Spatiotemporal Distribution and Analysis of Organophosphate Flame Retardants in the Environmental Systems: A Review
Abstract
:1. Introduction
2. Physicochemical Properties of OPFRs
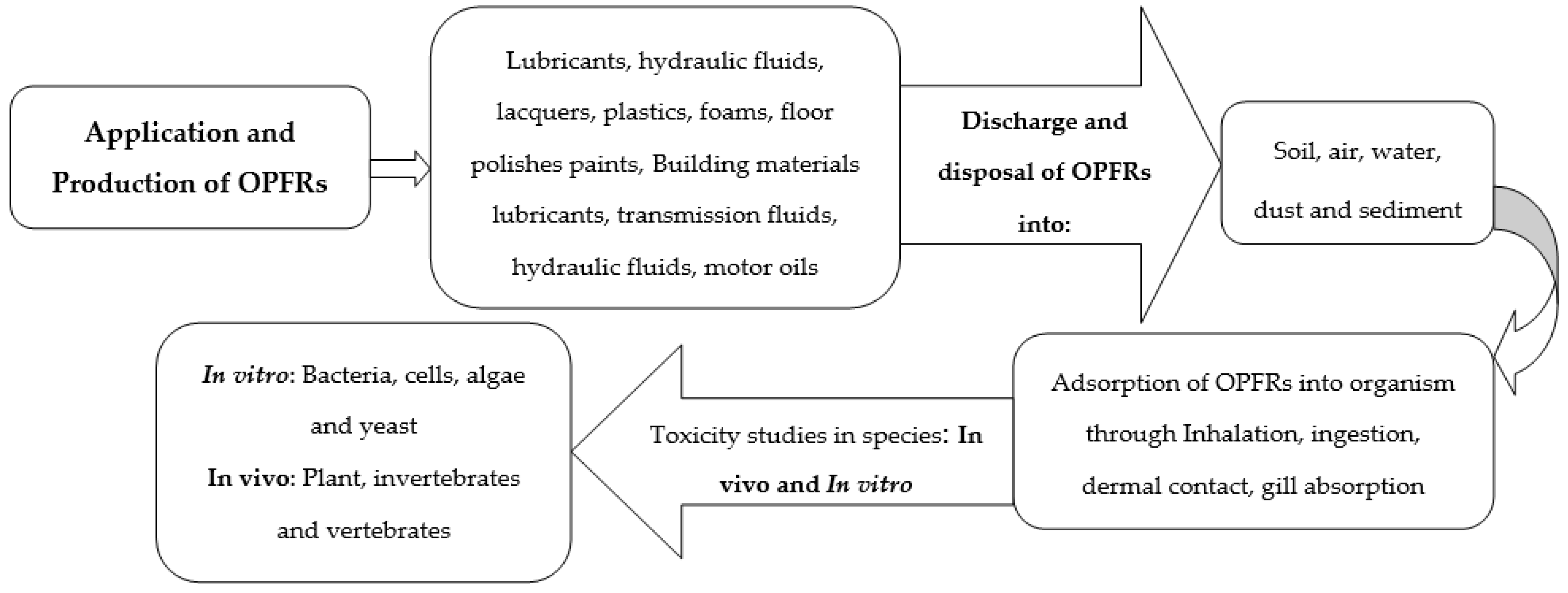
3. Application of OPFRs
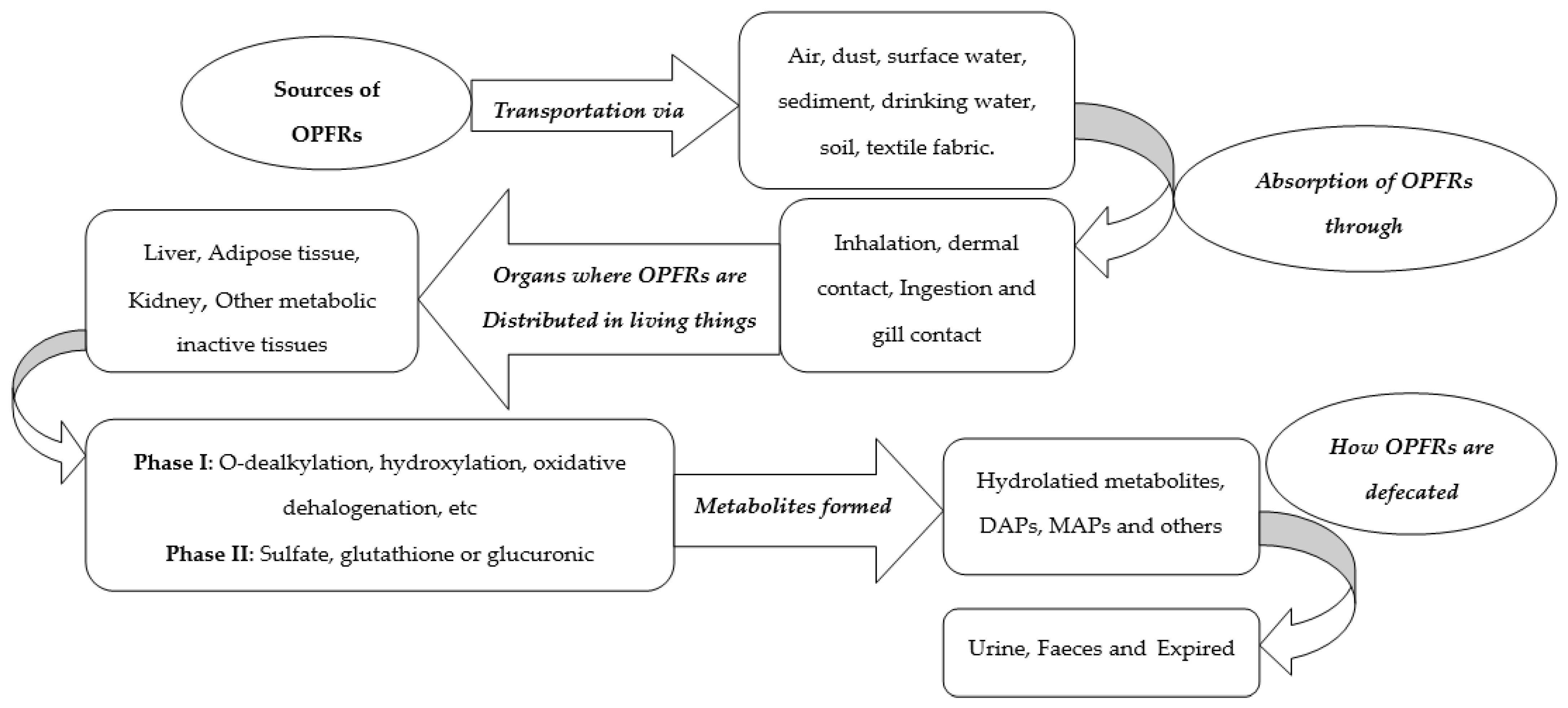
4. Sources of OPFRs
5. Bioresources and Biocomposites of OPFRs
6. Toxic Effects of OPFRs and Risk Exposure
6.1. Toxicity of OPFRs in Humans
6.2. Toxicity of OPFRs in Animals/Living Organisms
6.3. Risk Assessment of OPFRs
7. OPFRs Analysis
8. Extraction Methods for OPFRs in Different Environmental Media
9. Analytical Procedures for OPFRs in Water and Sediments
9.1. Gas Chromatographic Methods
9.2. Liquid Chromatographic Methods
9.3. Nitrogen Phosphorus Detector (NPD)
10. Levels of OPFRs in the Environment across the Globe
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Verbruggen, E.M.J.; Rila, J.P.; Traas, T.P.; Posthuma-Doodeman, C.J.A.M.; Posthumus, R. Environmental Risk Limits for Several Phosphate esters, with Possible Application as Flame Retardant; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Utrecht, The Netherlands, 2006. [Google Scholar]
- Yang, J.; Zhao, Y.; Li, M.; Du, M.; Li, X.; Li, Y. A review of a class of emerging contaminants: The classification, distribution, intensity of consumption, synthesis routes, environmental effects and expectation of pollution abatement to organophosphate flame retardants (OPFRs). Int. J. Mol. Sci. 2019, 20, 2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Lu, L.; Zhu, W.; Yang, B.; Lu, D.; Dan, S.F.; Zhang, S. Organophosphorus flame retardants (OPFRs) in the seawater and sediments of the Qinzhou Bay, Northern Beibu Gulf: Occurrence, distribution, and ecological risks. Mar. Pollut. Bull. 2021, 168, 112368. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Y.; Wang, Y.; Li, Z.; Yang, C.; Rodgers, T.F.; Tan, F. Occurrence and distribution of organophosphate flame retardants in the typical soil profiles of the Tibetan Plateau, China. Sci. Total Environ. 2022, 807, 150519. [Google Scholar] [CrossRef]
- Ma, Y.; Cui, K.; Zeng, F.; Wen, J.; Liu, H.; Zhu, F.; Ouyang, G.; Luan, T.; Zeng, Z. Microwave-assisted extraction combined with gel permeation chromatography and silica gel cleanup followed by gas chromatography–mass spectrometry for the determination of organophosphorus flame retardants and plasticizers in biological samples. Anal. Chim. Acta 2013, 786, 47–53. [Google Scholar] [CrossRef]
- Rigby, H.; Dowding, A.; Fernandes, A.; Humphries, D.; Jones, N.R.; Lake, I.; Petch, R.G.; Reynolds, C.K.; Rose, M.; Smith, S.R. Concentrations of organic contaminants in industrial and municipal bioresources recycled in agriculture in the UK. Sci. Total Environ. 2021, 765, 142787. [Google Scholar] [CrossRef]
- Reemtsma, T.; Quintana, J.B.; Rodil, R.; Garcı, M.; Rodrı, I. Organophosphorus flame retardants and plasticizers in water and air I. Occurrence and fate. TrAC Trends Anal. Chem. 2008, 27, 727–737. [Google Scholar] [CrossRef]
- Van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef] [PubMed]
- Pantelaki, I.; Voutsa, D. Organophosphate flame retardants (OPFRs): A review on analytical methods and occurrence in wastewater and aquatic environment. Sci. Total Environ. 2019, 649, 247–263. [Google Scholar] [CrossRef]
- Stockholm Convention. 2008. Available online: http://chm.pops.int/ (accessed on 15 March 2018).
- Du, J.; Li, H.; Xu, S.; Zhou, Q.; Jin, M.; Tang, J. A review of organophosphorus flame retardants (OPFRs): Occurrence, bioaccumulation, toxicity, and organism exposure. Environ. Sci. Pollut. Res. Int. 2019, 26, 22126–22136. [Google Scholar] [CrossRef] [PubMed]
- Marklund, A.; Andersson, B.; Haglund, P. Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere 2003, 53, 1137–1146. [Google Scholar] [CrossRef]
- Sundkvist, A.M.; Olofsson, U.; Haglund, P. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J. Environ. Monit. 2010, 12, 943–951. [Google Scholar] [CrossRef]
- Wang, Q.; Lam, J.C.W.; Man, Y.C.; Lai, N.L.S.; Kwok, K.Y.; yong Guo, Y.; Lam, P.K.S.; Zhou, B. Bioconcentration, metabolism and neurotoxicity of the organophorous flame retardant 1, 3-dichloro 2-propyl phosphate (TDCPP) to zebrafish. Aquat. Toxicol. 2015, 158, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.W.; Yin, Y.G. The pollution status and research progress on organophosphate esters flame retardants. Prog. Chem. 2010, 22, 1983. [Google Scholar]
- Shao, W.H.; Chen, J.J.; Fan, S.H.; Lei, Y.; Xu, H.B.; Zhou, J.; Cheng, P.F.; Yang, Y.T.; Rao, C.L.; Wu, B.; et al. Combined metabolomics and proteomics analysis of major depression in an animal model: Perturbed energy metabolism in the chronic mild stressed rat cerebellum. Omics A J. Integr. Biol. 2015, 19, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Sigma-Aldrich. 2011. Available online: http://www.sigmaaldrich.com/us-export.html (accessed on 11 November 2021).
- Van der Kolk, J. Inter-Organization Programme for the Sound Management of Chemicals and World Health Organization. In WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2009; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Liagkouridis, I.; Cousins, A.P.; Cousins, I.T. Physical–chemical properties and evaluative fate modelling of ‘emerging’and ‘novel’brominated and organophosphorus flame retardants in the indoor and outdoor environment. Sci. Total Environ. 2015, 524, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Gschwend, P.M.; Imboden, D.M. Environmental Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Hilal, S.H.; Karickhoff, S.W.; Carreira, L.A. Prediction of the vapor pressure boiling point, heat of vaporization and diffusion coefficient of organic compounds. QSAR Comb. Sci. 2003, 22, 565–574. [Google Scholar] [CrossRef]
- Hilal, S.H.; Karickhoff, S.W.; Carreira, L.A. Prediction of the solubility, activity coefficient and liquid/liquid partition coefficient of organic compounds. QSAR Comb. Sci. 2004, 23, 709–720. [Google Scholar] [CrossRef]
- Bergman, Å.; Rydén, A.; Law, R.J.; de Boer, J.; Covaci, A.; Alaee, M.; Birnbaum, L.; Petreas, M.; Rose, M.; Sakai, S.; et al. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environ. Int. 2012, 49, 57–82. [Google Scholar] [CrossRef] [Green Version]
- Liagkouridis, I.; Lazarov, B.; Giovanoulis, G.; Cousins, I. Chemical mass transfer of an organophosphate flame retardant between product source and dust in direct contact. Emerg. Contam. 2016. [Google Scholar]
- Liagkouridis, I.; Lazarov, B.; Giovanoulis, G.; Cousins, I.T. Mass transfer of an organophosphate flame retardant between product source and dust in direct contact. Emerg. Contam. 2017, 3, 115–120. [Google Scholar] [CrossRef]
- Andresen, J.A.; Grundmann, A.; Bester, K. Organophosphorus flame retardants and plasticisers in surface waters. Sci. Total Environ. 2004, 332, 155–166. [Google Scholar] [CrossRef] [PubMed]
- WHO Task Group on Environmental Health Criteria for Flame Retardants, Tris (chloropropyl) Phosphate, Tris (2-chloroethyl) Phosphate, World Health Organization and Inter-Organization Programme for the Sound Management of Chemicals. 1998. Flame Retardants: Tris (chloropropyl) Phosphate and tris (2-chloroethyl) Phosphate (No. 209). Environmental Health Criteria. Available online: https://inchem.org/pages/ehc.html (accessed on 11 November 2021).
- Zhang, D.; Li, S.; Zhu, F.; Li, C.; Xu, Y.; Qing, D.; Wang, J. The influence of an upgrade on the reduction of organophosphate flame retardants in a wastewater treatment plant. Chemosphere 2020, 256, 126895. [Google Scholar] [CrossRef]
- Lai, N.L.; Kwok, K.Y.; Wang, X.H.; Yamashita, N.; Liu, G.; Leung, K.M.; Lam, P.K.; Lam, J.C. Assessment of organophosphorus flame retardants and plasticizers in aquatic environments of China (Pearl River Delta, South China Sea, Yellow River Estuary) and Japan (Tokyo Bay). J. Hazard. Mater. 2019, 371, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, W.; Mu, L.; Chen, Y.; Ren, C.; Hu, X.; Zhou, Q. Rice ingestion is a major pathway for human exposure to organophosphate flame retardants (OPFRs) in China. J. Hazard. Mater. 2016, 318, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Cristale, J.; Vázquez, A.G.; Barata, C.; Lacorte, S. Priority and emerging flame retardants in rivers: Occurrence in water and sediment, Daphnia magna toxicity and risk assessment. Environ. Int. 2013, 59, 232–243. [Google Scholar] [CrossRef]
- Meyer, J.; Bester, K. Organophosphate flame retardants and plasticisers in wastewater treatment plants. J. Environ. Monit. 2004, 6, 599–605. [Google Scholar] [CrossRef]
- Hou, R.; Xu, Y.; Wang, Z. Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere 2016, 153, 78–90. [Google Scholar] [CrossRef]
- Aston, L.S.; Noda, J.; Seiber, J.N.; Reece, C.A. Organophosphate flame retardants in needles of Pinus ponderosa in the Sierra Nevada foothills. Bull. Environ. Contam. Toxicol. 1996, 57, 859–866. [Google Scholar] [CrossRef]
- Carlsson, H.; Nilsson, U.; Becker, G.; Östman, C. Organophosphate ester flame retardants and plasticizers in the indoor environment: Analytical methodology and occurrence. Environm. Sci. Technol. 1997, 31, 2931–2936. [Google Scholar] [CrossRef]
- Carlsson, H.; Nilsson, U.; Östman, C. Video display units: An emission source of the contact allergenic flame retardant triphenyl phosphate in the indoor environment. Environm. Sci. Technol. 2000, 34, 3885–3889. [Google Scholar] [CrossRef]
- Hoelting, L.; Klima, S.; Karreman, C.; Grinberg, M.; Meisig, J.; Henry, M.; Rotshteyn, T.; Rahnenführer, J.; Blüthgen, N.; Sachinidis, A.; et al. Stem cell-derived immature human dorsal root ganglia neurons to identify peripheral neurotoxicants. Stem Cells Transl Med. 2016, 5, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Huang, X.; Li, Z.; Cao, G.; Zhu, X.; She, S.; Huang, T.; Lu, G. Evaluation of hepatotoxicity induced by 2-ethylhexyldiphenyl phosphate based on transcriptomics and its potential metabolism pathway in human hepatocytes. J. Hazard. Mater. 2021, 413, 125281. [Google Scholar] [CrossRef] [PubMed]
- Chukwunwike, S.A.; Okafor, K.J. A review on some selected bio-based (Green) flame retardants. J. Eng. Technol. 2019, 8, 38–43. [Google Scholar]
- Davoodi, M.M.; Sapuan, S.M.; Ahmad, D.; Aidy, A.; Khalina, A.; Jonoobi, M. Concept selection of car bumper beam with developed hybrid bio-composite material. Mater. Des. 2011, 32, 4857–4865. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.Z.; Cai, G.P.; Mai, Y.W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Thomas, A.; Arun, M.; Moinuddin, K.; Joseph, P. Mechanistic Aspects of Condensed-and Gaseous-Phase Activities of Some Phosphorus-Containing Fire Retardants. Polymers 2020, 12, 1801. [Google Scholar] [CrossRef]
- Rabek, J.F. Contemporary Knowledge about Polymers; Wydawnictwo Naukowe PWN: Warszawa, Polska, 2008. (In Polish) [Google Scholar]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-Cuesta, J.M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Hull, T.R.; Stec, A.A. Polymers and fire. In Fire Retardancy of Polymers: New Strategies and Mechanisms; Royal Society of Chemistry: Cambridge, UK; London, UK, 2009. [Google Scholar]
- Kiliaris, P.; Papaspyrides, C.D. Polymers on fire. In Polymer Green Flame Retardants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–43. [Google Scholar]
- Matko, S.; Toldy, A.; Keszei, S.; Anna, P.; Bertalan, G.; Marosi, G. Flame retardancy of biodegradable polymers and biocomposites. Polym. Degrad. Stab. 2005, 88, 138–145. [Google Scholar] [CrossRef]
- Shaw, S. Halogenated flame retardants: Do the fire safety benefits justify the risks? Rev. Environ. Health 2010, 25, 261–306. [Google Scholar] [CrossRef]
- Suzuki, G.; Someya, M.; Takahashi, S.; Tanabe, S.; Sakai, S.; Takigami, H. Dioxin-like activity in Japanese indoor dusts evaluated by means of in vitro bioassay and instrumental analysis: Brominated dibenzofurans are an important contributor. Environ. Sci. Technol. 2010, 44, 8330–8336. [Google Scholar] [PubMed]
- Poston, R.G.; Saha, R.N. Epigenetic effects of polybrominated diphenyl ethers on human health. Int. J. Environ. Res. Public Health 2019, 16, 2703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Li, Y.; Ru, H.; Xie, H.; Yao, F.; Ni, Z.; Zhong, L. Parental exposure to 2, 2′, 4, 4′ 5-pentain polybrominated diphenyl ethers (BDE-99) causes thyroid disruption and developmental toxicity in zebrafish. Toxicol. Appl. Pharmacol. 2019, 372, 11–18. [Google Scholar] [CrossRef]
- Wu, Z.; He, C.; Han, W.; Song, J.; Li, H.; Zhang, Y.; Jing, X.; Wu, W. Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: A review. Environ. Res. 2020, 187, 109531. [Google Scholar] [CrossRef]
- Alexander, B.M.; Charles, A.W. Phosphorus-Based FRs. Non-Halogenated Flame Retardant Handbook. 2014, pp. 17–74. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781118939239.ch2 (accessed on 11 November 2021).
- Sienkiewicz, A.; Czub, P. Flame Retardancy of Biobased Composites—Research Development. Materials 2020, 13, 5253. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, J.; Lv, L.; Zhang, H. Exposure to organophosphate flame esters during early pregnancy and risk of spontaneous abortion: A case-control study. Chemosphere 2021, 268, 129375. [Google Scholar] [CrossRef]
- Blum, A.; Behl, M.; Birnbaum, L.S.; Diamond, M.L.; Phillips, A.; Singla, V.; Sipes, N.S.; Stapleton, H.M.; Venier, M. Organophosphate ester flame retardants: Are they a regrettable substitution for polybrominated diphenyl ethers? Environ. Sci. Technol. Lett. 2019, 6, 638–649. [Google Scholar] [CrossRef]
- Zhou, L.; Hiltscher, M.; Gruber, D.; Püttmann, W. Organophosphate flame retardants (OPFRs) in indoor and outdoor air in the Rhine/Main area, Germany: Comparison of concentrations and distribution profiles in different microenvironments. Environ. Sci. Pollut. Res. 2017, 24, 10992–11005. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Zheng, J.; Wang, T.; Shi, Y.G.; Chen, B.J.; Liu, B.; Ma, Y.H.; Li, M.; Zhuo, L.; Chen, S.J. Legacy and emerging contaminants in coastal surface sediments around Hainan Island in South China. Chemosphere 2019, 215, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Dishaw, L.V.; Powers, C.M.; Ryde, I.T.; Roberts, S.C.; Seidler, F.J.; Slotkin, T.A.; Stapleton, H.M. Is the PentaBDE replacement, tris (1, 3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol. Appl. Pharmacol. 2011, 256, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ji, K.; Choi, K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat. Toxicol. 2012, 114, 173–181. [Google Scholar] [CrossRef]
- Liu, X.; Ji, K.; Jo, A.; Moon, H.B.; Choi, K. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquat. Toxicol. 2013, 134, 104–111. [Google Scholar] [CrossRef]
- von der Ohe, P.C.; Dulio, V.; Slobodnik, J.; De Deckere, E.; Kühne, R.; Ebert, R.U.; Ginebreda, A.; De Cooman, W.; Schüürmann, G.; Brack, W. A new risk assessment approach for the prioritization of 500 classical and emerging organic microcontaminants as potential river basin specific pollutants under the European Water Framework Directive. Sci. Total Environ. 2011, 409, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hou, R.; Hong, X.; Yan, S.; Zha, J. Organophosphate flame retardants (OPFRs) induce genotoxicity in vivo: A survey on apoptosis, DNA methylation, DNA oxidative damage, liver metabolites, and transcriptomics. Environ. Int. 2019, 130, 104914. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yin, L.; Wen, X.; Jiang, C.; Long, Y.; Zhang, J.; Liu, R. Organophosphate Esters in China: Fate, Occurrence, and Human Exposure. Toxics 2021, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, N.; Guo, R.; Xu, H.; Zhang, Q.; Han, Z.; Feng, M.; Li, D.; Zhang, S.; Chen, J. Occurrence and partitioning behavior of organophosphate esters in surface water and sediment of a shallow Chinese freshwater lake (Taihu Lake): Implication for eco-toxicity risk. Chemosphere 2018, 202, 255–263. [Google Scholar] [CrossRef]
- Lian, M.; Lin, C.; Wu, T.; Xin, M.; Gu, X.; Lu, S.; Cao, Y.; Wang, B.; Ouyang, W.; Liu, X.; et al. Occurrence, spatiotemporal distribution, and ecological risks of organophosphate esters in the water of the Yellow River to the Laizhou Bay, Bohai Sea. Sci. Total Environ. 2021, 787, 147528. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, H. A pilot study of organophosphate esters in surface soils collected from Jinan City, China: Implications for risk assessments. Environ. Sci. Pollut. Res. 2021, 28, 3344–3353. [Google Scholar] [CrossRef]
- Jiao, E.; Hu, X.; Li, L.; Zhang, H.; Zhu, Z.; Yin, D.; Qiu, Y. Occurrence and risk evaluation of organophosphorus flame retardants in two urban rivers in Yangtze River Delta. Environ. Monit. Assess. 2021, 193, 1–11. [Google Scholar] [CrossRef]
- Usepa, U. Nanotechnology White Paper; US Environmental Protection Agency: Washington, DC, USA, 2007; p. 20460. [Google Scholar]
- Chokwe, T.B.; Okonkwo, J.O. Occurrence, distribution and ecological risk assessment of organophosphorus flame retardants and plasticizers in sediment samples along the Vaal River catchment, South Africa. Emerg. Contam. 2019, 5, 173–178. [Google Scholar] [CrossRef]
- Scudder, B.C.; Chasar, L.C.; DeWeese, L.; Brigham, M.E.; Wentz, D.A.; Brumbaugh, W.G. Procedures for Collecting and Processing Aquatic Invertebrates and Fish for Analysis of Mercury as Part of the National Water-Quality Assessment Program. US Geological Survey, 2008. Available online: https://pubs.usgs.gov/of/2008/1208/ (accessed on 11 November 2021).
- Giulivo, M.; Capri, E.; Kalogianni, E.; Milacic, R.; Majone, B.; Ferrari, F.; Eljarrat, E.; Barceló, D. Occurrence of halogenated and organophosphate flame retardants in sediment and fish samples from three European river basins. Sci. Total Environ. 2017, 586, 782–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Li, W.; Li, J.; Fan, S.; Shi, Z. Rapid determination of 13 organophosphorus flame retardants in milk by using modified quick, easy, cheap, effective, rugged, and safe technique, solid-phase extraction, and HPLC-MS/MS. J. Sep. Sci. 2021, 44, 2269–2278. [Google Scholar] [CrossRef]
- Drageset, A.; Bjørsvik, H.R. Continuous flow synthesis concatenated with continuous flow liquid–liquid extraction for work-up and purification: Selective mono-and di-iodination of the imidazole backbone. React. Chem. Eng. 2016, 1, 436–444. [Google Scholar] [CrossRef]
- Bokhary, A.; Leitch, M.; Liao, B.Q. Liquid–liquid extraction technology for resource recovery: Applications, potential, and perspectives. J. Water Process Eng. 2021, 40, 101762. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Cao, S.; Zeng, X.; Song, H.; Li, H.; Yu, Z.; Sheng, G.; Fu, J. Levels and distributions of organophosphate flame retardants and plasticizers in sediment from Taihu Lake, China. Environ. Toxicol. Chem. 2012, 31, 1478–1484. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochemistry 2021, 70, 105325. [Google Scholar] [CrossRef]
- Hou, L.; Jiang, J.; Gan, Z.; Dai, Y.; Yang, P.; Yan, Y.; Ding, S.; Su, S.; Bao, X. Spatial distribution of organophosphorus and brominated flame retardants in surface water, sediment, groundwater, and wild fish in Chengdu, China. Arch. Environ. Contam. Toxicol. 2019, 77, 279–290. [Google Scholar] [PubMed]
- De Castro, M.L.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- Soon, Z.Y.; Loh, A.J.Y.; Joseph, C.G.; Sakari, M. A Review on Available Techniques to Evaluate Polycyclic Aromatic Hydrocarbon Concentration Dependency on Certain Chemical Properties. Life Sci. J. 2021, 18. [Google Scholar] [CrossRef]
- Poole, C.F. New trends in solid-phase extraction. TrAC Trends Anal. Chem. 2003, 22, 362–373. [Google Scholar] [CrossRef]
- Sun, H.; Ge, X.; Lv, Y.; Wang, A. Application of accelerated solvent extraction in the analysis of organic contaminants, bioactive and nutritional compounds in food and feed. J. Chromatogr. A 2012, 1237, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Rodil, R.; Quintana, J.B.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Multi-residue analytical method for the determination of emerging pollutants in water by solid-phase extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 2958–2969. [Google Scholar] [CrossRef]
- Jin, T.; Cheng, J.; Cai, C.; Cheng, M.; Wu, S.; Zhou, H. Graphene oxide based sol-gel stainless steel fiber for the headspace solid-phase microextraction of organophosphate ester flame retardants in water samples. J. Chromatogr. A 2016, 1457, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.; Calvo, F.; Quintana, J.B.; Rubi, E.; Rodil, R.; Cela, R. Suitability of solid-phase microextraction for the determination of organophosphate flame retardants and plasticizers in water samples. J. Chromatogr. A 2006, 1108, 158–165. [Google Scholar] [CrossRef]
- Lorenzo, M.; Campo, J.; Picó, Y. Ultra-high-pressure liquid chromatography tandem mass spectrometry method for the determination of 9 organophosphate flame retardants in water samples. MethodsX 2016, 3, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Deng, Y.; Hu, X.; Yang, S.; Sun, C.; He, H. Determination of organophosphate esters in water samples using an ionic liquid-based sol–gel fiber for headspace solid-phase microextraction coupled to gas chromatography-flame photometric detector. J. Chromatogr. A 2013, 1300, 141–150. [Google Scholar] [CrossRef]
- Gustavsson, J.; Ahrens, L.; Nguyen, M.A.; Josefsson, S.; Wiberg, K. Development and comparison of gas chromatography–mass spectrometry techniques for analysis of flame retardants. J. Chromatogr. A 2017, 1481, 116–126. [Google Scholar] [CrossRef]
- Cristale, J.; Quintana, J.; Chaler, R.; Ventura, F.; Lacorte, S. Gas chromatography/mass spectrometry comprehensive analysis of organophosphorus, brominated flame retardants, by-products and formulation intermediates in water. J. Chromatogr. A 2012, 1241, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wolschke, H.; Sühring, R.; Xie, Z.; Ebinghaus, R. Organophosphorus flame retardants and plasticizers in the aquatic environment: A case study of the Elbe River, Germany. Environ. Pollut. 2015, 206, 488–493. [Google Scholar] [CrossRef]
- García-López, M.; Rodríguez, I.; Cela, R.; Kroening, K.K.; Caruso, J.A. Determination of organophosphate flame retardants and plasticizers in sediment samples using microwave-assisted extraction and gas chromatography with inductively coupled plasma mass spectrometry. Talanta 2009, 79, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.L.; Li, D.Q.; Zhuo, M.N.; Liao, Y.S.; Xie, Z.Y.; Guo, T.L.; Li, J.J.; Zhang, S.Y.; Liang, Z.Q. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut. 2015, 196, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Isobe, T.; Chang, K.H.; Amano, A.; Maneja, R.H.; Zamora, P.B.; Siringan, F.P.; Tanabe, S. Levels and distribution of organophosphorus flame retardants and plasticizers in fishes from Manila Bay, the Philippines. Environ. Pollut. 2011, 159, 3653–3659. [Google Scholar] [CrossRef]
- Cristale, J.; Ramos, D.D.; Dantas, R.F.; Junior, A.M.; Lacorte, S.; Sans, C.; Esplugas, S. Can activated sludge treatments and advanced oxidation processes remove organophosphorus flame retardants? Environ. Res. 2016, 144, 11–18. [Google Scholar] [CrossRef]
- Lee, S.; Cho, H.J.; Choi, W.; Moon, H.B. Organophosphate flame retardants (OPFRs) in water and sediment: Occurrence, distribution, and hotspots of contamination of Lake Shihwa, Korea. Mar. Pollut. Bull. 2018, 130, 105–112. [Google Scholar] [CrossRef]
- Li, S.; Zhu, F.; Zhang, D.; Li, C.; Xu, Y.; Qing, D.; Wang, J. Seasonal concentration variation and potential influencing factors of organophosphorus flame retardants in a wastewater treatment plant. Environ. Res. 2021, 199, 111318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, H.; Zhu, H.; Yao, Y.; Chen, H.; Ren, C.; Wu, F.; Kannan, K. Occurrence and distribution of organophosphate flame retardants (OPFRs) in soil and outdoor settled dust from a multi-waste recycling area in China. Sci. Total Environ. 2018, 625, 1056–1064. [Google Scholar] [PubMed]
- Sha, B.; Dahlberg, A.K.; Wiberg, K.; Ahrens, L. Fluorotelomer alcohols (FTOHs), brominated flame retardants (BFRs), organophosphorus flame retardants (OPFRs) and cyclic volatile methylsiloxanes (cVMSs) in indoor air from occupational and home environments. Environ. Pollut. 2018, 241, 319–330. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, H.; Xu, L.; Wang, Y. Uptake and translocation of organophosphate flame retardants (OPFRs) by hydroponically grown wheat (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2019, 174, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Lee, S.; Lee, H.K.; Moon, H.B. Organophosphate flame retardants and plasticizers in sediment and bivalves along the Korean coast: Occurrence, geographical distribution, and a potential for bioaccumulation. Mar. Pollut. Bull. 2020, 156, 111275. [Google Scholar] [CrossRef]
- Hu, M.; Li, J.; Zhang, B.; Cui, Q.; Wei, S.; Yu, H. Regional distribution of halogenated organophosphate flame retardants in seawater samples from three coastal cities in China. Mar. Pollut. Bull. 2014, 86, 569–574. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L.; Li, J.; Zhang, G. Organophosphate ester flame retardants in Nepalese soil: Spatial distribution, source apportionment and air-soil exchange assessment. Chemosphere 2018, 190, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, Y.; Yao, Y.; Ren, C.; Lan, Z.; Fang, X.; Zhang, K.; Sun, W.; Alder, A.C.; Sun, H. Occurrence of organophosphate flame retardants in farmland soils from Northern China: Primary source analysis and risk assessment. Environ. Pollut. 2019, 247, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Bao, L.J.; Wu, C.C.; Liu, L.Y.; Wong, C.S.; Zeng, E.Y. Organophosphate flame retardants emitted from thermal treatment and open burning of e-waste. J. Hazard. Mater. 2019, 367, 390–396. [Google Scholar] [CrossRef]
- Hao, C.; Helm, P.A.; Morse, D.; Reiner, E.J. Liquid chromatography-tandem mass spectrometry direct injection analysis of organophosphorus flame retardants in Ontario surface water and wastewater effluent. Chemosphere 2018, 191, 288–295. [Google Scholar] [CrossRef]
- Martínez-Carballo, E.; González-Barreiro, C.; Sitka, A.; Scharf, S.; Gans, O. Determination of selected organophosphate esters in the aquatic environment of Austria. Sci. Total Environ. 2007, 388, 290–299. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, W.; Kannan, K.; Moon, H.B. Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res. 2016, 103, 182–188. [Google Scholar] [CrossRef]
- Choo, G.; Cho, H.S.; Park, K.; Lee, J.W.; Kim, P.; Oh, J.E. Tissue-specific distribution and bioaccumulation potential of organophosphate flame retardants in crucian carp. Environ. Pollut. 2018, 239, 161–168. [Google Scholar] [CrossRef]
- Chen, R.; Hong, X.; Yan, S.; Zha, J. Three organophosphate flame retardants (OPFRs) reduce sperm quality in Chinese rare minnows (Gobiocypris rarus). Environ. Pollut. 2020, 263, 114525. [Google Scholar] [CrossRef] [PubMed]
- NICNAS, T. Priority Existing Chemical Assessment Report No. 30. In National Industrial Chemicals Notification and Assessment Scheme; Department of Health and Ageing, Australian Government: Sydney, Australia, 2009. [Google Scholar]
- Green, N.; Schlabach, M.; Bakke, T.; Brevik, E.; Dye, C.; Herzke, D.; Huber, S.; Plosz, B.; Remberger, M.; Schøyen, M.; et al. Screening of Selected Metals and New Organic Contaminants 2007. Phosphorus Flame Retardents, Polyfluorinated Organic Compounds, Nitro-Pahs, Silver, Platinum and Sucralose in Air, Wastewater Treatment Falcilities, and Freshwater and Marine Recipients; Norsk Institutt for Vannforskning: Oslo, Norway, 2008; Available online: https://niva.brage.unit.no/niva-xmlui/handle/11250/213998 (accessed on 11 November 2021).

| OPFRs | Full Name | MF | MW (g/mol) |
|---|---|---|---|
| TPP | Triphenyl phosphate | C15H33O4P | 308.4 |
| TBP | Tributyl phosphate | C12H27O4P | 266.3 |
| TBOEP | Tris (2-butoxyethyl) phosphate | C18H39O7P | 398.5 |
| TRCP | Tris (2-chloroethyl) phosphate | C6H12Cl13O4P | 285.5 |
| TEHP | Tris(2-ethylhexyl)-phosphate | C24H51O4P | 435.0 |
| TCPP | Tris(1-chloro-2-propyl)-phosphate | C9H18Cl3O4P | 327.6 |
| TOCP | Tri-o-cresyl phosphate | C21H21O4P | 368.4 |
| OPFRs | Henry’s Law Constant (atm.m3/mol) | Molecular Weight (g/mol) | Water Solubility (mg/L) at 25 °C; | Vapour Pressure (mm/ Hg) | Log KOW | Bioaccumulation Factor (BCF) |
|---|---|---|---|---|---|---|
| TRCP | 1.67 × 10−7 | 285.5 | 7000 | 0.061 | 1.63 | 0.425 |
| TBP | 1.4 × 10-6 | 266.32 | 280 | 1.13 × 10−3 | 4.00 | 39.81 |
| TBOEP | 1.2 × 10−11 | 398.5 | 1.100 | 0.03 | 3.00 | 25.56 |
| TEHP | 2.38 × 10−2 | 434.6 | 0.6 | 8.25 × 10−8 | 9.94 | 3.162 |
| TCEP | 1.67 × 10−7 | 250.2 | 7000 | 0.061 | 1.63 | 0.425 |
| TOCP | 9.21 × 10−7 | 368.4 | 0.3 | 1.10 × 10−7 | 6.34 | 2534 |
| TDCPP | 2.61 × 10−9 | 430.9 | 7.0 | 2.61 × 10−9 | 3.65 | 21.4 |
| TCIPP | 4.69 × 10−7 | 327.6 | 1200 | 5.64 × 10−5 | 2.89 | 3.27 |
| Congener | Matrices | PNEC (ng/g) | References |
|---|---|---|---|
| TCEP | Carassius auratus auratus Soil | 90,000 386 | [65] |
| TCPP | Carassius auratus auratus Soil | 30,000 1700 | [65] |
| TCIPP | Carassius auratus auratus Soil | 5100 320 | [65] |
| TMP | Pimephales promelas Soil | 7000 | [65] |
| TCrP | Carassius auratus auratus Soil | 110 - | [65] |
| TnBP | Carassius auratus auratus Soil | 880 - | [65] |
| TiBP | Carassius auratus auratus Soil | 20 - | [65] |
| EHDPP | crustacean Soil | 18 302 | [65] |
| TPHP | Carassius auratus auratus Soil | 700 130,000 | [65] |
| Type of Matrix | Example of Sites | Sample Collection | Storage | Extraction Method | References |
|---|---|---|---|---|---|
| Air | Private homes, indoor microenvironments, offices, day-care centres, private cars, schools, building material markets and floor/carpet stores | Vacuum pump connected with a gas meter | Quartz Fibre Filter (QFF) and Polyurethane Foam Plug (PUF- PAS) covered with aluminium foil. | Ultrasonic bath | [58] |
| Water | Waste water treatment plants (WWTPs), rivers, taps, surface water, sea and dams | Pre-cleaned 1 Litre amber glass bottle | Ice chest at 4 °C | Solid Phase Extraction | [3] |
| Sediments Soil | Dumpsite, river and terrestrial | Grab sampler Metallic spoon | Sealed in aluminium foil and stored in an ice chest | Ultrasonic bath, Ultrasound Assisted Extraction (UAE), Liquid–Liquid Extraction (LLE)and Microwave-Assisted Extraction (MAE) | [70] [71] [3] |
| Fishes/Other biota | Water environment | Gill or trap netting, electrofishing, tangling, gilling, filtering, spearing and pumping | Samples are preserved on dry ice | Soxhlet extraction (SE), Pressurised Liquid Extraction (PLE) | [72] [73] |
| Urine Breast milk Blood | Human | Metallic container Passive breast milk sampler or breast pump Syringe, needle and vein puncture | Pre-cleaned glass bottles | Solvent-induced phase transition extraction (SIPTE) Solid Phase Extraction (SPE) | [74] [56] |
| Extraction Method | Advantages | Disadvantages | Matrices that Can Be Extracted | References |
|---|---|---|---|---|
| Liquid–liquid extraction (LLE) | Remove inorganic compounds and can be used to deprotonate or protonate acids and bases | Challenging, time-wasting and demanding multiple extractions | Blood Water | [75] [76] |
| Ultrasonic assisted extraction (UAE) | Low-cost, appropriate, and suitable substitution to other extraction methods | Variables associated with UAE (i.e., frequency, power time etc) needs to be optimized for each product | Sediments Marine algae Fruit and Vegetables | [77] [78] [79] [80] |
| Microwave-assisted extraction (MAE) | Decrease the amount of solvent used and time, enhances reproducible results and helps in retrieving analytes from samples | To obtain results for OPFRs combine it with gel permeation chromatography and silica gel | Lipid samples | [2] [5] |
| Soxhlet extraction (SE) | Affordability and ease of operation, uninterrupted distinct method | Consumption of large volume of solvent, time-consuming and labour intensive | Solid samples (Sediments and soil) | [81] [82] [83] |
| Solid phase extraction (SPE) | Low consumption of solvent, efficient, cheap, convenient operation and short time-consuming. | Poor selectivity | Water, milk | [84] |
| Accelerated solvent extraction (ASE) | Uses less solvent, less extraction time, high throughput and automatic operation | It is costly | Solid samples, biotic matrices and food samples | [85] |
| Location | Sample Matrix | Congener | Concentration | Extraction Method | Instrument | Reference |
|---|---|---|---|---|---|---|
| Spain | Wastewater Sludge | 10 OPFRs congeners | 3.67–50 µgL−1 35.3–9980 ng g−1dw | a b | A | [97] |
| China | Rice | 6 OPFRs congeners | 0.004–287 ng/g | c | B | [30] |
| Qinzhou Bay | Sea water Sediments | 11 OPFRs congeners | 150–885 ng/L 32.3 ng/g dw | a d | C | [3] |
| Beijing of China | Wastewater Sludge | 10 OPFRs congeners | 600–838 ng/L | a f | K | [99] |
| Shanghai | Urine | 3 OPFRs congeners | 0.05–2.10 ng/mL | a | K | [56] |
| South Africa (Vaal River) | Sediment | 12 OPFRs congeners | 68–278 ng g−1 dw | d | C | [71] |
| China | Soil Outdoor dust | 12 OPFRs congeners | 37.7–2100 ng/g 9.14–42.700 ng/g | d | C | [100] |
| Sweden | Indoor air | TCEP | 310 ± 560 pg m−3 | e | C | [101] |
| China (Controlled environment growth) | Wheat (Triticum aestivum L.) | 14 OPFRs congeners | 0.18–0.37 μg/g | f | C | [102] |
| Korean coast | Sediment Bivalves | 18 OPFRs congeners | 2.18–347 ng/g dw 6.12–206 ng/g dw | f | B | [103] |
| China | Seawater | 4 OPFRs congeners | 91.87–1392 ng/L | a | D | [104] |
| Europe (European River basin) | Sediment Fish | 14 OPFRs congeners | 0.25–34.0 ng/g dw 9.32–461 ng/g lw | g | B | [73] |
| Nepal | Soil | 8 OPFRs congeners | 25–27,900 ng/g dw | e | C | [105] |
| Northern China (Beijing) | Farmland soil | 12 OPFRs congeners | 0.543 μg/kg–54.9 μg/kg | d | E | [106] |
| South China | e-waste (Thermal treatment) e-waste (Open burning) | 11 OPFRs congeners | 3.70 × 104–3.65 × 105 ng g−1 5.22 × 103–9.27 × 104 ng g−1 | d | C | [107] |
| Canada (Ontario) | Surface water Wastewater | 12 OPFRs congeners | 1.5–30 ng/L | _ | F | [108] |
| Austria | Wastewater Surface water Sediments | 9 OPFRs congeners | 4.1 and 13 ng/L 2.6 and 7.9 ng/L 0.48 and 11 μg/kg | h d | E G F | [109] |
| Korea | Drinking water | TCEP TCPP TBEP | <MDL-1660 ng/L | h | H | [110] |
| Korea (Shihwa lake) | Water Sediment | 18 OPFRs congeners | 28.3–16,000 ng/L 2.99–3800 ng/g dw | h e | B | [98] |
| South Korea (Nakdong River) | Fish (Crusian carp) | 9 OPFRs congeners | Liver: 6.2–18.1 ng/g ww Muscle: 4.2–7.8 ng/g ww | d | C | [111] |
| China (Chengdu) | Surface water Sediment Wild fish Groundwater | 13 OPFRs Congeners | 19.1–533 ng L−1 12.50–253 ng g−1 114–2108 ng g−1 lw 11.7–149 ng L−1 | a d e | I | [81] |
| Spain | Water Sediment | 10 OPFRs congeners | 0.0076–7.2 μg L−1 3.8–824 μg kg−1 | a e | I J | [31] |
| China | Rare minnows (Gobiocypris rarus) | TPHP TBOEP TDCIPP | 0.012 and 0.12 mg/L 0.24 and 2.4 mg/L 0.04 and 0.4 mg/L | i | D | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bika, S.H.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Spatiotemporal Distribution and Analysis of Organophosphate Flame Retardants in the Environmental Systems: A Review. Molecules 2022, 27, 573. https://doi.org/10.3390/molecules27020573
Bika SH, Adeniji AO, Okoh AI, Okoh OO. Spatiotemporal Distribution and Analysis of Organophosphate Flame Retardants in the Environmental Systems: A Review. Molecules. 2022; 27(2):573. https://doi.org/10.3390/molecules27020573
Chicago/Turabian StyleBika, Sinozuko Hope, Abiodun Olagoke Adeniji, Anthony Ifeanyi Okoh, and Omobola Oluranti Okoh. 2022. "Spatiotemporal Distribution and Analysis of Organophosphate Flame Retardants in the Environmental Systems: A Review" Molecules 27, no. 2: 573. https://doi.org/10.3390/molecules27020573
APA StyleBika, S. H., Adeniji, A. O., Okoh, A. I., & Okoh, O. O. (2022). Spatiotemporal Distribution and Analysis of Organophosphate Flame Retardants in the Environmental Systems: A Review. Molecules, 27(2), 573. https://doi.org/10.3390/molecules27020573







