Enhancing Removal of Cr(VI), Pb2+, and Cu2+ from Aqueous Solutions Using Amino-Functionalized Cellulose Nanocrystal
Abstract
:1. Introduction
2. Results and Discussion
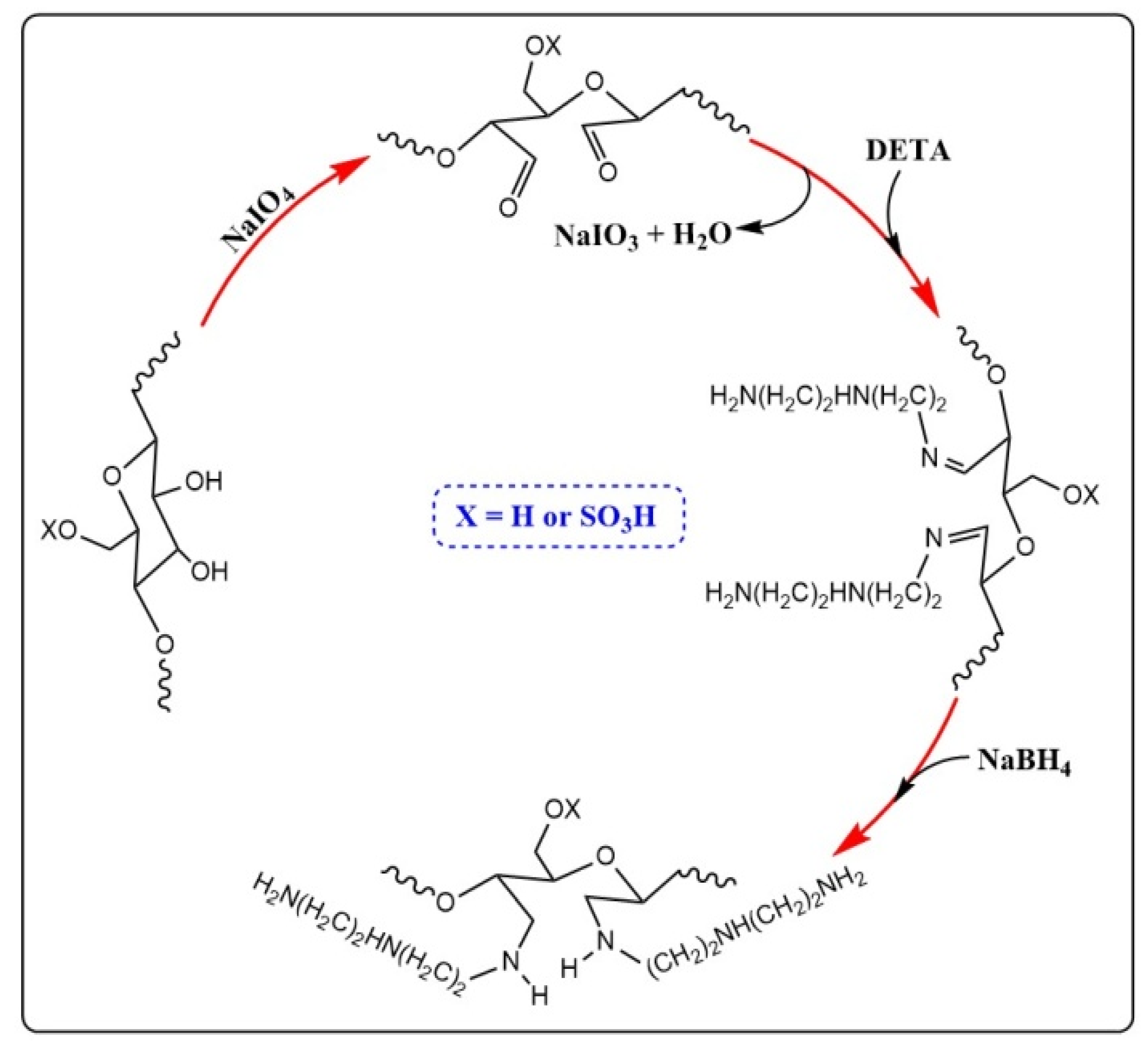
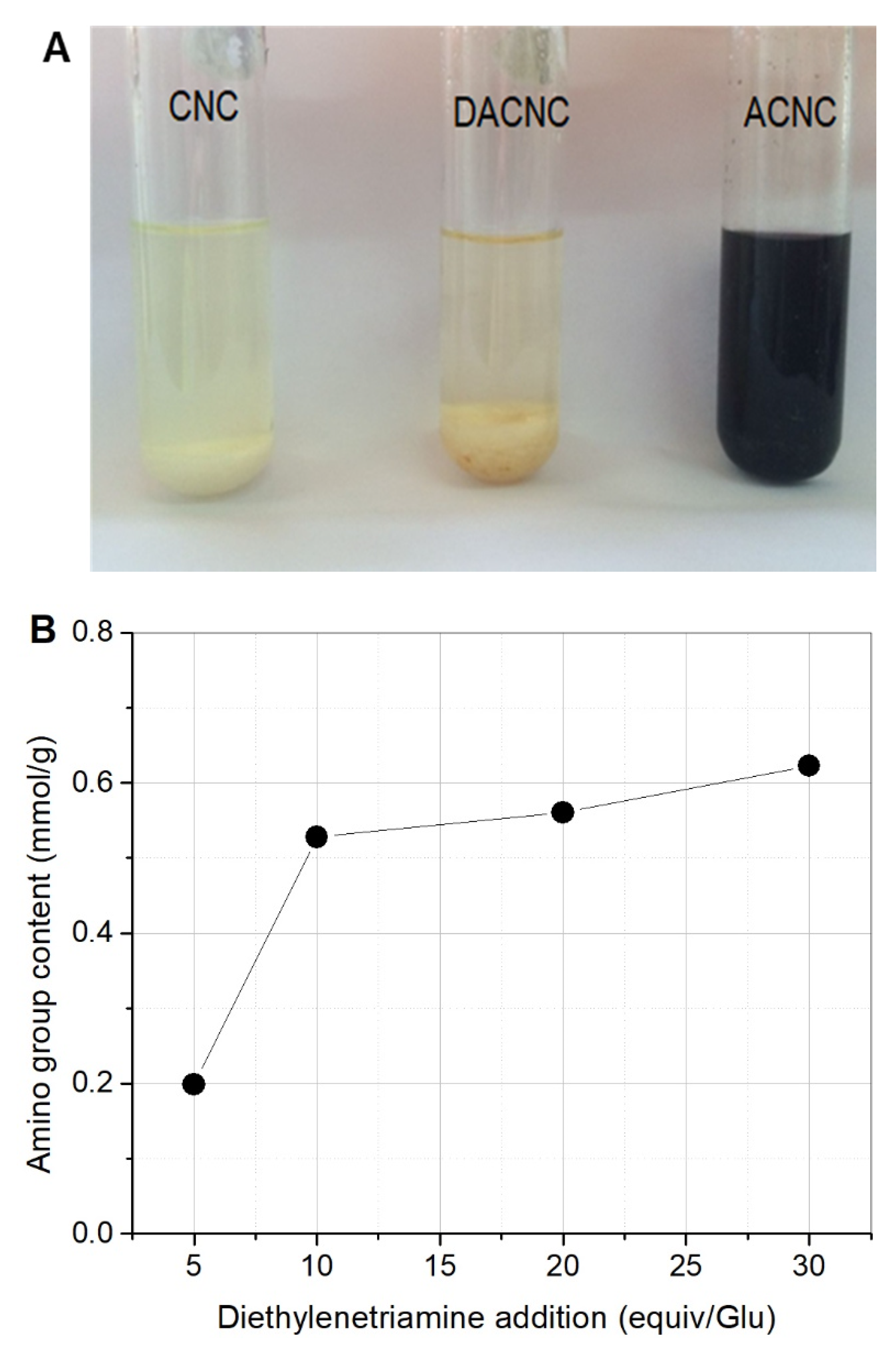
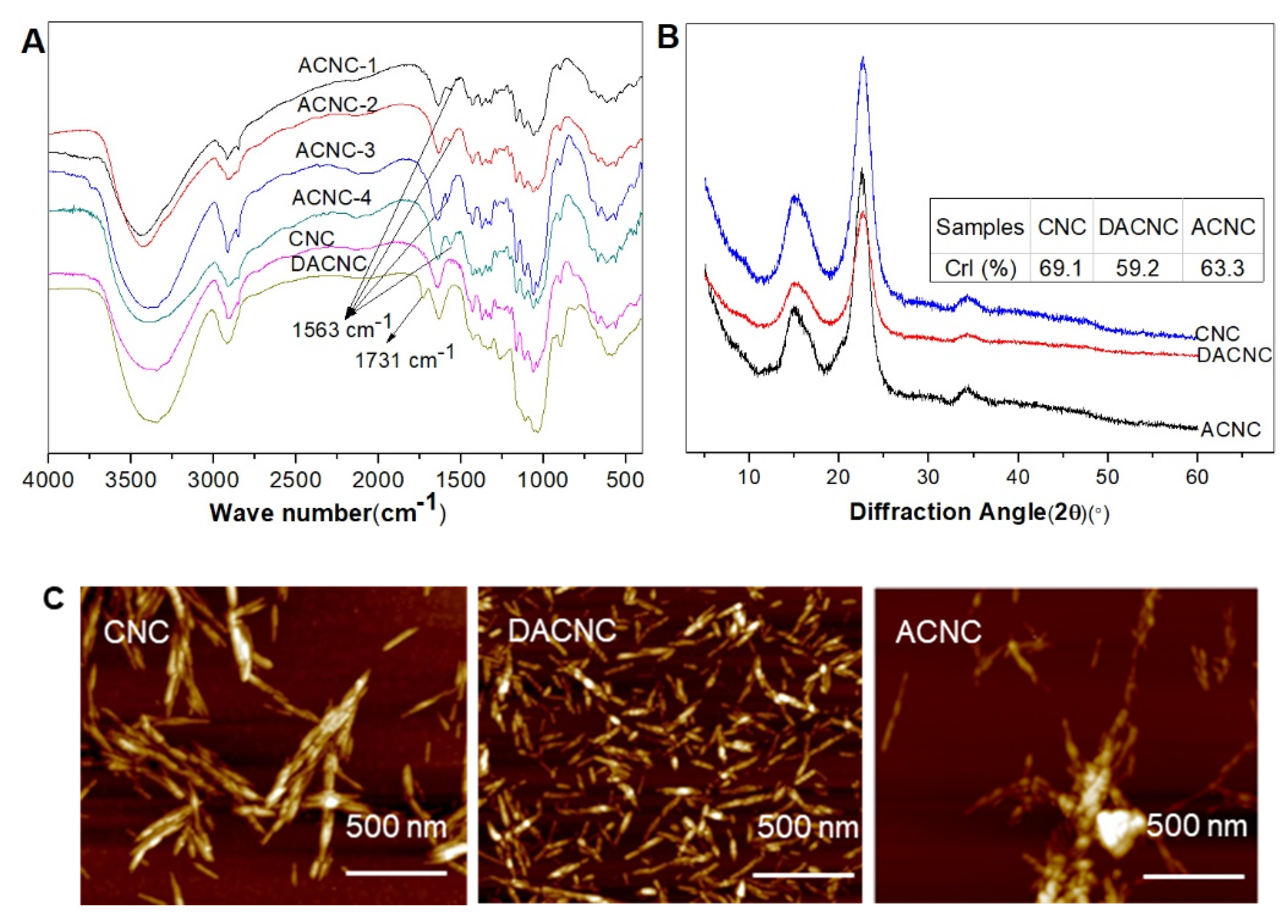
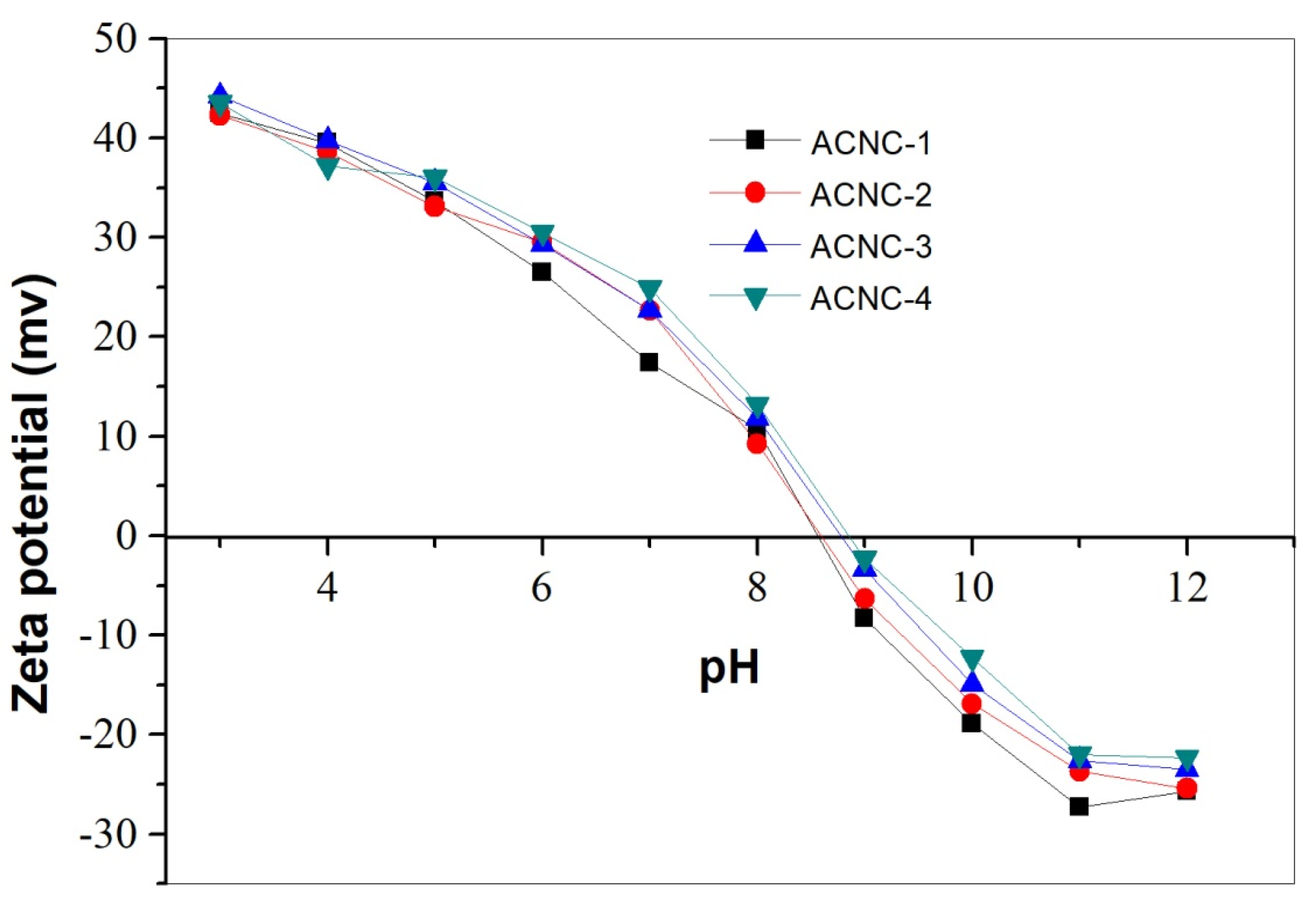
2.1. Preparation and Characterization of ACNC
2.2. Adsorption of Metal Ions
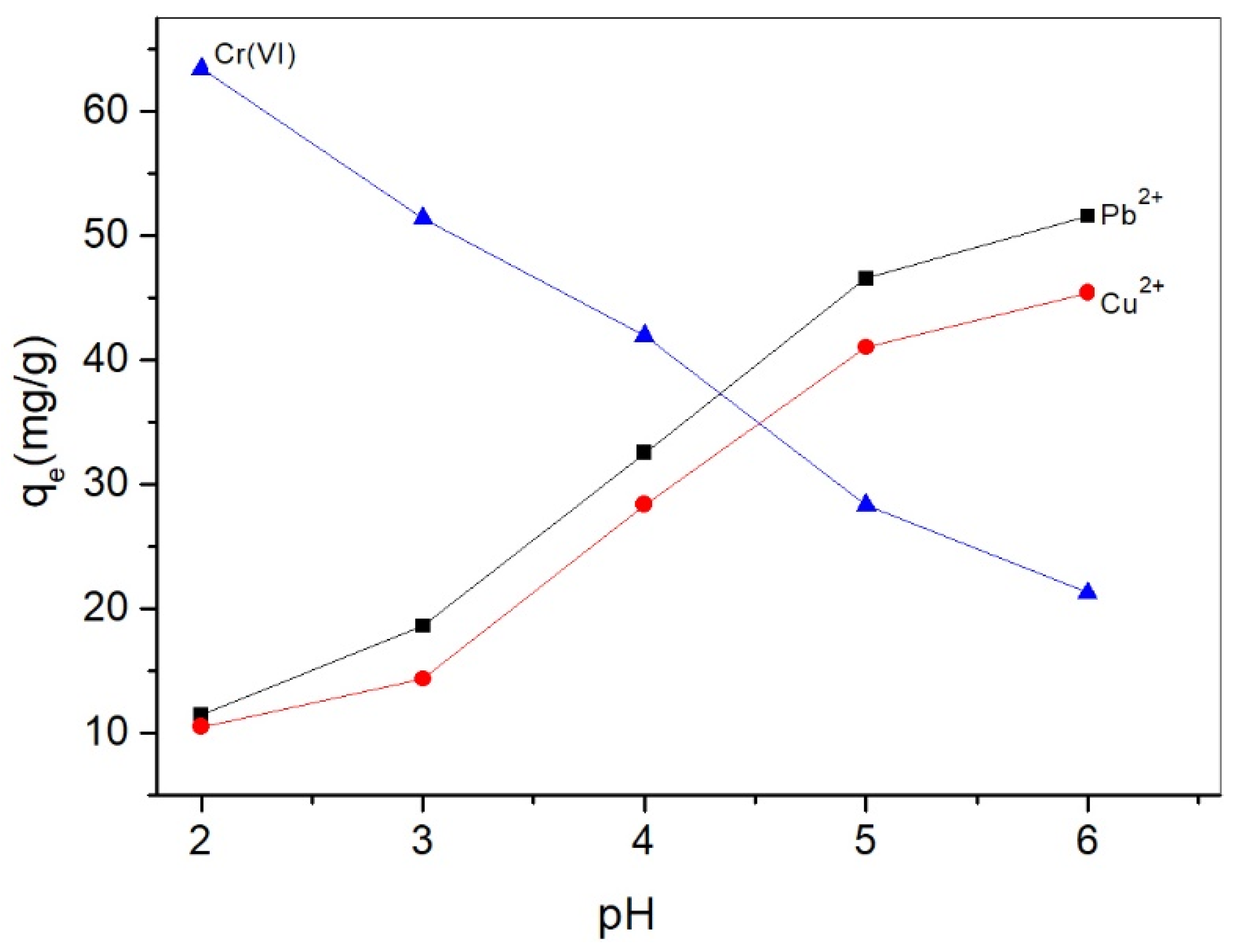
2.2.1. pH Effect on Adsorption
2.2.2. Adsorption Kinetics
2.2.3. Adsorption Isotherms
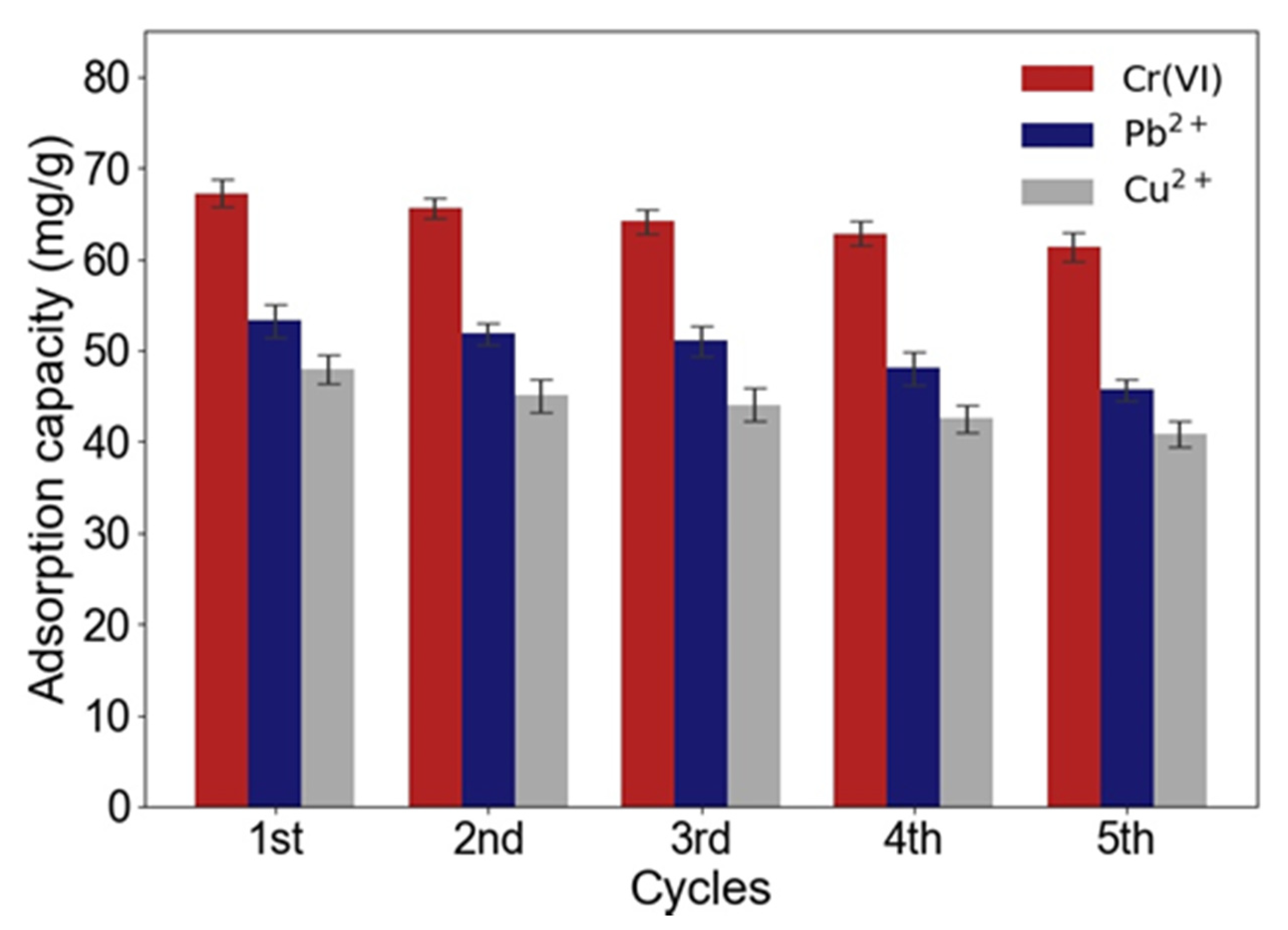
2.2.4. Reusability of ACNC
3. Materials and Methods
3.1. Materials
3.2. Preparation of DACNC
3.3. Amino Functionalization of DACNC with Diethylenetriamine (DETA)
3.4. Characterization
3.4.1. Elemental Analysis
3.4.2. Zeta Potential Measurement
3.4.3. FT-IR
3.4.4. X-ray Diffraction (XRD) Analysis
3.4.5. AFM Observation
3.5. Adsorption of Metal Ions
3.6. Regeneration Study
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mokhena, T.C.; John, M.J. Cellulose nanomaterials: New generation materials for solving global issues. Cellulose 2020, 27, 1149–1194. [Google Scholar] [CrossRef]
- Abou-Zeid, R.E.; Khiari, R.; El-Wakil, N.; Dufresne, A. Current state and new trends in the use of cellulose nanomaterials for wastewater treatment. Biomacromolecules 2019, 20, 573–597. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Z.X.; Sun, Y.; Wang, C.K.; Fan, J.L.; Kang, G.Y.; Zhang, R.; Dong, X.Y.; Li, Y.F. Nanocellulose-based magnetic hybrid aerogel for adsorption of heavy metal ions from water. J. Mater. Sci. 2019, 54, 6709–6718. [Google Scholar] [CrossRef]
- Wang, D. A critical review of cellulose-based nanomaterials for water purification in industrial processes. Cellulose 2019, 26, 687–701. [Google Scholar] [CrossRef]
- Qiao, A.H.; Cui, M.; Huang, R.l.; Ding, G.J.; Qi, W.; He, Z.M.; Klemes, J.J.; Su, R.X. Advances in nanocellulose-based materials as adsorbents of heavy metals and dyes. Carbohydr. Polym. 2021, 272, 118471. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Madhavan, A.; Pugazhendhi, A.; Sindhu, R.; Sirohif, R.; Awasthi, M.K.; Pandey, A.; Binod, P. Nanocellulose as green material for remediation of hazardous heavy metal contaminants. J. Hazard. Mater. 2022, 424, 127516. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Thielemans, W. Thermodynamics of adsorption on nanocellulose surfaces. Cellulose 2019, 26, 249–279. [Google Scholar] [CrossRef]
- Morantes, D.; Muñoz, E.; Kam, D.; Shoseyov, O. Highly charged cellulose nanocrystals applied as a water treatment flocculant. Nanomaterials 2019, 9, 272. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Sehaqui, H.; Tingaut, P.; Wichser, A.; Oksman, K.; Mathew, A.P. Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose 2014, 21, 449–461. [Google Scholar] [CrossRef]
- Kardam, A.; Raj, K.R.; Srivastava, S.; Srivastava, M.M. Nanocellulose fibers for biosorption of cadmium, nickel, and lead ions from aqueous solution. Clean Technol. Environ. Policy 2014, 16, 385–393. [Google Scholar] [CrossRef]
- Hokkanen, S.; Repo, E.; Westholm, L.J.; Lou, S.; Sainio, T.; Sillanpaa, M. Adsorption of Ni2+, Cd2+, PO43− and NO3− from aqueous solutions by nanostructured microfibrillated cellulose modified with carbonated hydroxyapatite. Chem. Eng. J. 2014, 252, 64–74. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K.; Bikiaris, D.N. N-(2-Carboxybenzyl) grafted chitosan as adsorptive agent for simultaneous removal of positively and negatively charged toxic metal ions. J. Hazard. Mater. 2013, 244–245, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, S.; Repo, E.; Liimatainen, H.; Liimatainen, H.; Niinimaa, J.; Sillanpää, M. Adsorption of Ni(II), Cu(II) and Cd(II) from aqueous solutions by amino modified nanostructured microfibrillated cellulose. Cellulose 2014, 21, 1471–1487. [Google Scholar] [CrossRef]
- Taleb, K.; Markovski, J.; Velickovic, Z.; Rusmirovic, J.; Rancic, M.; Pavlovic, V.; Marinkovic, A. Arsenic removal by magnetite-loaded amino modified nano/ microcellulose adsorbents: Effect of functionalization and media size. Arab. J. Chem. 2019, 12, 4675–4693. [Google Scholar] [CrossRef] [Green Version]
- Silva, N.H.C.S.; Figueir, P.; Fabre, E.; Pinto, R.J.B.; Pereira, M.E.; Silvestre, A.J.D.; Marrucho, I.M.; Vilela, C.; Freire, C.S.R. Dual nanofibrillar-based bio-sorbent films composed of nanocellulose and lysozyme nanofibrils for mercury removal from spring waters. Carbohyd. Polym. 2020, 238, 116210. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wan, L.; Xuan, J.; Wang, Y.W.; Xing, Z.Q.; Shan, W.J.; Lou, Z.N. Selective recovery of Ag(I) coordination anion from simulate nickel electrolyte using corn stalk based adsorbent modified by ammonia-thiosemicarbazide. J. Hazard. Mater. 2016, 301, 277–285. [Google Scholar] [CrossRef]
- Chai, F.; Wang, R.K.; Yan, L.L.; Li, G.H.; Cai, Y.Y.; Xi, C.Y. Facile fabrication of pH-sensitive nanoparticles based on nanocellulose for fast and efficient As(V) removal. Carbohyd. Polym. 2020, 245, 116511. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Mammadli, N.; Yildiz, U. Bioinspired modified nanocellulose adsorbent for enhanced boron recovery from aqueous media: Optimization, kinetics, thermodynamics and reusability study. J. Environ. Chem. Eng. 2019, 7, 103281. [Google Scholar] [CrossRef]
- Barazzouk, S.; Daneault, C. Amino acid and peptide immobilization on oxidized nanocellulose: Spectroscopic characterization. Nanomaterials 2012, 2, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.Q.; Sun, Q.C.; Xu, Q.H.; Xu, Y.J. Adsorptive removal of anionic dyes from aqueous solutions using microgel based on nanocellulose and polyvinylamine. Bioresour. Technol. 2015, 197, 348–355. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Edit. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Kim, U.J.; Kuga, S.; Wada, M.; Okano, T.; Kondo, T. Periodate oxidation of crystalline cellulose. Biomacromolecules 2000, 1, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.J.; Chavan, V.B. A study of crystallinity changes in oxidized celluloses. Polym. Degrad. Stabil. 1995, 49, 245–250. [Google Scholar] [CrossRef]
- Sun, B.; Hou, Q.X.; Liu, Z.H.; Ni, Y.H. Sodium periodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose 2015, 22, 1135–1146. [Google Scholar] [CrossRef]
- Goyal, P.; Sharma, P.; Srivastava, S.; Srivastava, M.M. Saraca indica leaf powder for decontamination of lead: Removal, recovery, adsorbent characterization and equilibrium modeling. Int. J. Environ. Sci. Technol. 2008, 5, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Takeshita, K.; Tsutsum, T. Adsorption mechanism of hexavalent chromium by redox with condensed-tannin gel. Water Res. 2001, 35, 496–500. [Google Scholar] [CrossRef]
- Sun, X.; Huang, X.; Liao, X.P.; Shi, B. Adsorptive removal of Cu(II) from aqueous solutions using collagen-tannin resin. J. Hazard. Mater. 2011, 186, 1058–1063. [Google Scholar] [CrossRef]
- Hubbe, M.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. Bioresources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- Nair, V.; Panigrahy, A.; Vinu, R. Development of novel chitosan-lignin composites for adsorption of dyes and metal ions from wastewater. Chem. Eng. J. 2014, 254, 491–502. [Google Scholar] [CrossRef]
- Silva, L.S.; Lima, L.C.B.; Silva, F.C.; Matos, J.M.E.; Santos, M.R.M.C.; Santos, L.S.S., Jr.; Sousa, K.S.; Filho, E.C.S. Dye anionic sorption in aqueous solution onto a cellulose surface chemically modified with aminoethanethiol. Chem. Eng. J. 2013, 218, 89–98. [Google Scholar] [CrossRef]
- Xu, Q.H.; Wang, Y.L.; Jin, L.Q.; Wang, Y.; Qin, M.H. Adsorption of Cu (II), Pb (II) and Cr (VI) from aqueous solutions using black wattle tannin-immobilized nanocellulose. J. Hazard. Mater. 2017, 339, 91–99. [Google Scholar] [CrossRef]
- Singh, K.; Arora, J.K.; Sinha, T.J.M.; Srivastava, S. Functionalization of nanocrystalline cellulose for decontamination of Cr(III) and Cr(VI) from aqueous system: Computational modeling approach. Clean Technol. Environ. Policy 2014, 16, 1179–1191. [Google Scholar] [CrossRef]
- Yang, R.; Aubrecht, K.B.; Ma, H.; Wang, R.; Grubbs, R.B.; Hsiao, B.S.; Chu, B. Thiol-modified cellulose nanofibrous composite membranes for chromium (VI) and lead (II) adsorption. Polymer 2014, 55, 1167–1176. [Google Scholar] [CrossRef]
- Liu, P.; Garrido, B.; Oksman, K.; Mathew, A.P. Adsorption isotherms and mechanisms of Cu(ii) sorption onto TEMPO-mediated oxidized cellulose nanofibers. RSC Adv. 2016, 6, 107759–107767. [Google Scholar] [CrossRef]
- Zhang, N.; Zang, G.L.; Shi, C.; Yu, H.Q.; Sheng, G.P. A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. J. Hazard. Mater. 2016, 316, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Maaloul, N.; Oulego, P.; Rendueles, M.; Ghorbal, A.; Díaz, M. Novel biosorbents from almond shells: Characterization and adsorption properties modeling for Cu(II) ions from aqueous solutions. J. Environ. Chem. Eng. 2017, 5, 2944–2954. [Google Scholar] [CrossRef]
- Shen, W.; Chen, S.; Shi, S.; Li, X.; Zhang, X.; Hu, W.; Wang, H. Adsorption of Cu(II) and Pb(II) onto diethylenetriamine-bacterial cellulose. Carbohydr. Polym. 2009, 75, 110–114. [Google Scholar] [CrossRef]
- Filpponen, I.; Argyropoulos, D.S. Regular linking of cellulose nanocrystals via click chemistry: Synthesis and formation of cellulose nanoplatelet gels. Biomacromolecules 2010, 11, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]


| Samples | C | H | O | N | S |
|---|---|---|---|---|---|
| CNC | 39.96 | 10.02 | 49.82 | 0 | 0.20 |
| DACNC | 39.75 | 9.27 | 50.73 | 0 | 0.25 |
| ACNC-1 | 41.64 | 9.30 | 47.23 | 1.59 | 0.24 |
| ACNC-2 | 41.24 | 9.66 | 47.10 | 1.71 | 0.29 |
| ACNC-3 | 41.38 | 10.07 | 46.35 | 1.92 | 0.28 |
| ACNC-4 | 41.64 | 10.40 | 45.63 | 2.12 | 0.21 |
| Models | Parameters | Cr(VI) | Pb2+ | Cu2+ |
|---|---|---|---|---|
| Pseudo first-order | R2 | 0.660 | 0.614 | 0.598 |
| k1 (h−1) | 0.466 | 0.388 | 0.415 | |
| qe cal (mg/g) | 5.981 | 7.863 | 7.155 | |
| Pseudo second-order | R2 | 0.999 | 0.999 | 0.999 |
| k2 (g/mg·h) | 0.122 | 0.162 | 0.112 | |
| qe cal (mg/g) | 64.103 | 51.813 | 45.660 | |
| qe exp (mg/g) | 63.608 ± 1.645 | 51.571 ± 2.271 | 45.364 ± 2.072 | |
| Models | Parameters | Cr(VI) | Pb2+ | Cu2+ |
|---|---|---|---|---|
| Langmuir | R2 | 0.971 | 0.905 | 0.928 |
| KL (L/g) | 0.0434 | 0.0331 | 0.0299 | |
| qmax (mg/g) | 77.560 | 65.215 | 58.501 | |
| Freundlich | R2 | 0.809 | 0.709 | 0.759 |
| Kf (mg/g) | 15.243 | 10.711 | 8.870 | |
| 1/n | 0.296 | 0.321 | 0.333 | |
| Sips | R2 | 0.987 | 0.995 | 0.980 |
| qs (mg/g) | 70.503 | 54.115 | 49.600 | |
| Ks (mL/mg) | 0.0164 | 0.0127 | 0.0271 | |
| m | 1.378 | 2.119 | 1.799 | |
| qe exp (mg/g) | 67.216 ± 1.688 | 53.258 ± 1.787 | 47.913 ± 1.267 |
| Adsorbents | Metal Ions | qe (mg/g) | Reference |
|---|---|---|---|
| Amination CNCs with acrylamide and ethylenediamine | Cr(VI) | 2.77 | [33] |
| Polycysteine-grafted CNFs | Cr(VI) | 87.5 | [34] |
| carboxylic nanocellulose | Cu(II) | 75.0 | [35] |
| Pristine CNCs | Pb(II) | 9.42 | [9] |
| Cu(II) | 19.7 | [8] | |
| TEMPO-oxidized CNFs modified with PEI | Cu(II) | 52.3 | [36] |
| TEMPO-modified CNF | Cu(II) | 23.8 | [37] |
| Diethylenetriamine- BC (EABC) | Pb(II) | 12 | [38] |
| Cu(II) | 63.1 | ||
| ACNC | Cu(II) | 49.6 | This work |
| Pb(II) | 54.1 | ||
| Cr(VI) | 70.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Huang, X.; Guo, L.; Wang, Y.; Jin, L. Enhancing Removal of Cr(VI), Pb2+, and Cu2+ from Aqueous Solutions Using Amino-Functionalized Cellulose Nanocrystal. Molecules 2021, 26, 7315. https://doi.org/10.3390/molecules26237315
Xu Q, Huang X, Guo L, Wang Y, Jin L. Enhancing Removal of Cr(VI), Pb2+, and Cu2+ from Aqueous Solutions Using Amino-Functionalized Cellulose Nanocrystal. Molecules. 2021; 26(23):7315. https://doi.org/10.3390/molecules26237315
Chicago/Turabian StyleXu, Qinghua, Xiaodi Huang, Lukuan Guo, Yu Wang, and Liqiang Jin. 2021. "Enhancing Removal of Cr(VI), Pb2+, and Cu2+ from Aqueous Solutions Using Amino-Functionalized Cellulose Nanocrystal" Molecules 26, no. 23: 7315. https://doi.org/10.3390/molecules26237315





