mg2+; Fe2+, Ni2+, Zn2+, Cu2+) | Mineral, tap and well waters | Dilution in the ratio 1: 10 for tap water | Coated capillary: 60 (50) cm, 75 μm I.D. BGE: 20 mM benzimidazole,75 mM AcOH, 0.6 mM 18-crown-6 (pH 4.22) V: 25 kV | Indirect UV 254 nm 0.015–0.10 mg·L−1 | Kowalski, 2017 | [18] |
| Cations (NH4+, K+, Ca2+, Na+, Mg2+, Pb2+) | Natural, and potable waters, wastewater | Capillary: 64.5 (56) cm, 75 μm I.D. BGE: 15 mM imidazole, 8 mM malonic acid, 2 mM 18-crown-6 ether, 10% v/v MeOH (pH 4.35) V: −20 kV | Indirect UV 214 nm 0.023–0.084 mg·L−1 | Varden, 2017 | [19] | |
| Cations (K+, Na+, Ca+2, Mg+2) | Sea water | Dilution 1:10 (v/v) | Capillary: 50 (40) cm, 50 μm I.D. BGE: 200 mM 2,4,6-trimethylpyridine, 250 mM lactic acid, 5% v/v MeOH (pH 4.5) V:25 kV | Indirect UV 230 nm ~10 mg·L−1 | Lancioni, 2021 | [20] |
| Cations (Ca2+, Mg2+, Cu2+, Zn2+, Ni2+, Co2+, Mn2+, Cd2+, Pb2+) | River water | Derivatization | Capillary: 60 (46.5) cm, 50 μm I.D. BGE: 50 mM borate buffer (pH 10.09), 0.05% PB, 1.0 mM DOTA V: 20 kV | LIF low ng·L−1 levels | Saito, 2011 | [34] |
| Cations (Mn2+, Cd2+, Zn2+, Co2+, Pb2+, Cu2+, Ni2+) | Drinking and sea water | EME | Capillary: 50 (37) cm, 50 μm I.D. BGE: 5.2 M AcOH V: 20 kV | C4D 1–2.6 nM | Silva, 2018 | [41] |
| Cations (Na+, K+, Li+, Ca2+, Mg2+, NH4+) | Ocean and lake waters | Dilution | Capillary: 64(43) cm, 50 μm I.D. BGE: 5 M AcOH, 10 mM 18-crown-6 and 10% ACN V: 30 kV | C4D 1.0 μM | Ferreira, 2018 | [42] |
| Cations (Mn2+, Cd2+, Zn2+, Co2+, Pb2+, Cu2+, Ni2+) | Tap water | EME | Capillary: 50 (37) cm, 50 μm I.D. BGE: 20 mM l-His and 17 mM AcOH (pH 5.6) V: 25 kV | C4D 25–200 nM | Kuban, 2011 | [46] |
| Cations (Na+, K+, NH4+, Ca2+, Mg2+) | Porewater of lake sediment core | Micro-injection | Capillary: 60 (49) cm, 25 μm I.D., BGE: 30 mM MES/His (pH 6), 2 mM 18-crown-6 V: −30 kV | C4D 10 μM | Saiz, 2015 | [51] |
| Cations (Na+, K+, Li+, Ca2+, Mg2+, NH4+) | Drinking water and soil extracts | Capillary: 126 × 8 mm I.D. channels and 49.1 cm in length BGE: 20 mM MES/His (pH 6.1), 2 mM 18-crown-6. V: 286 mV/cm | C4D NH4+–3.2 μM Na+–2 μM | Nakatani, 2019 | [60] | |
| Fluoride | Seawater | 10-fold dilution/tITP | Capillary 87.4 (75) cm, 75 µm I.D. BGE: 5 mM PDC (pH 3.5), 0.03% m/v HPMC V: 23 kV | indirect UV 200 0.024 mg·L−1 | Fukushi, 2018 | [26] |
| Mercury speciation (Hg(II), MeHg, EtHg and PhHg) | Tap, sea and surface waters | DLLME | Capillary: 60.2 (50) cm, 75 μm I.D. BGE: of 75 mM boric acid, 10% (v/v) MeOH (pH 9.0) V: 20kV | UV 210 nm Hg(II)–1.5, MeHg–1.79, EtHg–1.62 and PhHg–0.23 μg·L−1 EFs 46, 102, 118 and 547 | Yang, 2014 | [27] |
| Mercury speciation Hg(II) MeHg, EtHg and PhHg | River and lake waters | HF-LLLME | Capillary: 48.5 (40) cm, 50 μm I.D. BGE: 35 mM borate buffer (pH 9.10) V: 25 kV | UV 200 nm Sub μg·L−1 level EF 2195 | Li, 2015 | [28] |
| Mercury speciation(Hg(II), MeHg, EtHg and PhHg) | River and lake waters | PT-LLLME and LVSS | Capillary: 64.5 (56) cm, 50 μm I.D. BGE: 35 mM borate buffer (pH 9.10) V: 25 kV | UV 200 nm Sub μg·L−1 level EFs up to 12,138 | Li, 2011 | [29] |
| Mercury speciation (Hg(II), MeHg, EtHg and PhHg) | River water | HF-LLLMME | Capillary: 48 cm, 75 μm I.D. BGE: 100 mM borate buffer (pH 9) V: 15 kV | UV 210 nm 0.07–1.0 μg·L−1 (as Hg) EFs: Hg(II)–103, MeHg–265, EtHg–511 and PhHg–683 | Chen, 2013 | [30] |
| Mercury speciation (Hg(II), MeHg, EtHg) | Tap water | DSPE and FASI | Capillary: 90 cm, 75 μm I.D. BGE: 50 mM H3BO3—12.5 mM Na2B4O7 (pH 9.20) V: 12 kV | ICP-MS 9–11 ng·L−1 | Chen, 2016 | [65] |
| Mercury speciation (Hg(II) and MeHg) | River water | - | Capillary: 16 cm, 75 μm I.D. BGE: 30 mM boric acid and 5% (v/v) MeOH (pH 8.6) V: 21 kV | ICP-MS 9.7 mg·L−1 MeHg 12.0 mg·L−1 Hg(II) | Li, 2011 | [66] |
| Selenium speciation (Se(IV) and Se(VI)) | River, spring, and tap waters | SPME and FASS | Capillary: 64.5 (50) cm, 75 μm I.D. BGE: 0.2 M Tris-phosphate (pH 2.5), 0.1 mM CTAB V: −20 kV | UV 200 nm Se(IV)–57 ng·L−1 EF 41367 Se(VI)–ng·L−1 71 EF 61935 | Duan, 2012 | [31] |
| Selenium speciation (Se(IV), Se(VI)m SeMet, SeCys2) | Wastewater | MSPE | Capillary: 80 cm, 75 μm I.D. BGE: 20 mM phosphate buffer (pH 10.6), 0.2 mM CTAB V: 25 kV | ETAAS Se(VI)–0.18 μg·L−1, Se(IV)–0.17 μg·L−1 | Yan, 2015 | [73] |
| Analyte(s) | Sample | System Configuration | Separation Conditions | Detection, LOD | First Author, Year of Publication | Ref. |
|---|---|---|---|---|---|---|
| Anion HPO42− Cation Ca2+ | River water | On-column complexation | Capillary: 55.0 (48.5) * cm, 50 μm I.D. BGE: 10 mM PDCA, 0.75 mM TTAB (pH 7.0) V: −20 kV | UV 214 nm 5 μM for [Ca(PDCA)2]2− 2 μM for HPO42− | Wang, 2011 | [23] |
| Anions (NO3−, SO42−) Cations (K+, NH4+) | Fertiliser solution | Dual capillary system | Capillary: 10.5 (8.0) cm, 25 μm I.D. BGE: 500 mM AcOH, 20 mM Tris, 2 mM 18-crown-6 (pH 3.3) V: ±10 kV | C4D 6.9 μM K+ 10.6 μM NH4+ | Opekar, 2016 | [44] |
| Anions (Cl−, NO3−, SO42−, NO2−, F−, PO43−) Cations (Na+, K+, Li+, Ca2+, Mg2+, NH4+) | Creek water | SIA-CE | Capillary: 60 (35) cm, 50 μm I.D. BGE:12 mM His, 2 mM 18-crown-6 (pH 4) V: ±20 kV | C4D Anions: 0.7–2.0 μM Cations: 13–40 μM | Mai, 2010 | [48] |
| Anions (NO3−, NO2−, Cl−, Br−, F-, SO42−, PO43−, ClO4−, ClO3−, CrO42−, MoO42−) Cations (NH4+, K+, Na+, Mg2+, Ca2+, Mn2+, Zn2+, Sr2+, Cd2+, Fe2+) | Tap and process waters | Dual capillary—SI | LPA coated capillaries: Cations—55 (35) cm, 50 μm I.D., Anions—50 (28) cm, 50 μm I.D., BGE: 50 mM AcOH,10 mM His, 2.5 mM 18-crown-6 (pH 4.2) V: ±30kV | C4D Anions: 5–61 μg·L−1 Cations: 13–40 μg·L−1 | Gaudry, 2013 | [54] |
| Anions (NO3−, NO2−) Cation (NH4+) | Contaminated groundwater | Dual capillary system | Capillary: 55 (40) cm, 50 μm I.D. BGE: 12 mM His, 2 mM 18-crown-6 (pH 4) V: ±15 kV | C4D 5.0 μM NH4+ 6.0 μM NO3− 7.5 μM NO2− | Pham, 2014 | [55] |
| Anions (Cl−, NO3−, SO42−) Cations (K+, Na+, Mg2+, Ca2+) | Mineral and tap waters | GEMBE | Capillary: 5.0 cm, 15 μm I.D. BGE: 100 mM AcOH and 10 mM His (pH 3.76) V: 20 kV | C4D Anions: 0.34–1.13 mg·L−1 Cations: 0.76–3.09 mg·L−1 | Flanigan, 2010 | [57] |
| Anions (Cl−, NO3−, SO42−) Cations (NH4+, K+, Na+, Ca2+, Mg2+, Mn2+, Zn2+, Cd2+, Ba2+ | Tap water | SIA/Dual single-end injections | Capillary: 50 cm, 10 μm I.D, Leff for cations—43 cm and for anions—35 cm; BGE: 12 mM His, 2 mM 18-crown-6 (pH 4) V: 20 kV | Dual C4D Anions: 1.5–2.0 μM Cations: 0.3–1.5 μM | Mai, 2012 | [58] |
| Anions (NO3−, NO2−) Cation (NH4+) | Water quality monitoring after wastewater treatment | SIA-CE | Capillary: 68.0 cm, 20 μm I.D. BGE: 100 mM His, 100 mM MES, 0.13 mM CTAB, 1.5 mM 18-crown-6 (pH 6) V: 24 kV | C4D 0.03 mg⋅L−1 NO2−, 0.08 mg⋅L−1NO3−, 0.11 mg⋅L−1 NH4+ | Fuiko, 2019 | [61] |
| Anions (Cl−, Br−, NO3−, NO2−, SO42−, PO43−) Cations (NH4+, K+, Na+, Li+, Mg2+, Ca2+) | Drinking water (domestic well) | DOI dual opposite end injection | PVA coated capillary: 60 cm, 50 μm I.D. BGE: 15 mM PMA, 10 mM citric acid, 2 mM 18-crown-6 (pH 3.70 adjusted with His) V: 30 kV | C4D Anions: 0.076–2.51 mg·L−1 Cations: 0.075–2.33 mg·L−1 | Neaga, 2014 | [62] |
| Anions (Cl−, NO3−, SO42−) Cations (NH4+, Na+, K+, Ca2+, Mg2+, Mn2+, Zn2+, Cu2+) | Sediment porewater, well and mining pond water | Dual-channel portable | Capillary: 90 (80) cm, 25 μm I.D. BGE for anions: 7.5 mM His and 40 mM AcOH BGE for cations: 9 mM His, 4.6 mM lactic acid, 25 mM AcOH, 1mM 18-crown-6 V: ±25 kV | C4D Anions: 10–12 μM Cations: 2.8–4.8 μM | Koenka, 2016 | [63] |
| Anions (Cl−, NO3−, SO42−) Cations (Na+, K+, NH4+, Ca2+, Mg2+) | Mineral and tap waters | CFM | Capillary: 35.0 (20 and 15) cm, 25 μm I.D. BGE: 18 mM His, 130 mM malic acid, 100 mM DDAPS, 3 mM18-crown-6 (pH 3.6) V: 30 kV | C4D Anions: 0.4–0.6 mg·L−1 Cations: 0.4–0.6 mg·L−1 | Yamamoto, 2019 | [85] |
| Analyte(s) | Sample | Chip | Separation Conditions | Detection, LOD | First Author, Year of Publication | Ref. |
|---|---|---|---|---|---|---|
| Ammonium | Wastewater | CZE and ITP-CZE PMMA | Channel C1—59 mm × 0.2 mm–0.5 mm × 0.14 mm–0.2 mm; channel C2—56 mm × 0.2 mm–0.5 mm × 0.14 mm–0.2 mm) BGE1: LE: 1.25 mM ethylenediamine, 3.75 mM acetic acid, 50 mM 18-crown-6, 0.1% v/v PEG, pH 5.4; TE: 10 mM sodium acetate, 10 mM AcOH, 0.1% v/v PEG (pH 4.8), I = 15 µA BGE2: 50 mM AcOH, 25 mM 18-crown-6 and 4 mM tartaric acid, 0.1% v/v PEG (pH 3.0) I = 25 µA | C4D 20 µg·L−1 CZE 40 µg·L−1 ITP-CZE | Luc, 2011 | [99] |
| Anions (Cl−, NO3−, SO42−) | Drinking water | PMMA | Channel: 85 (65) mm, 50 × 50 µm BGE: 18 mM aspartate (pH 4.15), 0.1% MHEC, 5.94 mM Bis-tris propane, 100 mM DDAPS I: 40 μA | C4D 40–120 µg·L−1 | Masar, 2012 | [97] |
| Anions (Cl−, NO3−, F−, SO42−, SCN−, PO43−) | Mineral water, tap water | PDMS | Channel: 65 mm, 100 µm BGE: 50 mM MES/His (pH 6.0), 0.5% PVP V: 2 kV | C4D with in-plane electrodes 3.6–14.7 μM | Koczka, 2016 | [100] |
| Anions (Br−, Cl−, NO3−, NO2−, F−, SO42−) Cations (NH4+, K+, Na+, Li+, Ca2+, Mg2+) | Bottled drinking water | PMMA | Channel: 85 (65) mm, 50 × 50 µm BGE: 30 mM MES/His (pH 6), 2mM 18-crown-6 V: ±4 kV | C4D dual top–bottom cell 0.3 µM cations, 0.15 µM anions | Mahabadi, 2010 | [101] |
| Anions (Cl−, NO3−, NO2−, SO42−) | Aquarium, river water | Borosilicate glass | Channel: 33 mm, 10 × 100 µm BGE: 30 mM latic acid and 15 mM His (pH 3.8) V: −1.0 kV | C4D 2.0 to 4.9 μM | Freitas, 2016 | [102] |
| Cations (K+, Na+, Li+, Ca2+, Mg2+, Zn2+, Cd2+, Cu2+) | River water | PDMS/PET | Channel: 50 mm, 50 × 50 µm BGE1: 10 mM MES, 10 mM His BGE2: 0.1 M acetic buffer pH 4.0 V: 5kV | C4D with ITO-coated films electrodes 5.8 μM K+, 8.0 μM Na+, 14.3 μM Li+ | Yan, 2015 | [98] |
| Cations (Ag+, Hg2+) | Tap water and river water | Quartz | Channel: 23 mm, 104 × 48 μm BGE: phosphate buffer saline (pH 7.4) V: 250 V | LED LIF 0.038 nM Ag+, 0.054 nM Hg2+ | Chen, 2019 | [105] |
| Nitrite | Well water | PDMS | Channel: 50 mm, 15 × 15 μm BGE: 5 mM phosphate (pH 7.5), 200 mM CTAB V: −1.0 kV | Amperometric detection pencil graphite electrode 2.8 μM | Da Silva, 2017 | [103] |
| Nitrite | Drinking water | PDMS | Channel: 50 mm, 15 × 15 μm BGE: 5 mM phosphate (pH 6.85), 200 mM CTAB V: −1.2 kV | Amperometric detection screen-printed carbon-based electrode 8.2 μM | Petroni, 2017 | [104] |
| Perchlorate | Drinking water | PDMS | Channel: 20 mm, 50 × 50 µm BGE:10 mM nicotinic acid, 1.0 mM TDAPS (pH 3.6); V: −700 V | C4D 5.6 ± 1.7 µg·L−1 | Gertsch, 2010 | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poboży, E.; Trojanowicz, M. Application of Capillary Electrophoresis for Determination of Inorganic Analytes in Waters. Molecules 2021, 26, 6972. https://doi.org/10.3390/molecules26226972
Poboży E, Trojanowicz M. Application of Capillary Electrophoresis for Determination of Inorganic Analytes in Waters. Molecules. 2021; 26(22):6972. https://doi.org/10.3390/molecules26226972
Chicago/Turabian StylePoboży, Ewa, and Marek Trojanowicz. 2021. "Application of Capillary Electrophoresis for Determination of Inorganic Analytes in Waters" Molecules 26, no. 22: 6972. https://doi.org/10.3390/molecules26226972
APA StylePoboży, E., & Trojanowicz, M. (2021). Application of Capillary Electrophoresis for Determination of Inorganic Analytes in Waters. Molecules, 26(22), 6972. https://doi.org/10.3390/molecules26226972







