Neuroprotective Effect of SGLT2 Inhibitors
Abstract
:1. Introduction
2. Neurological Potential of SGLT2 Inhibitors
3. Atherosclerosis, Cognitive Impairment, and SGLT2i
4. Inflammation
5. Oxidative Stress and Mitochondrial Dysfunction
6. mTOR Signaling
7. Cerebrovascular Dysfunction
8. The Effect of SGLT2i on Alzheimer’s Disease Pathology
9. Summary
Funding
Conflicts of Interest
References
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dearborn, J.L.; Qiao, Y.; Suri, M.F.K.; Liu, L.; Mosley, T.H.; Alonso, A.; Knopman, D.S. Intracranial atherosclerosis and dementia The Atherosclerosis Risk in Communities (ARIC) Study. Am. Acad. Neurol. 2017, 88, 1556–1563. [Google Scholar]
- Iadecola, C. Revisiting atherosclerosis and dementia. Nat. Neurosci. 2020, 23, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Bertoluci, M.C.; Rocha, V.Z. Cardiovascular risk assessment in patients with diabetes. Diabetol. Metab. Syndr. 2017, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Van Giau, V. Type 3 diabetes and its role implications in alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, C.; Hua, S.; Liao, H.; Wang, M.; Xiong, Y.; Cao, F. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res. Clin. Pract. 2017, 124, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Exalto, L.G.; Biessels, G.J.; Karter, A.J.; Huang, E.S.; Katon, W.J.; Minkoff, J.R.; Whitmer, R.A. Risk score for prediction of 10 year dementia risk in individuals with type 2 diabetes: A cohort study. Lancet Diabetes Endocrinol. 2013, 1, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An Update on SGLT2 Inhibitors for the Treatment of Diabetes Mellitus. Curr Opin Endocrinol Diabetes Obes 2017, 24, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. Z. Gefassmedizin 2016, 13, 17–18. [Google Scholar] [CrossRef]
- Mahaffey, K.W.; Neal, B.; Perkovic, V.; De Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Fabbrini, E.; Sun, T.; Li, Q.; et al. Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018, 137, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Hierro-bujalance, C.; Infante-garcia, C.; Marco, A.; Herrera, M.; Carranza-naval, M.J.; Suarez, J.; Alves-martinez, P.; Lubian-lopez, S.; Garcia-alloza, M. Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer’ s disease and type 2 diabetes. Alzheimer’s Res. Ther. 2020, 4, 1–13. [Google Scholar]
- McGill, J.B.; Subramanian, S. Safety of Sodium-Glucose Co-Transporter 2 Inhibitors. Am. J. Cardiol. 2019, 124, S45–S52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahara, A.; Takasu, T.; Yokono, M.; Imamura, M.; Kurosaki, E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors in pharmacokinetics, pharmacodynamics, and pharmacologic effects. J. Pharmacol. Sci. 2016, 130, 159–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K.; DeSilva, S.; Abbruscato, T. The role of glucose transporters in brain disease: Diabetes and Alzheimer’s disease. Int. J. Mol. Sci. 2012, 13, 12629–12655. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; LOO, D.D.F.L.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poppe, R.; Karbach, U.; Gambaryan, S.; Wiesinger, H.; Lutzenburg, M.; Kraemer, M.; Witte, O.W.; Koepsell, H. Expression of the Na+-D-glucose cotransporter SGLT1 in neurons. J. Neurochem. 1997, 69, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H. Glucose transporters in brain in health and disease. Pflugers Arch. Eur. J. Physiol. 2020, 472, 1299–1343. [Google Scholar] [CrossRef] [PubMed]
- Enerson, B.E.; Drewes, L.R. The rat blood-brain barrier transcriptome. J. Cereb. Blood Flow Metab. 2006, 26, 959–973. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Wen, S.; Gong, M.; Yuan, X.; Xu, D.; Wang, C.; Jin, J.; Zhou, L. Dapagliflozin activates neurons in the central nervous system and regulates cardiovascular activity by inhibiting sglt-2 in mice. Diabetes, Metab. Syndr. Obes. Targets Ther. 2020, 13, 2781–2799. [Google Scholar] [CrossRef] [PubMed]
- Gaur, A.; Pal, G.K.; Ananthanarayanan, P.H.; Pal, P. Role of Ventromedial hypothalamus in high fat diet induced obesity in male rats: Association with lipid profile, thyroid profile and insulin resistance. Ann. Neurosci. 2014, 21, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Oerter, S.; Förster, C.; Bohnert, M. Validation of sodium/glucose cotransporter proteins in human brain as a potential marker for temporal narrowing of the trauma formation. Int. J. Legal Med. 2019, 133, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, A.; Greve, F.; Gölz, C.; Förster, C.Y.; Koepsell, H.; Thal, S.C. RS1 (Rsc1A1) deficiency limits cerebral SGLT1 expression and delays brain damage after experimental traumatic brain injury. J. Neurochem. 2018, 147, 190–203. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, A.; Kudyar, S.; Gupta, A.; Kudyar, R.; Malhotra, P. Sodium glucose co-transporter inhibitors—A new class of old drugs. Int. J. Appl. Basic Med. Res. 2015, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Cinti, F.; Moffa, S.; Impronta, F.; Cefalo, C.M.; Sun, V.A.; Sorice, G.P.; Mezza, T.; Giaccari, A. Spotlight on ertugliflozin and its potential in the treatment of type 2 diabetes: Evidence to date. Drug Des. Devel. Ther. 2017, 11, 2905–2919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakil, S. Molecular Interaction of Anti-Diabetic Drugs with Acetylcholinesterase and Sodium Glucose Co-Transporter 2. J. Cell. Biochem. 2017, 118, 3855–3865. [Google Scholar] [CrossRef]
- Shaikh, S.; Rizvi, S.M.; Suhail, T.; Shakil, S.; Abuzenadah, A.; Anis, R.; Naaz, D.; Dallol, A.; Haneef, M.; Ahmad, A.; et al. Prediction of Anti-Diabetic Drugs as Dual Inhibitors Against Acetylcholinesterase and Beta-Secretase: A Neuroinformatics Study. CNS Neurol. Disord.—Drug Targets 2016, 15, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Koibuchi, N.; Hasegawa, Y.; Sueta, D.; Toyama, K.; Uekawa, K.; Ma, M.J.; Nakagawa, T.; Kusaka, H.; Kim-Mitsuyama, S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 2014, 13, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdogan, M.A.; Yusuf, D.; Christy, J.; Solmaz, V.; Erdogan, A.; Taskiran, E.; Erbas, O. Highly selective SGLT2 inhibitor dapagliflozin reduces seizure activity in pentylenetetrazol-induced murine model of epilepsy. BMC Neurol. 2018, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Akhanli, P.; Hepsen, S.; Emre, A.I.; Duger, H.; Bostan, H.; Kizilgul, M.; Ucan, B.; Cakal, E. AEP816: 24-week impact of dapagliflozin treatment on body weight, body composition, and cardiac risk indicators of patients with type-2 diabetes mellitus. In Endocrine Abstracts; Bioscientifica: Bristol, UK, 2020. [Google Scholar]
- Irace, C.; Casciaro, F.; Scavelli, F.B.; Oliverio, R.; Cutruzzolà, A.; Cortese, C.; Gnasso, A. Empagliflozin influences blood viscosity and wall shear stress in subjects with type 2 diabetes mellitus compared with incretin-based therapy. Cardiovasc. Diabetol. 2018, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019, 62, 1154–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, L.; Yuan, X.; Zhang, S.; Zhao, X. Investigating the Effects of Dapagliflozin on Cardiac Function, Inflammatory Response, and Cardiovascular Outcome in Patients with STEMI Complicated with T2DM after PCI. Evidence-Based Complement. Altern. Med. 2021, 2021, 9388562. [Google Scholar] [CrossRef] [PubMed]
- Ganbaatar, B.; Fukuda, D.; Shinohara, M.; Yagi, S.; Kusunose, K.; Yamada, H.; Soeki, T.; Hirata, K.-i.; Sata, M. Empagliflozin ameliorates endothelial dysfunction and suppresses atherogenesis in diabetic apolipoprotein E-deficient mice. Eur. J. Pharmacol. 2020, 875, 173040. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sridhar, V.S.; Lovblom, L.E.; Lytvyn, Y.; Burger, D.; Burns, K.; Brinc, D.; Lawler, P.R.; Cherney, D.Z.I. Markers of Kidney Injury, Inflammation, and Fibrosis Associated with Ertugliflozin in Patients With CKD and Diabetes. Kidney Int. Reports 2021, 6, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Bajaj, M.; Yang, H.C.; Perez-Polo, J.R.; Birnbaum, Y. SGLT-2 Inhibition with Dapagliflozin Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Cardiomyopathy in Mice with Type 2 Diabetes. Further Augmentation of the Effects with Saxagliptin, a DPP4 Inhibitor. Cardiovasc. Drugs Ther. 2017, 31, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, S.G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Song, C.; Zeng, Y.; Li, Y.; Li, H.; Liu, B.; Dai, M.; Pan, P. Canagliflozin alleviates LPS-induced acute lung injury by modulating alveolar macrophage polarization. Int. Immunopharmacol. 2020, 88. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, S.J.; Lee, J.J.; Kim, J.S.; Lee, O.H.; Kim, C.K.; Kim, D.; Lee, Y.H.; Oh, J.; Park, S.; et al. Anti-inflammatory effect for atherosclerosis progression by sodium-glucose cotransporter 2 (SGLT-2) inhibitor in a normoglycemic rabbit model. Korean Circ. J. 2020, 50, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nagata, N.; Chen, G.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Sakai, Y.; Kaneko, S.; Ota, T. Empagliflozin reverses obesity and insulin resistance through fat browning and alternative macrophage activation in mice fed a high-fat diet. BMJ Open Diabetes Res. Care 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Bode, D.; Semmler, L.; Wakula, P.; Hegemann, N.; Primessnig, U.; Beindorff, N.; Powell, D.; Dahmen, R.; Ruetten, H.; Oeing, C.; et al. Dual SGLT-1 and SGLT-2 inhibition improves left atrial dysfunction in HFpEF. Cardiovasc. Diabetol. 2021, 20, 1–14. [Google Scholar] [CrossRef]
- Hasan, R.; Lasker, S.; Hasan, A.; Zerin, F.; Zamila, M. Canagliflozin ameliorates renal oxidative stress and inflammation by stimulating AMPK—Akt—eNOS pathway in the isoprenaline - induced oxidative stress model. Sci. Rep. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zaibi, N.; Li, P.; Xu, S.Z. Protective effects of dapagliflozin against oxidative stress-induced cell injury in human proximal tubular cells. PLoS ONE 2021, 16, 1–17. [Google Scholar] [CrossRef]
- Iannantuoni, F.; De Marañon, A.M.; Diaz-morales, N.; Falcon, R.; Hernandez-mijares, A.; Rovira-llopis, S. The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes. J. Clin. Med. 2019, 8, 1814. [Google Scholar] [CrossRef] [Green Version]
- Croteau, D.; Luptak, I.; Chambers, J.M.; Hobai, I.; Panagia, M.; Pimentel, D.R.; Siwik, D.A.; Qin, F.; Colucci, W.S. Effects of sodium-glucose linked transporter 2 inhibition with ertugliflozin on mitochondrial function, energetics, and metabolic gene expression in the presence and absence of diabetes mellitus in mice. J. Am. Heart Assoc. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; Demarco, V.G. Empagliflozin Ameliorates Type 2 Diabetes-Induced Ultrastructural Remodeling of the Neurovascular Unit and Neuroglia in the Female db/db Mouse. Brain Sci. 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bdel-Latif, R.G.; Rifaai, R.A.; Amin, E.F. Empagliflozin alleviates neuronal apoptosis induced by cerebral ischemia/reperfusion injury through HIF-1α/VEGF signaling pathway. Arch. Pharm. Res. 2020, 43, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhu, J.; Yu, S.; Ma, H.; Chen, J.; Ding, X.; Chen, G.; Liang, Y.; Zhang, Q. Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed. Pharmacother. 2020, 132, 110821. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Han, F.; Lu, Q.; Li, X.; Ren, D.; Zhang, J.; Han, Y.; Xiang, Y.K.; Li, J. Empagliflozin Ameliorates Obesity-Related Cardiac Dysfunction by Regulating Sestrin2-Mediated AMPK-mTOR Signaling and Redox Homeostasis in High-Fat Diet—Induced Obese Mice. Diabetes 2020, 69, 1292–1305. [Google Scholar] [CrossRef] [PubMed]
- Moellmann, J.; Mann, P.; Krueger, K.; Klinkhammer, B.; Boor, P.; Marx, N.; Lehrke, M. The SGLT2 inhibitor ertugliflozin causes a switch of cardiac substrate utilization leading to reduced cardiac mTOR-signaling, unfolded protein response and apoptosis. Eur. Heart J. 2021, 42, 3289. [Google Scholar] [CrossRef]
- Szekeres, Z.; Toth, K.; Szabados, E. The effects of sglt2 inhibitors on lipid metabolism. Metabolites 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.A. Atherosclerosis in epilepsy: Its causes and implications. Epilepsy Behav. 2014, 41, 290–296. [Google Scholar] [CrossRef]
- Chiba, Y.; Sugiyama, Y.; Nishi, N.; Nonaka, W.; Murakami, R.; Ueno, M. Sodium/glucose cotransporter 2 is expressed in choroid plexus epithelial cells and ependymal cells in human and mouse brains. Neuropathology 2020, 40, 482–491. [Google Scholar] [CrossRef]
- Pearson, A.; Ajoy, R.; Crynen, G.; Reed, J.M.; Algamal, M.; Mullan, M.; Purohit, D.; Crawford, F.; Ojo, J.O. Molecular abnormalities in autopsied brain tissue from the inferior horn of the lateral ventricles of nonagenarians and Alzheimer disease patients. BMC Neurol. 2020, 20, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Shakil, S.; Biswas, D.; Shakil, S.; Shaikh, S.; Bagga, P.; Kamal, M. Invokana (Canagliflozin) as a Dual Inhibitor of Acetylcholinesterase and Sodium Glucose Co-Transporter 2: Advancement in Alzheimer’s Disease- Diabetes Type 2 Linkage via an Enzoinformatics Study. CNS Neurol. Disord.—Drug Targets 2014, 13, 447–451. [Google Scholar] [CrossRef]
- Behnammanesh, G.; Durante, Z.E.; Peyton, K.J.; Martinez-Lemus, L.A.; Brown, S.M.; Bender, S.B.; Durante, W. Canagliflozin inhibits human endothelial cell proliferation and tube formation. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Riyaz, S.; Biswas, D.; Jahan, R. Forxiga (dapagliflozin): Plausible role in the treatment of diabetes-associated neurological disorders. Biotechnol. Appl. Biochem. 2016, 63, 145–150. [Google Scholar] [CrossRef]
- Ferreira-Vieira, H.T.; Guimaraes, M.I.; Silva, R.F.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Arafa, N.M.S.; Ali, E.H.A.; Hassan, M.K. Canagliflozin prevents scopolamine-induced memory impairment in rats: Comparison with galantamine hydrobromide action. Chem. Biol. Interact. 2017, 277, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical Implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.F.; Zhang, J.; Liu, X.Y.; Fang, H.; Tian, L.B.; Zhou, D.H.; Kosten, T.R.; Zhang, X.Y. Low BDNF is associated with cognitive deficits in patients with type 2 diabetes. Psychopharmacology 2013, 227, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Fu, Y.; Li, B. Brain-derived neurotrophic factor alleviates diabetes mellitus-accelerated atherosclerosis by promoting M2 polarization of macrophages through repressing the STAT3 pathway. Cell. Signal. 2020, 70, 109569. [Google Scholar] [CrossRef] [PubMed]
- Bos, D.; Vernooij, M.W.; De Bruijn, R.F.A.G.; Koudstaal, P.J.; Hofman, A.; Franco, O.H.; Van Der Lugt, A.; Ikram, M.A. Atherosclerotic calcification is related to a higher risk of dementia and cognitive decline. Alzheimer’s Dement. 2015, 11, 639–647.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingo, A.P.; Fan, W.; Duong, D.M.; Gerasimov, E.S.; Dammer, E.B.; Liu, Y.; Harerimana, N.V.; White, B.; Thambisetty, M.; Troncoso, J.C.; et al. Shared proteomic effects of cerebral atherosclerosis and Alzheimer’s disease on the human brain. Nat. Neurosci. 2020, 23, 696–700. [Google Scholar] [CrossRef]
- Sabia, S.; Fayosse, A.; Dumurgier, J.; Schnitzler, A.; Empana, J.P.; Ebmeier, K.P.; Dugravot, A.; Kivimäki, M.; Singh-Manoux, A. Association of ideal cardiovascular health at age 50 with incidence of dementia: 25 Year follow-up of Whitehall II cohort study. BMJ 2019, 366, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Ma, X.; Ilyas, I.; Zheng, X.; Luo, S.; Little, P.J.; Kamato, D.; Sahebkar, A.; Wu, W.; Weng, J.; et al. Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: From pharmacology to pre-clinical and clinical therapeutics. Theranostics 2021, 11, 4502–4515. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Manolio, T.A.; Burke, G.L.; Wolfson, S.K. Carotid-Artery Intima and Media Thickness as a Risk Factor for Myocardial Infarction and Stroke in Older Adults. N. Engl. J. Med. 1999, 340, 14–22. [Google Scholar] [CrossRef]
- Feinkohl, I.; Keller, M.; Robertson, C.M.; Morling, J.R.; Williamson, R.M.; Nee, L.D.; McLachlan, S.; Sattar, N.; Welsh, P.; Reynolds, R.M.; et al. Clinical and subclinical macrovascular disease as predictors of cognitive decline in older patients with type 2 diabetes: The Edinburgh type 2 diabetes study. Diabetes Care 2013, 36, 2779–2786. [Google Scholar] [CrossRef] [Green Version]
- Suridjan, I.; Pollock, B.G.; Verhoeff, N.P.L.G.; Voineskos, A.N.; Chow, T.; Rusjan, P.M.; Lobaugh, N.J.; Houle, S.; Mulsant, B.H.; Mizrahi, R. In-vivo imaging of grey and white matter neuroinflammation in Alzheimer’s disease: A positron emission tomography study with a novel radioligand, “18 F”-FEPPA. Mol. Psychiatry 2015, 20, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Ficek, B.N.; Westbrook, R. Understanding the Role of Systemic Inflammation in Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 3340–3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, K.A.; Gottesman, R.F.; Wu, A.; Knopman, D.S.; Gross, A.L.; Mosley, T.H.; Selvin, E.; Windham, B.G. Systemic inflammation during midlife and cognitive change over 20 years: The ARIC Study. Neurology 2019, 92, E1256–E1267. [Google Scholar] [CrossRef] [PubMed]
- Rochfort, K.D.; Cummins, P.M. The blood-brain barrier endothelium: A target for pro-inflammatory cytokines. Biochem. Soc. Trans. 2015, 43, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Vogels, T.; Murgoci, A.N.; Hromádka, T. Intersection of pathological tau and microglia at the synapse. Acta Neuropathol. Commun. 2019, 7, 109. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 1–15. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M. G and Garrett, W.S, 2016 The Role of Cytokines in the Development of Atherosclerosis. Biochemistry 2016, 176, 1358–1370. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Fu, J. Novel Insights into the NLRP3 Inflammasome in Atherosclerosis. J. Am. Heart Assoc. 2019, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Van Der Heijden, T.; Kritikou, E.; Venema, W.; Van Duijn, J.; Van Santbrink, P.J.; Slütter, B.; Foks, A.C.; Bot, I.; Kuiper, J. NLRP3 Inflammasome Inhibition by MCC950 Reduces Atherosclerotic Lesion Development in Apolipoprotein E–Deficient Mice—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1457–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejera, D.; Mercan, D.; Sanchez-caro, J.M.; Hanan, M.; Greenberg, D.; Soreq, H.; Latz, E.; Golenbock, D.; Heneka, M.T. Systemic inflammation impairs microglial A b clearance through NLRP 3 inflammasome. EMBO J. 2019, 38, e101064. [Google Scholar] [CrossRef] [PubMed]
- Lonnemann, N.; Hosseini, S.; Marchetti, C.; Skouras, D.B.; Stefanoni, D.; D’Alessandro, A.; Dinarello, C.A.; Korte, M. The NLRP3 inflammasome inhibitor OLT1177 rescues cognitive impairment in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2020, 117, 32145–32154. [Google Scholar] [CrossRef] [PubMed]
- Lathe, R.; Sapronova, A.; Kotelevtsev, Y. Atherosclerosis and Alzheimer—Diseases with a common cause? Inflammation, oxysterols, vasculature. BMC Geriatr. 2014, 14, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.G.; Wang, Z.H.; Song, M.; Yu, S.P.; Kang, S.S.; Liu, X.; Zhang, Z.; Xie, M.; Liu, G.P.; et al. δ-Secretase-cleaved Tau stimulates Aβ production via upregulating STAT1-BACE1 signaling in Alzheimer’s disease. Mol. Psychiatry 2021, 26, 586–603. [Google Scholar] [CrossRef]
- Li, X.; Hong, X.; Wang, Y.; Zhang, S.; Zhang, J.; Li, X.; Liu, Y.; Sun, D.; Feng, Q.; Ye, J.; et al. Tau accumulation triggers STAT 1-dependent memory deficits by suppressing NMDA receptor expression. EMBO Rep. 2019, 20, 1–18. [Google Scholar] [CrossRef]
- Lee, N.; Heo, Y.J.; Choi, S.E.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kang, Y.; Lee, K.W.; Kim, H.J. Anti-inflammatory Effects of Empagliflozin and Gemigliptin on LPS-Stimulated Macrophage via the IKK/NF- κ B, MKK7/JNK, and JAK2/STAT1 Signalling Pathways. J. Immunol. Res. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Faraco, G.; Sugiyama, Y.; Lane, D.; Garcia-Bonilla, L.; Chang, H.; Santisteban, M.M.; Racchumi, G.; Murphy, M.; Van Rooijen, N.; Anrather, J.; et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J. Clin. Invest. 2016, 126, 4674–4689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Mack, J.J.; Güç, E.; Warren, C.M.; Squadrito, M.L.; Kilarski, W.W.; Baer, C.; Freshman, R.D.; McDonald, A.I.; Ziyad, S.; et al. Perivascular Macrophages Limit Permeability. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2203–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerkhofs, D.; Van Hagen, B.T.; Milanova, I.V.; Schell, K.J.; Van Essen, H.; Wijnands, E.; Goossens, P.; Blankesteijn, W.M.; Unger, T.; Prickaerts, J.; et al. Pharmacological depletion of microglia and perivascular macrophages prevents Vascular Cognitive Impairment in Ang II-induced hypertension. Theranostics 2020, 10, 9512–9527. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalba, G. Oxidative Stress in Vascular Pathophysiology: Still Much to Learn. Antioxidants 2021, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.E.N.J.; Zhang, X.I.A.; Chen, W.E.I.W.E.I. Role of oxidative stress in Alzheimer’ s disease (Review). Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Hajjar, I.; Hayek, S.S.; Goldstein, F.C.; Martin, G.; Jones, D.P.; Quyyumi, A. Oxidative stress predicts cognitive decline with aging in healthy adults: An observational study. J. neuroinflammation 2018, 15, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaribeygi, H.; Atkin, S.L.; Butler, A.E. Sodium—Glucose cotransporter inhibitors and oxidative stress: An update. J. Cell. Physiol. 2019, 234, 3231–3237. [Google Scholar] [CrossRef] [PubMed]
- Cenini, G.; Voos, W. Mitochondria as potential targets in Alzheimer disease therapy: An update. Front. Pharmacol. 2019, 10, 1–20. [Google Scholar] [CrossRef]
- Khacho, M.; Clark, A.; Svoboda, D.S.; MacLaurin, J.G.; Lagace, D.C.; Park, D.S.; Slack, R.S. Mitochondrial dysfunction underlies cognitive defects as a result of neural stem cell depletion and impaired neurogenesis. Hum. Mol. Genet. 2017, 26, 3327–3341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sa-nguanmoo, P.; Tanajak, P.; Kerdphoo, S.; Jaiwongkam, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol. Appl. Pharmacol. 2017, 333, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Zhang, W. Role of mTOR in Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2018, 19, 2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, S.; Bin-jumah, M.N. Multifarious roles of mTOR signaling in cognitive aging and cerebrovascular dysfunction of Alzheimer’ s disease. Iubmb Life 2020, 72, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Van Skike, C.E.; Galvan, V. A Perfect sTORm: The Role of the Mammalian Target of Rapamycin ( mTOR ) in Cerebrovascular Dysfunction of Alzheimer ’ s Disease: A Mini-Review. Gerontology 2018, 64, 205–211. [Google Scholar] [CrossRef]
- Esterline, R.; Oscarsson, J.; Burns, J. International Review of Neurobiology, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 113–140. [Google Scholar]
- Stanciu, G.D.; Rusu, R.N.; Bild, V.; Filipiuc, L.E.; Tamba, B.I.; Ababei, D.C. Systemic actions of sglt2 inhibition on chronic mtor activation as a shared pathogenic mechanism between alzheimer’s disease and diabetes. Biomedicines 2021, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: A paradigm shift in understanding their mechanism of action. Diabetes Care 2020, 43, 508–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Hamed, F.A.; Elewa, H. Potential Therapeutic Effects of Sodium Glucose-linked Cotransporter 2 Inhibitors in Stroke. Clin. Ther. 2020, 42, e242–e249. [Google Scholar] [CrossRef]
- Yan, C.; Zhou, Y.; Chen, Q.; Luo, Y.; Zhang, J.H.; Huang, H.; Shao, A. Dysfunction of the neurovascular unit in diabetes-related neurodegeneration. Biomed. Pharmacother. 2020, 131, 110656. [Google Scholar] [CrossRef]
- Shabir, O.; Berwick, J.; Francis, S.E. Neurovascular dysfunction in vascular dementia, Alzheimer’ s and atherosclerosis. BMC Neurosci. 2018, 19, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhu, Y.; Wu, Y.; Liu, Y.; Teng, Z.; Hao, Y. Association of carotid atherosclerosis and recurrent cerebral infarction in the Chinese population: A meta-analysis. Neuropsychiatr Dis Treat. 2017, 13, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Tan, L.; Yu, J. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann. Transl. Med. 2014, 2. [Google Scholar] [CrossRef]
- Usman, M.S.; Siddiqi, T.J.; Memon, M.M.; Khan, M.S.; Rawasia, W.F.; Talha Ayub, M.; Sreenivasan, J.; Golzar, Y. Sodium-glucose co-transporter 2 inhibitors and cardiovascular outcomes: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2018, 25, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Wajngarten, M.; Sampaio Silva, G. Hypertension and stroke: Update on treatment. Eur. Cardiol. Rev. 2019, 14, 111–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briasoulis, A.; Al Dhaybi, O.; Bakris, G.L. SGLT2 Inhibitors and Mechanisms of Hypertension. Curr. Cardiol. Rep. 2018, 20, 8–10. [Google Scholar] [CrossRef] [PubMed]
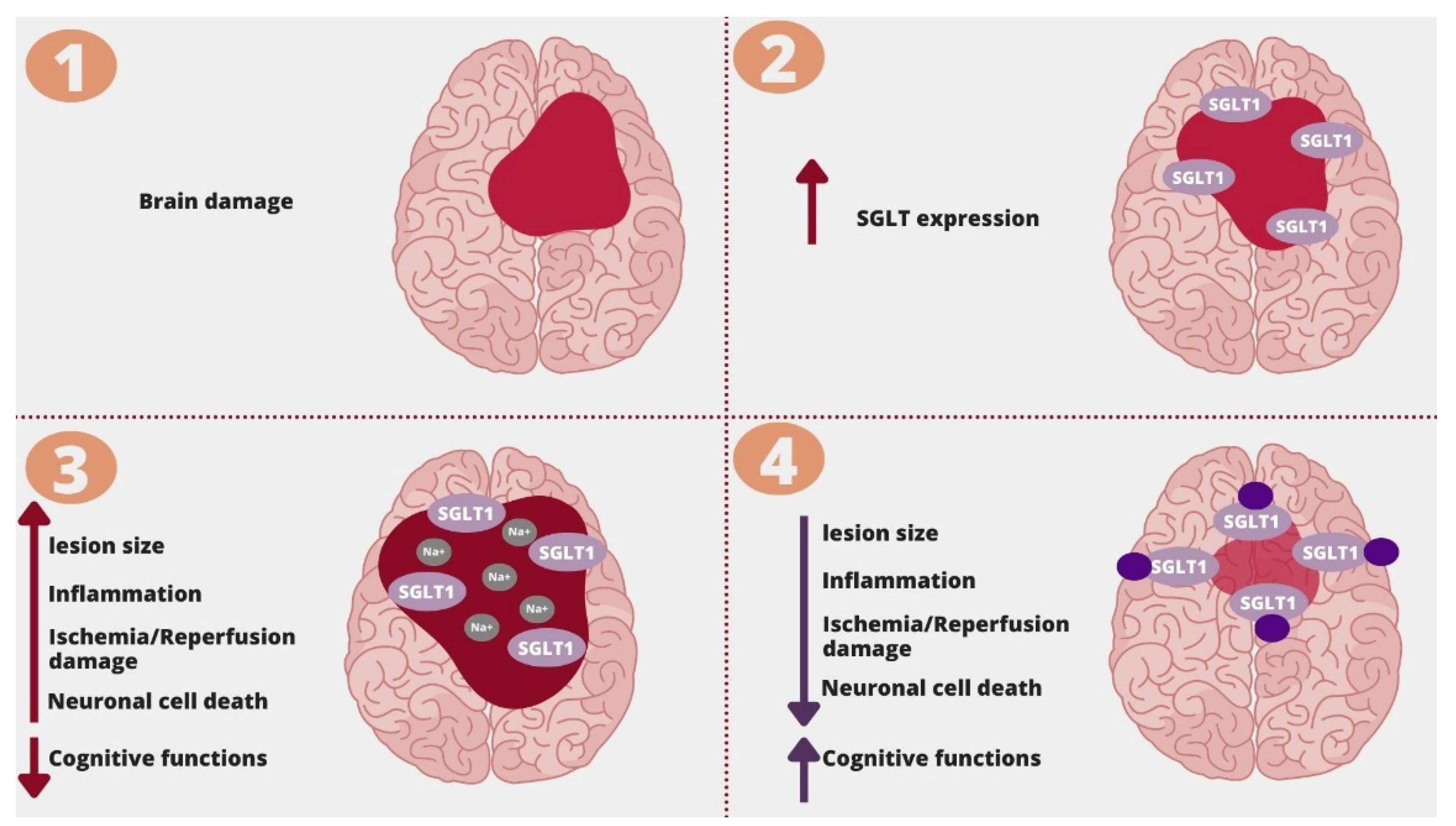
- Ishida, N.; Saito, M.; Sato, S.; Koepsell, H.; Taira, E.; Hirose, M. SGLT1 participates in the development of vascular cognitive impairment in a mouse model of small vessel disease. Neurosci. Lett. 2020, 727, 134929. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Harada, S.; Wada, T.; Hagiwara, T.; Yoshida, S.; Tokuyama, S. Sodium influx through cerebral sodium-glucose transporter type 1 exacerbates the development of cerebral ischemic neuronal damage. Eur. J. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sim, A.Y.; Barua, S.; Kim, J.Y.; Lee, Y.H.; Lee, J.E. Role of DPP-4 and SGLT2 Inhibitors Connected to Alzheimer Disease in Type 2 Diabetes Mellitus. Front. Neurosci. 2021, 15, 1–11. [Google Scholar] [CrossRef]
- Langbaum, J.B.S.; Chen, K.; Lee, W.; Reschke, C.; Bandy, D.; Fleisher, A.S.; Alexander, G.E.; Foster, N.L.; Weiner, M.W.; Koeppe, R.A.; et al. Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Neuroimage 2009, 45, 1107–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthony, K.; Reed, L.J.; Dunn, J.T.; Bingham, E.; Hopkins, D.; Marsden, P.K.; Amiel, S.A. The Cerebral Basis for Impaired Control of Food Intake in. Diabetes 2006, 55, 2986–2992. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, F.; Lucas, J.J.; Avila, J. GSK3 and tau: Two convergence points in Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 33, 141–144. [Google Scholar] [CrossRef] [Green Version]
- Inaba, Y.; Hashiuchi, E.; Watanabe, H.; Kimura, K.; Sato, M.; Kobayashi, M.; Matsumoto, M.; Kitamura, T.; Kasuga, M.; Inoue, H. Hepatic Gluconeogenic Response to Single and Long-Term SGLT2 Inhibition in Lean/Obese Male Hepatic G6pc-Reporter Mice. Endocrinology 2019, 160, 2811–2824. [Google Scholar] [CrossRef]



| Sotagliflozin | Canagliflozin | Dapagliflozin | Empagliflozin | Ertugliflozin | |
|---|---|---|---|---|---|
| SGLT2 Selectivity over SGLT1 | 20 fold [28] | 250 fold [28] | 1200 fold [28] | 2500 fold [28] | 2500 fold [28] |
| Brain/Serum Ratio | n/a | 0.3 | 0.3 | 0.5 | n/a |
| AChE Inhibition | Ki 5.6µM [29] | The most potent, even called a dual inhibitor Ki 0.13 µM [29] | Ki 25.02µM [29] | Ki 0.177µM [30] | Ki 31.69µM [29] |
| BDNF Increase | n/a | n/a | n/a | Yes [31] | n/a |
| Anti-epileptic Potential | n/a | n/a | Yes [32] | n/a | n/a |
| CIMT Regression | n/a | n/a | Yes [33] | Yes [34] | n/a |
| Anti-inflammatory | n/a | Yes [35] | Yes [36] | Yes [37] | No|n/a [38] |
| Blood-brain Barrier Protection | n/a | n/a | n/a | Yes [37] | n/a |
| NLRP3 Inflammasome Inhibition | n/a | n/a | Yes [39] | Yes [40] | n/a |
| Promoting M2 Macrophages Polarization | n/a | Yes [41] | Yes [42] | Yes [43] | n/a |
| Oxidative Stress Reduction | Yes [44] | Yes [45] | Yes [46] | Yes [47] | Yes [48] |
| Neurovascular Unit Remodeling | n/a | n/a | n/a | Yes [49] | n/a |
| Cerebral Ischemia/Reperfusion Damage Reduction | n/a | n/a | n/a | Yes [50] | n/a |
| Reduced mTOR Signaling | n/a | Yes [51] | Yes [51] | Yes [52] | Yes [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlos, A.; Broncel, M.; Woźniak, E.; Gorzelak-Pabiś, P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules 2021, 26, 7213. https://doi.org/10.3390/molecules26237213
Pawlos A, Broncel M, Woźniak E, Gorzelak-Pabiś P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules. 2021; 26(23):7213. https://doi.org/10.3390/molecules26237213
Chicago/Turabian StylePawlos, Agnieszka, Marlena Broncel, Ewelina Woźniak, and Paulina Gorzelak-Pabiś. 2021. "Neuroprotective Effect of SGLT2 Inhibitors" Molecules 26, no. 23: 7213. https://doi.org/10.3390/molecules26237213
APA StylePawlos, A., Broncel, M., Woźniak, E., & Gorzelak-Pabiś, P. (2021). Neuroprotective Effect of SGLT2 Inhibitors. Molecules, 26(23), 7213. https://doi.org/10.3390/molecules26237213







