Andrographolide: A Herbal-Chemosynthetic Approach for Enhancing Immunity, Combating Viral Infections, and Its Implication on Human Health
Abstract
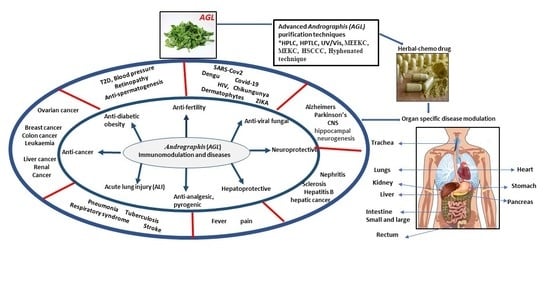
:1. Introduction
2. Functional Prospective of Andrographolide (AGL) on Human Health
Effectiveness of Andrographolide (AGL) with New Emerging Viruses and Their Frequent Mutant Counterparts (SARS-CoV-2 and COVID-19)
3. Modification of Andrographolide (AGL) and Its Constituents: Semi-Synthetic Antiviral Compounds
4. Prospective of Herbal-Chemo Drug Development: A Potential Approach for Human Immunity Enhancement
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Okhuarobo, A.; Ehizogie Falodun, J.; Erharuyi, O.; Imieje, V.; Falodun, A.; Langer, P. Harnessing the medicinal properties of andrographis paniculata for diseases and beyond: A review of its phytochemistry and pharmacology. Asian Pac. J. Trop. Dis. 2014, 4, 213–222. [Google Scholar] [CrossRef]
- Sinha, R.K.; Sharma, S.N.; Verma, S.S.; Zha, J. Effects of lovastin, fosmidomycin and methyl jasmonate on andrographolide biosynthesis in the andrographis paniculata. Acta Physiol. Plant 2018, 40, 1–11. [Google Scholar]
- Shaik, H.A.; Mishra, A.; Sehnal, F. Silk recycling in larvae of the wax moth, galleria mellonella (lepidoptera: Pyralidae). Eur. J. Entomol. 2017, 114, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Shaik, H.A.; Mishra, A.; Sehadová, H.; Kodrík, D. Responses of sericotropin to toxic and pathogenic challenges: Possible role in defense of the wax moth galleria mellonella. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 227, 108633. [Google Scholar] [CrossRef] [PubMed]
- Shaik, H.A.; Mishra, A.; Hussein, H.M.; Skoková Habuštová, O.; Sehnal, F. Competitive interactions between entomopathogenic nematodes and parasitoid venom. J. Appl. Entomol. 2020, 144, 481–490. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-Ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Kar, A.; Mukherjee, P.K.; Haldar, P.K.; Sharma, N.; Katiyar, C.K. Immunoprotective potential of ayurvedic herb kalmegh (andrographis paniculata) against respiratory viral infections—LC-MS/MS and network pharmacology analysis. Phytochem. Anal. 2021, 32, 629–639. [Google Scholar] [CrossRef]
- Lim, J.C.W.; Chan, T.K.; Ng, D.S.W.; Sagineedu, S.R.; Stanslas, J.; Wong, W.S.F. Andrographolide and its analogues: Versatile bioactive molecules for combating inflammation and cancer. Clin. Exp. Pharmacol. Physiol. 2012, 39, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Chen, S.-R.; Chai, L.; Zhao, J.; Wang, Y.; Wang, Y. Overview of pharmacological activities of and its major compound andrographolide. Crit. Rev. Food Sci. Nutr. 2019, 59, S17–S29. [Google Scholar] [CrossRef]
- Worasuttayangkurn, L.; Nakareangrit, W.; Kwangjai, J.; Sritangos, P.; Pholphana, N.; Watcharasit, P.; Rangkadilok, N.; Thiantanawat, A.; Satayavivad, J. Acute oral toxicity evaluation of standardized first true leaf ethanolic extract. Toxicol. Rep. 2019, 6, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.K.; Jiang, F.; Eudes, F. TALE protein mediated overexpression of embryogenesis related marker genes in wheat microspores. S. Afr. J. Bot. 2021, 138, 50–56. [Google Scholar] [CrossRef]
- Hua, Z.; Frohlich, K.M.; Zhang, Y.; Feng, X.; Zhang, J.; Shen, L. Andrographolide inhibits intracellular chlamydia trachomatis multiplication and reduces secretion of proinflammatory mediators produced by human epithelial cells. Pathog. Dis. 2015, 73, 1–11. [Google Scholar] [CrossRef]
- Calabrese, C.; Berman, S.H.; Babish, J.G.; Ma, X.; Shinto, L.; Dorr, M.; Wells, K.; Wenner, C.A.; Standish, L.J. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother. Res. 2000, 14, 333–338. [Google Scholar] [CrossRef]
- Seubsasana, S.; Pientong, C.; Ekalaksananan, T.; Thongchai, S.; Aromdee, C. A potential andrographolide analogue against the replication of herpes simplex virus type 1 in vero cells. Med. Chem. 2011, 7, 237–244. [Google Scholar] [CrossRef]
- Chen, H.; Ma, Y.B.; Huang, X.Y.; Geng, C.A.; Zhao, Y.; Wang, L.J.; Guo, R.H.; Liang, W.J.; Zhang, X.M.; Chen, J.J. Synthesis, structure-activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis b virus agents. Bioorg. Med. Chem. Lett. 2014, 24, 2353–2359. [Google Scholar] [CrossRef]
- Lee, J.C.; Tseng, C.K.; Young, K.C.; Sun, H.Y.; Wang, S.W.; Chen, W.C.; Lin, C.K.; Wu, Y.H. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 Pathway in human hepatoma cells. Br. J. Pharmacol. 2014, 171, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Xue, H.J.; Ye, W.C.; Fang, B.H.; Liu, Y.H.; Yuan, S.H.; Yu, P.; Wang, Y.Q. Activity of andrographolide and its derivatives against Influenza virus in vivo and in vitro. Biol. Pharm. Bull. 2009, 32, 1385–1391. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Khanom, W.; Sun, X.; Paemanee, A.; Roytrakul, S.; Wang, D.; Smith, D.R.; Zhou, G.-C. Andrographolide and its 14-aryloxy analogues inhibit zika and dengue virus infection. Molecules 2020, 25, 5037. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mishra, K.P.; Kumar, B.; Singh, S.B.; Ganju, L. Andrographolide mitigates unfolded protein response pathway and apoptosis involved in chikungunya virus infection. Comb. Chem. High Throughput Screen 2021, 24, 849–859. [Google Scholar] [CrossRef]
- Edwin, E.S.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Thanigaivel, A.; Ponsankar, A.; Pradeepa, V.; Selin-Rani, S.; Kalaivani, K.; Hunter, W.B.; Abdel-Megeed, A.; et al. Anti-dengue efficacy of bioactive andrographolide from andrographis paniculata (lamiales: Acanthaceae) against the Primary dengue vector aedes aegypti (diptera: Culicidae). Acta Trop. 2016, 163, 167–178. [Google Scholar] [CrossRef]
- Ramalingam, S.; Karupannan, S.; Padmanaban, P.; Vijayan, S.; Sheriff, K.; Palani, G.; Krishnasamy, K.K. Anti-dengue activity of Extracts and quantification of dengue viral inhibition by SYBR green reverse transcription polymerase chain reaction. AYU 2018, 39, 87–91. [Google Scholar] [PubMed]
- Kaushik, S.; Dar, L.; Kaushik, S.; Yadav, J.P. Identification and characterization of new potent inhibitors of dengue virus NS5 Proteinase from andrographis paniculata supercritical extracts on in animal cell culture and in silico approaches. J. Ethnopharmacol. 2021, 267, 113541. [Google Scholar] [CrossRef]
- Enmozhi, S.K.; Raja, K.; Sebastine, I.; Joseph, J. Andrographolide as a Potential inhibitor of SARS-CoV-2 main protease: An in silico approach. J. Biomol. Struct. Dyn. 2021, 39, 3092–3098. [Google Scholar] [CrossRef]
- Sharma, A.; Vora, J.; Patel, D.; Sinha, S.; Jha, P.C.; Shrivastava, N. Identification of natural inhibitors against prime targets of SARS-CoV-2 Using molecular docking, molecular dynamics simulation and MM-PBSA approaches. J. Biomol. Struct. Dyn. 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sukardiman, M.E.; Fadhil Pratama, M.R.; Poerwono, H.; Siswodihardjo, S. The Coronavirus disease 2019 main protease inhibitor from (Burm. F) ness. J. Adv. Pharm. Technol. Res. 2020, 11, 157–162. [Google Scholar] [CrossRef]
- Gao, F.; Liu, X.; Shen, Z.; Jia, X.; He, H.; Gao, J.; Wu, J.; Jiang, C.; Zhou, H.; Wang, Y. Andrographolide sulfonate attenuates acute lung injury by reducing expression of myeloperoxidase and neutrophil-derived proteases in mice. Front. Physiol. 2018, 9, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, L.; Xia, N.; Chen, X.; Li, Y.; Hong, Y.; Liu, Y.; Wang, Z.; Liu, Y. Activity of antibacterial, antiviral, anti-inflammatory in compounds andrographolide salt. Eur. J. Pharmacol. 2014, 740, 421–427. [Google Scholar] [CrossRef]
- Malik, Z.; Parveen, R.; Parveen, B.; Zahiruddin, S.; Aasif Khan, M.; Khan, A.; Massey, S.; Ahmad, S.; Husain, S.A. Anticancer potential of andrographolide from andrographis paniculata (Burm.f.) nees and its mechanisms of action. J. Ethnopharmacol. 2021, 272, 113936. [Google Scholar] [CrossRef]
- Geng, J.; Liu, W.; Gao, J.; Jiang, C.; Fan, T.; Sun, Y.; Qin, Z.-H.; Xu, Q.; Guo, W.; Gao, J. Andrographolide alleviates parkinsonism in MPTP-PD Mice via targeting mitochondrial fission mediated by dynamin-related protein 1. Br. J. Pharmacol. 2019, 176, 4574–4591. [Google Scholar] [CrossRef]
- Plešingerová, H.; Janovská, P.; Mishra, A.; Smyčková, L.; Poppová, L.; Libra, A.; Plevová, K.; Ovesná, P.; Radová, L.; Doubek, M.; et al. Expression of COBLL1 encoding novel ROR1 Binding partner is robust predictor of survival in chronic lymphocytic leukemia. Haematologica 2018, 103, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.H.; Yu, S.L.; Chen, H.Y.; Wang, C.C.; Chen, H.W.; Chen, J.J.W. The HLJ1-Targeting drug screening identified Chinese Herb andrographolide that can suppress tumour growth and invasion in non-small-cell lung cancer. Carcinogenesis 2013, 34, 1069–1080. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, M.A.; Romero, A.; Figueroa, J.; Cortés, P.; Concha, I.I.; Hancke, J.L.; Burgos, R.A. Andrographolide interferes with Binding of nuclear factor-kappaB to DNA in HL-60-Derived neutrophilic cells. Br. J. Pharmacol. 2005, 144, 680–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.W.; Lin, A.H.; Chu, H.C.; Li, C.C.; Tsai, C.W.; Chao, C.Y.; Wang, C.J.; Lii, C.K.; Liu, K.L. Inhibition of TNF-α-induced Inflammation by andrographolide via down-regulation of the PI3K/Akt signaling pathway. J. Nat. Prod. 2011, 74, 2408–2413. [Google Scholar] [CrossRef]
- Yen, T.L.; Chen, R.J.; Jayakumar, T.; Lu, W.J.; Hsieh, C.Y.; Hsu, M.J.; Yang, C.H.; Chang, C.C.; Lin, Y.K.; Lin, K.H.; et al. Andrographolide stimulates p38 Mitogen-activated protein kinase-nuclear factor erythroid-2-related factor 2-heme oxygenase 1 signaling in Primary cerebral endothelial cells for definite protection against ischemic stroke in rats. Transl. Res. 2016, 170, 57–72. [Google Scholar] [CrossRef]
- Iruretagoyena, M.I.; Tobar, J.A.; González, P.A.; Sepúlveda, S.E.; Figueroa, C.A.; Burgos, R.A.; Hancke, J.L.; Kalergis, A.M. Andrographolide interferes with T Cell activation and reduces experimental autoimmune encephalomyelitis in the mouse. J. Pharmacol. Exp. Ther. 2005, 312, 366–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Gui, L.; Xu, Y.; Wu, T.; Liu, D. Preventive Effects of andrographolide on the development of diabetes in autoimmune diabetic NOD mice by Inducing immune tolerance. Int. Immunopharmacol. 2013, 16, 451–456. [Google Scholar] [CrossRef]
- Su, H.; Mo, J.; Ni, J.; Ke, H.; Bao, T.; Xie, J.; Xu, Y.; Xie, L.; Chen, W. Andrographolide Exerts antihyperglycemic effect through Strengthening intestinal barrier function and increasing microbial composition of Akkermansia Muciniphila. Oxid. Med. Cell. Longev. 2020, 2020, 6538930. [Google Scholar] [CrossRef] [PubMed]
- Gherardelli, C.; Cisternas, P.; Gutiérrez, J.; Martinez, M.; Inestrosa, N.C. Andrographolide Restores glucose uptake in rat hippocampal neurons. J. Neurochem. 2021, 157, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Anantharaman, S.; Rego, R.; Muthakka, M.; Anties, T.; Krishna, H. Andrographis paniculata-mediated synthesis of silver nanoparticles: Antimicrobial properties and computational studies. SN Appl. Sci. 2020, 2, 1618. [Google Scholar] [CrossRef]
- Roy, P.; Das, S.; Auddy, R.G.; Saha, A.; Mukherjee, A. Engineered andrographolide nanoparticles mitigate paracetamol hepatotoxicity in mice. Pharm. Res. 2013, 30, 1252–1262. [Google Scholar] [CrossRef]
- Roy, P.; Das, S.; Mondal, A.; Chatterji, U.; Mukherjee, A. Nanoparticle engineering enhances anticancer efficacy of andrographolide in MCF-7 Cells and mice bearing EAC. Curr. Pharm. Biotechnol. 2012, 13, 2669–2681. [Google Scholar] [CrossRef] [PubMed]
- Sanati, P.; Chua, L.S.; Nasiri, R.; Hashemi, S.-S. Nanoencapsulation of Andrographolide rich extract for the inhibition of cervical and neuroblastoma cancer cells. J. Biomed. Nanotechnol. 2020, 16, 1370–1380. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qu, X.; Qian, W.; Song, Y.; Wang, C.; Liu, W. Andrographolide-loaded solid lipid nanoparticles enhance anti-cancer activity against head and neck cancer and precancerous cells. Oral Dis. 2020, 00, 1–8. [Google Scholar] [CrossRef]
- Ahiwale, R.J.; Chellampillai, B.; Pawar, A.P. Investigation of 1,2-Dimyristoyl-Sn-Glycero-3-Phosphoglycerol-Sodium (DMPG-Na) Lipid with various metal cations in nanocochleate preformulation: Application for Andrographolide oral delivery in cancer therapy. AAPS Pharm. Sci. Tech. 2020, 21, 279. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.P.; Tee, W.; Ng, D.S.W.; Chan, T.K.; Peh, H.Y.; Ho, W.E.; Cheng, C.; Mak, J.C.; Wong, W.S.F. Andrographolide protects against cigarette smoke-induced Oxidative lung injury via augmentation of Nrf2 activity. Br. J. Pharmacol. 2013, 168, 1707–1718. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Ehsan, I.; Mukherjee, B.; Mondal, L.; Roy, S.; Saha, K.D.; Paul, B.; Debnath, M.C.; Bera, T. Therapeutic potential of Andrographolide-loaded nanoparticles on a murine asthma model. Nanomedicine 2019, 20, 102006. [Google Scholar] [CrossRef]
- Chen, P.; Zeng, Z.; Du, H. Establishment and Validation of a drug-target microarray for SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 530, 4–9. [Google Scholar] [CrossRef]
- Sa-ngiamsuntorn, K.; Suksatu, A.; Pewkliang, Y.; Thongsri, P.; Kanjanasirirat, P.; Manopwisedjaroen, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Pitiporn, S.; Khemawoot, P.; et al. Anti-SARS-CoV-2 Activity of andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J. Nat. Prod. 2021, 84, 1261–1270. [Google Scholar] [CrossRef]
- Rehan, M.; Ahmed, F.; Howladar, S.M.; Refai, M.Y.; Baeissa, H.M.; Zughaibi, T.A.; Kedwa, K.M.; Jamal, M.S. A Computational Approach identified andrographolide as a potential drug for suppressing COVID-19-Induced cytokine storm. Front. Immunol. 2021, 12, 648250. [Google Scholar] [CrossRef]
- Srivastava, N.; Garg, P.; Srivastava, P.; Seth, P.K. A Molecular dynamics simulation study of the ACE2 Receptor with screened Natural inhibitors to identify novel drug candidate against COVID-19. PeerJ 2021, 9, e11171. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac injury with Mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [Green Version]
- Junior, A.G.; Tolouei, S.E.L.; Dos Reis Lívero, F.A.; Gasparotto, F.; Boeing, T.; de Souza, P. Natural Agents modulating ACE-2: A Review of compounds with potential against SARS-CoV-2 infections. Curr. Pharm. Des. 2021, 27, 1588–1596. [Google Scholar] [CrossRef]
- Alazmi, M.; Motwalli, O. Molecular Basis for drug repurposing to study the interface of the S protein in SARS-CoV-2 and human ACE2 through Docking, characterization, and molecular dynamics for natural drug candidates. J. Mol. Model. 2020, 26, 338. [Google Scholar] [CrossRef]
- Rajagopal, K.; Varakumar, P.; Baliwada, A.; Byran, G. Activity of Phytochemical constituents of curcuma longa (turmeric) and andrographis paniculata against Coronavirus (COVID-19): An in silico approach. Futur. J. Pharm. Sci. 2020, 6, 104. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Zhang, Z.; Ren, J.; Peluffo, A.E.; Zhang, W.; Zhao, Y.; Wu, J.; Yan, K.; Cohen, D.; et al. Network bioinformatics Analysis provides insight into drug repurposing for COVID-19. Med. Drug Discov. 2021, 10, 100090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Lv, L.; Zhou, Y.L.; Xie, L.D.; Xu, Q.; Zou, X.F.; Ding, Y.; Tian, J.; Fan, J.L.; Fan, H.W.; et al. Efficacy and safety of xiyanping injection in the treatment of COVID-19: A Multicenter, prospective, open-label and randomized controlled trial. Phytother. Res. 2021, 35, 4401–4410. [Google Scholar] [CrossRef]
- Jiang, M.; Sheng, F.; Zhang, Z.; Ma, X.; Gao, T.; Fu, C.; Li, P. Andrographis paniculata (Burm.f.) Nees and its major constituent Andrographolide as potential antiviral agents. J. Ethnopharmacol. 2021, 272, 113954. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Si, Y.; Xu, H. Andrographolide and Its derivatives: Current Achievements and future perspectives. Eur. J. Med. Chem. 2021, 224, 113710. [Google Scholar] [CrossRef] [PubMed]
- Murugan, N.A.; Pandian, C.J.; Jeyakanthan, J. Computational Investigation on phytochemicals to evaluate their potency against SARS-CoV-2 in Comparison to known antiviral compounds in drug trials. J. Biomol. Struct. Dyn. 2021, 39, 4415–4426. [Google Scholar] [CrossRef] [PubMed]
- Ekalaksananan, T.; Sookmai, W.; Fangkham, S.; Pientong, C.; Aromdee, C.; Seubsasana, S.; Kongyingyoes, B. Activity of andrographolide and its derivatives on HPV16 pseudovirus infection and viral Oncogene expression in cervical carcinoma cells. Nutr. Cancer 2015, 67, 687–696. [Google Scholar] [CrossRef]
- Khanal, P.; Dey, Y.N.; Patil, R.; Chikhale, R.; Wanjari, M.M.; Gurav, S.S.; Patil, B.M.; Srivastava, B.; Gaidhani, S.N. Combination of System biology to probe the anti-viral activity of andrographolide and its derivative against COVID-19. RSC Adv. 2021, 11, 5065–5079. [Google Scholar] [CrossRef]
- Aromdee, C. Modifications of Andrographolide to increase some biological activities: A patent review (2006–2011). Expert Opin. Ther. Pat. 2012, 22, 169–180. [Google Scholar] [CrossRef]
- Chao, W.W.; Lin, B.-F. Isolation and identification of bioactive compounds in andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aromdee, C.; Suebsasana, S.; Ekalaksananan, T.; Pientong, C.; Thongchai, S. Stage of Action of naturally occurring andrographolides and Their semisynthetic analogues against herpes simplex virus type 1 in vitro. Planta Med. 2011, 77, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Liu, Y.; Wang, B.; Gu, G.; Yang, L.; Zheng, Y.; Qian, H.; Huang, W. Synthesis and Biological evaluation of andrographolide Derivatives as potent anti-HIV agents. Arch. Pharm. 2012, 345, 647–656. [Google Scholar] [CrossRef]
- Uttekar, M.M.; Das, T.; Pawar, R.S.; Bhandari, B.; Menon, V.; Nutan; Gupta, S.K.; Bhat, S.V. Anti-HIV Activity of semisynthetic Derivatives of andrographolide and computational study of HIV-1 gp120 protein binding. Eur. J. Med. Chem. 2012, 56, 368–374. [Google Scholar] [CrossRef]
- Li, F.; Lee, E.M.; Sun, X.; Wang, D.; Tang, H.; Zhou, G.-C. Design, Synthesis and discovery of andrographolide derivatives against zika virus infection. Eur. J. Med. Chem. 2020, 187, 111925. [Google Scholar] [CrossRef] [PubMed]
- Priengprom, T.; Ekalaksananan, T.; Kongyingyoes, B.; Suebsasana, S.; Aromdee, C.; Pientong, C. Synergistic effects of acyclovir and 3,19-Isopropylideneandrographolide on herpes simplex virus wild types and drug-resistant strains. BMC Complement. Altern. Med. 2015, 15, 56. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Zhang, C.; Sun, H.; Liu, Q.; Huang, J.; Sheng, L.; Lin, B.; Wang, J.; Chen, L. The Semi-synthesis of novel andrographolide analogues and Anti-Influenza Virus activity evaluation of their derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 769–773. [Google Scholar] [CrossRef]
- Wang, D.; Guo, H.; Chang, J.; Wang, D.; Liu, B.; Gao, P.; Wei, W. Andrographolide prevents EV-D68 replication by inhibiting the acidification of virus-containing endocytic vesicles. Front. Microbiol. 2018, 9, 2407. [Google Scholar] [CrossRef]
- Cai, W.; Wen, H.; Zhou, Q.; Wu, L.; Chen, Y.; Zhou, H.; Jin, M. 14-Deoxy-11,12-didehydroandrographolide inhibits apoptosis in influenza A(H5N1) virus-infected human lung epithelial cells via the caspase-9-dependent intrinsic apoptotic pathway which contributes to its antiviral activity. Antiviral Res. 2020, 181, 104885. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Jain, V. Andrographolide: A review of analytical methods. J. Chromatogr. Sci. 2021, 59, 191–203. [Google Scholar] [CrossRef]
- Pancham, Y.; Patil, N.; Girish, B.; Mannur, V. Development and validation of analytical method for determination of andrographolide in Bulk powder. Int. J. Pharm. Res. Health Sci. 2019, 7, 2899–2903. [Google Scholar] [CrossRef]
- Pundarikakshudu, K.; Shah, K.; Trivedi, P.; Shivprakash, K. Spectrophotometric determination of andrographolides in Andrographis Paniculata nees and its formulation. Indian J. Pharm. Sci. 2007, 69, 457. [Google Scholar] [CrossRef] [Green Version]
- Indrati, O.; Martien, R.; Rohman, A.; Nugroho, A.K. Employment of ATR-FTIR and HPLC-UV method for detection and quantification of andrographolide. Int. J. Appl. Pharm. 2018, 10, 135. [Google Scholar] [CrossRef]
- Shivali, G.; Praful, L.; Vijay, G. A Validated Fourier transform infrared spectroscopy method for quantification of total lactones in inula racemosa and andrographis paniculata. Phytochem. Anal. 2012, 23, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Hao, Z.; Wu, Q.; Li, Y.; Liu, H.; Yan, L. A novel flow-injection chemiluminescence method for determination of andrographolide in andrographis tablets. Drug Test. Anal. 2013, 5, 340–345. [Google Scholar] [CrossRef]
- Zhao, Q.; Ding, J.; Jin, H.; Ding, L.; Ren, N. A Green method using a micellar system for determination of andrographolide and dehydroandrographolide in human plasma. J. Chromatogr. Sci. 2013, 51, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Stanković, D.M.; Samphao, A.; Kuzmanović, D.; Kalcher, K. Novel electroanalyical method for the determination of andrographolide from andrographis paniculata extract and urine samples. Microchem. J. 2015, 122, 16–19. [Google Scholar] [CrossRef]
- Yanfang, Z.; Xingping, L.; Zongde, Z.; Liren, C.; Yongmin, L. Simultaneous determination of andrographolide and dehydroandrographolide in andrographis paniculata and Chinese medicinal preparations by microemulsion electrokinetic chromatography. J. Pharm. Biomed. Anal. 2006, 40, 157–161. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, G.; Liu, H.; Wang, D.; Song, X.; Chen, Y. Determination of andrographolide, deoxyandrographolide and neoandrographolide in the Chinese herb andrographis paniculata by micellar electrokinetic capillary chromatography. Phytochem. Anal. 2002, 13, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Qizhen, D.; Jerz, G.; Winterhalter, P. Separation of andrographolide and neoandrographolide from the leaves of andrographis Paniculata using high-speed counter-current chromatography. J. Chromatogr. A 2003, 984, 147–151. [Google Scholar]
- Jain, P.K.; Ravichandran, V.; Jain, P.K.; Agrawal, R.K. High-performance thin layer chromatography method for estimation of andrographolide in herbal extract and polyherbal formulations. J. Saudi Chem. Soc. 2010, 14, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Chavan, A.K.; Nirmal, S.A.; Pattan, S.R. Development and validation of HPTLC method to detect curcumin and gallic acid in polyherbal microencapsulated formulation. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1213–1217. [Google Scholar] [CrossRef]
- Phattanawasin, P.; Burana-Osot, J.; Sotanaphun, U.; Kumsum, A. Stability-indicating TLC—image analysis method for determination of andrographolide in bulk drug and andrographis paniculata formulations. Acta Chromatogr. 2016, 28, 525–540. [Google Scholar] [CrossRef] [Green Version]
- Syukri, Y.; Afetma, D.W.; Sirin, M.; Fajri, R.; Ningrum, A.D.K.; Setiawan, S.D.; Wibowo, A. Validation of a simple HPLC-UV method for the quantification of andrographolide in self-nano emulsifying drug delivery system (Snedds) for dissolution study. Int. J. Drug Deliv. Technol. 2017, 7, 239–243. [Google Scholar]
- Bhope, S.G.; Kuber, V.V.; Nagore, D.H.; Gaikwad, P.S.; Patil, M.J. Development and validation of RP-HPLC method for simultaneous analysis of andrographolide, phyllanthin, and hypophyllanthin from herbal hepatoprotective formulation. Acta Chromatogr. 2013, 25, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Kotagiri, R.; Kanaaujia, A.; Singh, P.; Thakur, D. Validated RP-HPLC method for the quantification of andrographolide in toxiroak premix, a polyherbal mycotoxin inhibitor. Int. J. Pharm. Sci. Res. 2013, 4, 2623–2628. [Google Scholar]
- Suo, X.B.; Zhang, H.; Wang, Y.Q. HPLC Determination of Andrographolide in Rat Whole Blood: Study on the pharmacokinetics of andrographolide incorporated in liposomes and tablets. Biomed. Chromatogr. 2007, 21, 730–734. [Google Scholar] [CrossRef]
- Levita, J.; Juwita, T.; Ramadhani, S.; Saptarini, N.; Mutakin, M. Chromatogram profiles of andrographolide in A23187-induced New Zealand rabbit’s urine and faeces. J. App. Pharm. Sci. 2017, 7, 156–159. [Google Scholar] [CrossRef] [Green Version]
- Chandra, P.; Kannujia, R.; Pandey, R.; Shukla, S.; Bahadur, L.; Pal, M.; Kumar, B. Rapid quantitative analysis of multi-components in andrographis paniculata using UPLC-QqQLIT-MS/MS: Application to soil sodicity and organic farming. Ind. Crops Prod. 2016, 83, 423–430. [Google Scholar] [CrossRef]
- Bera, R.; Ahmed, S.K.M.; Sarkar, L.; Sen, T.; Karmakar, S. Pharmacokinetic analysis and tissue distribution of andrographolide in rat by a validated LC-MS/MS method. Pharm. Biol. 2014, 52, 321–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pholphana, N.; Panomvana, D.; Rangkadilok, N.; Suriyo, T.; Ungtrakul, T.; Pongpun, W.; Thaeopattha, S.; Satayavivad, J. A simple and sensitive LC-MS/MS method for determination of four major active diterpenoids from andrographis paniculata in human plasma and its application to a pilot study. Planta Med. 2016, 82, 113–120. [Google Scholar] [CrossRef]
- Nugraha, R.V.; Ridwansyah, H.; Ghozali, M.; Khairani, A.F.; Atik, N. Traditional herbal medicine candidates as complementary treatments for COVID-19: A review of their mechanisms, pros and cons. Evid. Based Complementary Altern. Med. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Lim, X.Y.; Chan, J.S.W.; Tan, T.Y.C.; Teh, B.P.; Razak, M.R.M.; Mohamad, S.; Mohamed, A.F.S. Andrographis paniculata (Burm. F.) wall. ex nees, andrographolide, and andrographolide analogues as SARS-CoV-2 antivirals? A rapid review. Nat. Prod. Commum. 2021, 16. [Google Scholar] [CrossRef]
- Çubuk, H.; Özbİl, M. Comparison of clinically approved molecules on SARS-CoV-2 drug target proteins: A molecular docking study. Turk. J. Chem. 2021, 45, 35–41. [Google Scholar] [CrossRef]
- Uzunova, K.; Filipova, E.; Pavlova, V.; Vekov, T. Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2. Biomed. Pharmacother. 2020, 131, 110668. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef] [PubMed]
- Basak, A.; Li, S.; Banik, U.K. A new combination drugs using andrographolide derived natural prod-559 uct restomune for management of HIV. Case Rep. Clin. Pract. Rev. 2003, 4, 223–233. [Google Scholar]


| AGL Analogues $/ Derivatives # | Virus | Targeted Cells | CC50 (µM) | EC50 (µM) | Cytotoxicity Assay | Selective Index (SI)/Therapeutic Index (TI) | Reference |
|---|---|---|---|---|---|---|---|
| 14-aryloxy analogues ZAD-1 $ | ZIKV DENV | 136.3 ± 6 | [18] | ||||
| 510.3 ± 53 | 27.9 ± 1.7 | 9.8 (SI) | |||||
| 272.9 ± 22 | 22.6 ± 1.8 | 6.6 (SI) | |||||
| 148.8 ± 40 | |||||||
| BHK-21 Vero | |||||||
| 14-aryloxy analogues ZAD-2 $ | 217.7 ± 16 | - | MTT Assay | - | |||
| 179.2 ± 13 | |||||||
| 194.2 ± 17 | |||||||
| 196.8 ± 7 | |||||||
| A549 HEK293T/17 | |||||||
| 14-aryloxy analogues ZAD-3 $ | 175.0 ± 7 | - | - | ||||
| 186.1 ± 25 | |||||||
| 190.1 ± 22 | |||||||
| 201.4 ± 25 | |||||||
| Human immunodeficiency virus HIV-1 | 23.74 (TI) | [65] | |||||
| 3-O-Nicotinoyl-19-O-(n-decanoyl)-dehydroandrographolide # | 103.5 ± 128.73 | 82.89 ± 7.33 | |||||
| 34.07 (TI) | |||||||
| 3-O-Nicotinoyl-19-O-(1-naphthalene acetyl)-dehydroandrographolide # | >200 | 5.87 ± 1.96 | 18.02 (TI) | ||||
| 3-O-Nicotinoyl-19-O-phenylacetyldehydroandrographolide | C8166 | >200 | 11.34 ± 2.56 | MTT Assay | 16.8 (TI) | ||
| 3-O-Nicotinoyl-19-O-(3,4-dimethoxyphenylacetyl) dehydroandrographolide | >200 | ||||||
| 3-O-Nicotinoyl-19-O-(3,4-dimethoxyphenylacetyl) dehydroandrographolide | >200 | 11.76 ± 3.66 | |||||
| 14-deoxyandrographolide (DAD) $ | Herpes simplex virus type 1 (HSV 1) | 80 | [14] | ||||
| 3,19-isopropylideneandrographolide (IPAD) $ | 40 | ||||||
| 3,19-dipalmitoylandrographolide $ | 4.2 | - | - | ||||
| 14-acetyl-3,19-isopropylideneandrographolide $ | Vero | 5.9 | Anti-HSV-1 assay | [64] | |||
| 3,14,19-triacetylamdrographolide $ | 6.4 | ||||||
| 14-dehydroxyandrographolide- 12-sulfonic acid sodium salt (DASS) # | H9N2 H5N1 H1N1 | MDCK | 3720 ± 725 | 142.2 ± 10.3 | MTT Assay | 26 (SI) | [17] |
| 222.9 ± 14.9 | 17 (SI) | ||||||
| 171.1 ± 13.5 | 22 (SI) | ||||||
| 14-a-lipoyl andrographolide (AL-1) # | 785 ± 330 | 8.4 ± 2.4 | 93(SI) | ||||
| 15.2 ± 4.09 | 51(SI) | ||||||
| 7.2 ± 1.5 | 109(SI) | ||||||
| 19-O-(3′, 4′, 5′-Trimethoxy) cinnamoyl dehydroandrographolide # | Hepatitis B (HBV) | HepG2 C8166 | >1706 | MTT Assay | 165.1 (SI) | [15] | |
| 19-O-(2′-Thenoyl)-14-deoxy-14,15-didehydroandrographolide # | 2466 | 104.9 (SI) | |||||
| 19-O-Nicotinoyl-14-deoxy-14,15-didehydroandrographolide # | 2054 | - | 126.0 (SI) | ||||
| 19-O-Cinnamoyl dehydroandrographolide # | 183 | 15.5 (SI) | |||||
| 3,19-(30-Nitrobenzylidene)-andrographolide # | Human immunodeficiency virus (HIV) | 745 | - | MTT Assay | 1460 (TI) | [66] | |
| 14-(20,60-Dichloronicotinoyl) ester of andrographolide # | TZM-bl cells | 10354 | 12,474 (TI) | ||||
| (14α)-(Quinolyl-5′,7′-dichloro-8′-oxy)-19-acetoxyandrographolide # | ZIKV | SNB-19 Vero | 88.7 ± 1.1 | 4.5 ± 0.2 | ZIKV titer assay | 19.7 (SI) | [67] |
| 85.0 ± 1.6 | 18.9 (SI) | ||||||
| 14β-(8′-quinolyloxy)-3,19-diol # | 22.7 ± 1.1 | 1.3 ± 0.1 | >16 | ||||
| 20.8 ± 0.5 | |||||||
| (14β)-(Quinolyl-5′,7′-dichloro-8′-oxy)andrographolide # | >100 | 13.3 ± 0.5 | >7.5 | ||||
| (14α)-(Quinolyl-5′,7′-dichloro-8′-oxy)andrographolide # | 85.2 ± 1 | 7.8 ± 0.4 | 10.9 | ||||
| 82.5 ± 2.2 | 7.5 | ||||||
| 3,19-isopropylideneandrographolide (IPAD) $ | HSV 2 | Vero | 39.71 | - | Anti-HSV-1 assay | 2.20 (SI) | [18,68] |
| HSV 1-KOS | 2.34 | ||||||
| HSV 1-dxpIII | 2.32 |
| Source | Method/Instrument | Solvent/Instrument Detection | Limit of Detection (LOD)/Limit of Quantification (LOQ) | Reference |
|---|---|---|---|---|
| A. paniculate bulk powder | UV/VIS Spectroscopy | Methanol: water (50:50 v/v) | [73] | |
| Herbs and herbal formulations | 95% ethanol, methanol, aqueous sodium hydroxide, picric acid, Baljet reagent dinitrobenzoic acid and KOH solution | 1.2/4.23 μg | [74] | |
| Andrographolide in plant material | FT/IR spectroscopy | FTIR single reflectance horizontal ATR cell spectrometer (Perkin Elmer) | 1.0/3.34 μg/mL | [75] |
| Andrographolide Powder | Perkin Elmer spectrometer with KBr Optics and mercury cadmium telluride A detector | 1.5/15 μg | [76] | |
| Andrographis tablets (Commercial) | Flow-injection chemiluminescence | Chemiluminescence analysis system (IFFM-E mode flow injection) LC-20 A HPLC, fluorescence spectrophotometer, UV/VIS spectrophotometer | 0.0742 μg/mL | [77] |
| Human plasma (Andrographolide treated) | Cloud Point Extraction (CPE) | Triton X-114 (5%, v/v), 0.45 g NaCl, Agilent 1100 liquid chromatograph M.P.-methanol-acetonitrile-0.5% formic acid aqueous solution (40:17:43, v/v/v), SB C18 column (5 μM) | 0.032 μg/mL | [78] |
| Human urine (Andrographolide treated), A. paniculata oil extract | Pulse Voltammetry Measurements | Autolab Pgstat 302 N Electrochemical system, Methrom Autolab B.V. (The Netherlands), pH meter, Boron-doped diamond electrode | 0.75 μM | [79] |
| Heptane (0.81%, w/w), SDS (3.31%, w/w), butan-1-ol (6.61%, w/w) Sodium tetraborate buffer (10 mM), pH 9.2. MDQ CE instrument (PDA detector) | ||||
| Andrographolide (standards), dehydro-andrographolide commercial tablets. | Microemulsion electrokinetic chromatography (MEEKC) | 0.30 and 1.0 μg/mL | [80] | |
| Andrographolide (standards), dehydro-andrographolide commercial tablets. | Micellar Electrokinetic Chromatography (MEKC) | SDS (15 mM) in 30 mM borate buffer pH 9.5. Waters (Milford, MA, USA) fixed wavelength with UV detector Quanta 4000E CE system | 8.64–60.1 mg/L | [81] |
| A. paniculate (plant material) | High-speed Counter-Current Chromatography (HSCCC) | Multilayer coil counter-current chromatograph (Potomac, MD, USA), Water/methanol/ethyl acetate/n-hexane (2.5:2.5:4:1) 1.5 mL/min | - | [82] |
| Herbal extract and multi-herbal formulations, | High-performance Thin-layer Chromatography (HPTLC) | Silica gel 60 F254 Coated TLC Aluminium plates. Chloroform:Toluene:Methanol (66:26:8, v/v/v). | 3.5 and 11.7 ng | [83] |
| Polyherbal Livogat capsule, | Toluene:Acetone:Formic acid (9:7:1, v/v/v) | 62.91 and 209.7 ng per spot | [84] | |
| Bulk Drug and A. paniculata formulations determination. | High-performance liquid chromatography (HPLC) | Dichloromethane-Toluene-Ethanol (6:3:1, v/v/v), | 34–109 and 112–363 ng per spot | [85] |
| Andrographolide in Self-Nano Emulsifying Drug Delivery System (Snedds) | Isocratic methanol:Water (70:30) 0.8 mL/min, Xterra MS C 18 column (150 mm × 4.6 mm, 5 μm) | 1.95 and 3.13 μg/mL | [86] | |
| Andrographolide hypophyllanthin and phyllanthin (herbal liver protective formulations) | Gradient-0.1% orthophosphoric acid (sol. A) and (1:1) acetonitrile: methanol (sol. B), Symmetry C8 column (250 mm × 4.6 mm, 5 μm) | 20 and 60 ng | [87] | |
| Toxiroak Premix (Polyherbal mycotoxin inhibitor) | Isocratic acetonitrile:ortho-phosphoric acid (0.1%), 40:60 v/v 1.0 mL/min, C18 column Phenomenex luna (250 mm × 4.6 mm, 5 μm) | 0.06 and 0.2 μg/mL | [88] | |
| Rat whole blood administered with Andrographolide containing liposomes and commercial tablets | Isocratic methanol:Water (52:48 v/v) 0.8 mL/min, Chromasil ODS Column (25 mm × 4.6 mm, 5 μm) | 0.015 and 0.053 μg/mL | [89] | |
| Urine and faeces of New Zealand rabbit’s (23187-INDUCED and treated with andrographolide) | Isocratic methanol:water (55:45) 1 mL/min 0.5 mL/min, C 18 column (250 mm in, 5-μm and 120 Å pore size) | 1.87 and 5.45 μg/mL | [90] | |
| A. paniculata fresh leaves and stem | Hyphenated technique | Gradient A:0.1% formic acid in water (B) 0.1% formic acid in acetonitrile 0.3 mL/min, Acquity BEH C18 (2.1 mm × 50 mm, 1.7 μm) | 0.18 and 0.75 ng/mL | [91] |
| Pharmaco-kinetic analysis and distribution of andrographolide in rat tissues | Gradient-2 mM ammonium acetate buffer (A), mixture of acetonitrile and solvent A (80:20, v/v) (B) 0.8 mL/min, C18 column (2 mm × 30 mm, 5 μm) | 3.91 ng/mL (LOQ) | [92] | |
| Human plasma determination of four major active diterpenoids from A. paniculata | Gradient: water (A) acetonitrile(B) 0.5 mL/min, Kinetex column (4.6 mm × 150 mm, 2.6 μm) | 2.50 ng/mL (LOQ) | [93] |
| Company/Manufacturer | Drug/Supplements | Composition of the AGL Based Combo-Drug’s Active Principles | Proposed Functional Role |
|---|---|---|---|
| EU Nature | Armor2 Andrographis Pure 800 MG | AP (Plant extract) 600 mg, 10% Andrographolides (200 mg), Gelatine | Strengthens immunity, seasonal protection, cold and flu |
| https://ecosupplements.eu/product/eu-natural-amor-2-andrographis-800mg-60-vcaps/ (accessed on 20 November 2021) | |||
| Bixa Botanicals | Andrographis | AP (Plant extract) 450 mg, 20% AGL, Gelatin | Fever, liver ailments, blood sugar control, headache, anti-inflammation |
| https://bixabotanical.com/search?collection=all&type=product&x=0&y=0&q=Andrographis (accessed on 20 November 2021) | |||
| Nine Life | Andrographis | Andrographis 900 mg, Gelatin, Rice Powder | Supports healthy immune and liver function |
| https://www.ninelife.eu/search?type=product&q=andrographis (accessed on 20 November 2021) | |||
| Terry Naturally Vitamins | Andrographis and Ashwagandha | AP (leaf extract) 200 mg, Ashwagandha (leaf and root extract) 150 mg, Hydroxypropyl methylcellulose, microcrystalline cellulose, magnesium stearate, silica, maltodextrin | Immune defence, antistress, energy and endurance, focus and clarity |
| https://www.terrynaturallyvitamins.com/andrographis-and-ashwagandha (accessed on 20 November 2021) | |||
| Terry Naturally Vitamins | Andrographis EP80TM immune | AP (Leaf extract) 60 mg, Melatonin 5 mg, Selenium 65 mcg and Zn 15 mg | Upper respiratory function support, cellular level support, restorative sleep |
| https://www.terrynaturallyvitamins.com/andrographis-immune (accessed on 20 November 2021) | Hydroxypropyl methylcellulose, microcrystalline cellulose, silica, cellulose powder, citric acid | ||
| Terry Naturally Vitamins | Andrographis EP80™ Extra Strength | AP (Leaf extract) 40 mg, Hydroxypropyl methylcellulose, microcrystalline cellulose, magnesium stearate, silica | Immune function and upper respiratory tract health, joint health, daily energy and adaptability, intensive cellular health and DNA protection from oxidative stress, mental clarity and brain function |
| https://www.terrynaturallyvitamins.com/andrographis-ep80-extra-strength (accessed on 20 November 2021) | |||
| Peak performance | Andrographis +Echinacea Vegan Capsules | AP (aerial extract) 200 mg, Echinacea purpurea root 200 mg | Immune support, stress relief respiratory support |
| https://www.ninelife.eu/products/andrographis-echinacea-vegan-capsules-made-with-organic-echinacea-pure-andrographis-paniculata-nilavembu-green-chiretta-complex-from-natural-plant-extracts-immune-respiratory-support-pills?_pos=34&_sid=b865e717f&_ss=r (accessed on 20 November 2021) | |||
| Swanson | Full spectrum Andrographis paniculata | AP (aerial parts) 400 mg, Gelatin, microcrystalline cellulose, magnesium stearate, silica | Immune system support |
| https://ecosupplements.eu/?s=Full+spectrum+Andrographis+paniculata&post_type=product (accessed on 20 November 2021) | |||
| One planet nutrition | Nano Andrographis | Aqueous nano Andrographis 250 mg, rice flour | Immune support, superior absorption, and bioavailability |
| https://www.oneplanetnutrition.com/shop#!/Nano-Andrographis-120-Caps-250-mg/p/192856679/category=27372415 (accessed on 20 November 2021) | |||
| Solaray | |||
| https://solaray.com/products/andrographis-aerialextract?_pos=2&_sid=aeaccba57&_ss=r (accessed on 20 November 2021) | Andrographis aerial extract | AP (aerial extract) 300 mg, AGL 4% | Immunity booster, respiratory tract benefit |
| Natur’s Way | Andrographis | AP (stem, leaf, flower) 100 mg, 10% AGL | Immune support, seasonal protection against cold and flu |
| https://www.iherb.com/search?kw=Andrographis (accessed on 20 November 2021) | |||
| Cardiovascular Research | Restenoril Andrographolide | AP (stem) 500 mg, Gelatin, Methocel, Potassium sorbate | Supports a healthy immune response, promotes healthy cardiovascular function, provides antioxidant support |
| https://www.nhc.com/restenoril-andrographolide-by-cardiovascular-research (accessed on 20 November 2021) | |||
| Herbadiet | Andrographis Extract | AP (leaf extract) 500 mg | Liver detoxification; immunity booster; fights cold and flu |
| https://herbadiet.in/search?q=Andrographis+Extract (accessed on 20 November 2021) | |||
| Piping Rock | AP Extract | AP (stem) 400 mg, Rice Powder, Gelatin Capsule, Vegetable Magnesium Stearate, Silica. | Immune support and liver function, dietary supplement |
| https://cz.pipingrock.com/andrographis?keywords=Andrographis%20Paniculata%20Extract&qid=1600435411 (accessed on 20 November 2021) | |||
| Oriental Botanicals | ViraForce | AGL 62.5 mg in combination with Olive leaf 1.25 g, Honeysuckle flower bud 1 g, Echinacea root 750 mg, vitamin C (250 mg), Zinc (8 mg). | Immunity against viral and bacterial infections, common cold, influenza (flu), tonsillitis and sinusitis, fever and headache, sore throat, |
| https://www.orientalbotanicals.com.au/products/viraforce (accessed on 20 November 2021) | |||
| Fusion Health | ActiViral | AP leaf 4.5 g (ai 62.5 mg) combined with olive leaf (1250 mg), oleuropein (30 mg), Honeysuckle flower bud (1000 mg), Echinacea root 750 mg, Vitamin C (250 mg), Zinc glycinate (equiv. to 8 mg) | Antiviral and immune support during infections. Multiple disease and infections. |
| https://www.fusionhealth.com.au/products/activiral (accessed on 20 November 2021) | |||
| Sears | Andrographis | AP plant extract 500 mg combined with wood pulp, Colloidal silicon dioxide, Magnesium stearate, Dicalcium phosphate, Sodium benzoate | Liver support, multiple disease and infections. |
| https://www.sears.com/search=Andrographis (accessed on 20 November 2021) | |||
| Panaxea International | AntiVirii | AGL (95%), Taraxasterol (20%), Chlorogenic acid (25%), Lonerica Japonica (contains: standardized Chlorogenic acid 25%) | Boosts immunity, influenza, virus, COVID-19, SARS-CoV-1, SARS-CoV-2, anti-inflammatory properties, pulmonary protective |
| https://antivirii.com/ (accessed on 20 November 2021) | |||
| NHR Science | Andrographis | AP (leaf extract) 300 g; (Bioactive14-Neo-Andro Compound) Vegetarian capsules (Hydroxypropyl Methylcellulose), Non-GMO Rice Flour | Healthy inflammatory response, maintains bone mass and strength, immune response, supports nose, throat, and respiratory health |
| https://nhrscience.com/products/paractin-andrographis-paniculata-leaf-extract (accessed on 20 November 2021) | |||
| MediHerb | Andrographis Complex. Herbal blend of chinacea root, Holy Basil leaf, and AGL | Calcium, Echinacea root 4:1 extract from Echinacea angustifolia root 500 mg; Holy Basil herb 5:1 extract; from Ocimum tenuiflorum herb 500 mg; AGL herb 10:1 extract from AP herb 1.0 g Containing AGL 10 mg; Holy Basil (Ocimum tenuiflorum) herb essential oil | Boosts immunity, supports healthy respiratory system function, helps in maintaining body temperature, encourages adaptive response to occasional everyday stress, promotes healthy liver function |
| https://www.natures-source.com/45126-mediherb-andrographis-complex-60tabs.html (accessed on 20 November 2021) | |||
| Life extension® | Immune Protect with PARACTIN® | Vitamin C (camu-camu extract) 50 mg; Camu-camu extract (wildcrafted berry) 250 mg; Wellmune® (highly purified Beta 1,3/1,6 Glucan from Yeast) 100 mg; PARACTIN® PARACTIN® mixed AGL extract from different source and analogues (25 mg) | Promotes immunity, encourages macrophage activity and natural killer cell function, provides potent antioxidants as well as vitamin C |
| https://www.lifeextensioneurope.com/immune-protect-with-paractin-30-vegetarian-capsules (accessed on 20 November 2021) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, A.; Shaik, H.A.; Sinha, R.K.; Shah, B.R. Andrographolide: A Herbal-Chemosynthetic Approach for Enhancing Immunity, Combating Viral Infections, and Its Implication on Human Health. Molecules 2021, 26, 7036. https://doi.org/10.3390/molecules26227036
Mishra A, Shaik HA, Sinha RK, Shah BR. Andrographolide: A Herbal-Chemosynthetic Approach for Enhancing Immunity, Combating Viral Infections, and Its Implication on Human Health. Molecules. 2021; 26(22):7036. https://doi.org/10.3390/molecules26227036
Chicago/Turabian StyleMishra, Archana, Haq Abdul Shaik, Rakesh Kumar Sinha, and Bakht Ramin Shah. 2021. "Andrographolide: A Herbal-Chemosynthetic Approach for Enhancing Immunity, Combating Viral Infections, and Its Implication on Human Health" Molecules 26, no. 22: 7036. https://doi.org/10.3390/molecules26227036
APA StyleMishra, A., Shaik, H. A., Sinha, R. K., & Shah, B. R. (2021). Andrographolide: A Herbal-Chemosynthetic Approach for Enhancing Immunity, Combating Viral Infections, and Its Implication on Human Health. Molecules, 26(22), 7036. https://doi.org/10.3390/molecules26227036