Amine- and Amino Acid-Based Compounds as Carbonic Anhydrase Activators
Abstract
:1. Introduction
1.1. Amino Acids and Biogenic Amines
1.2. CA Families
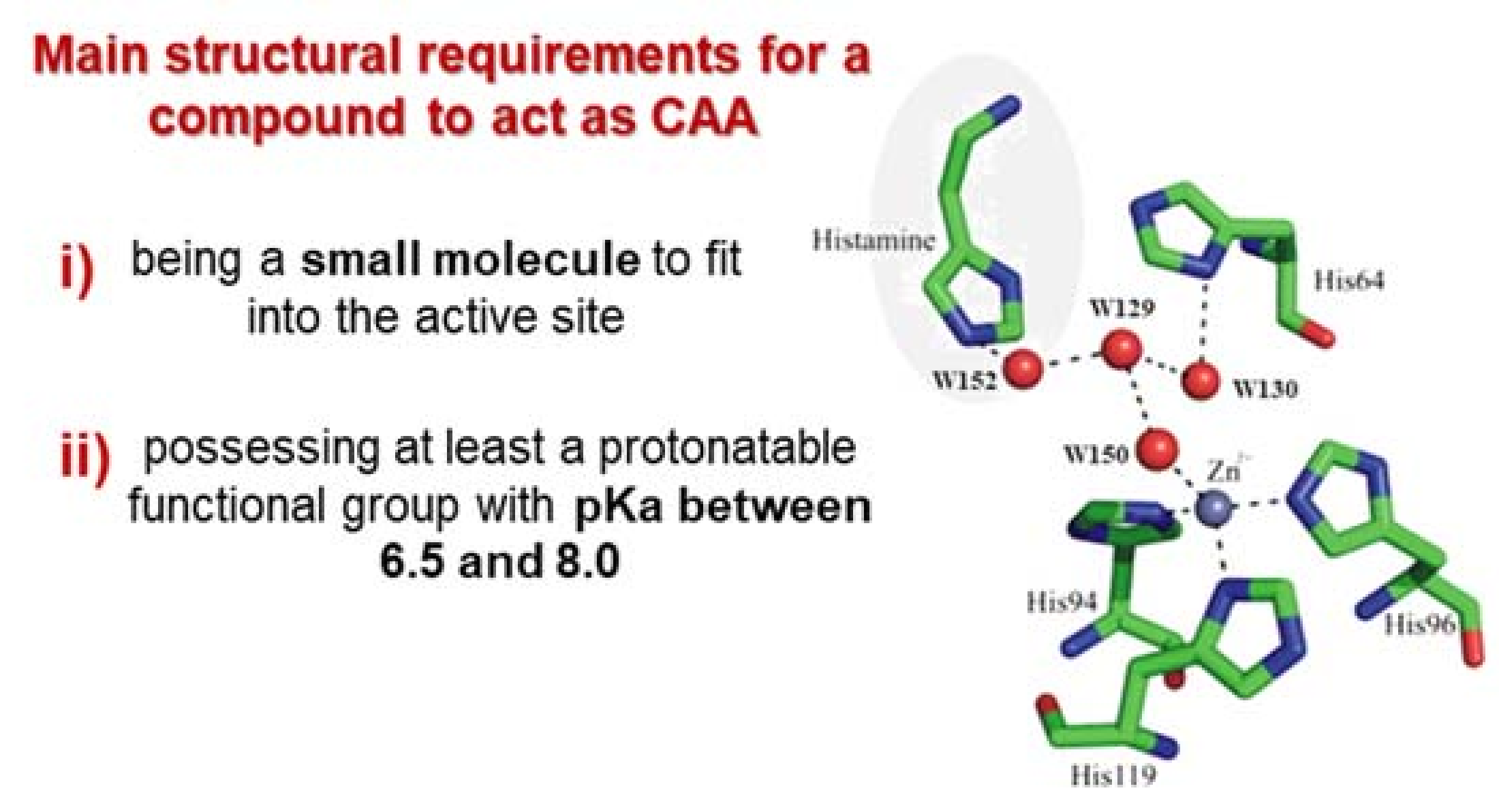
1.3. Carbonic Anhydrase Activators (CAAs)
[EZn2+ − HO− − AH+] ↔ EZn2+ − HO− + AH+
Enzyme-activator complexes
2. Activation Assay
3. Activation Studies on Human CAs
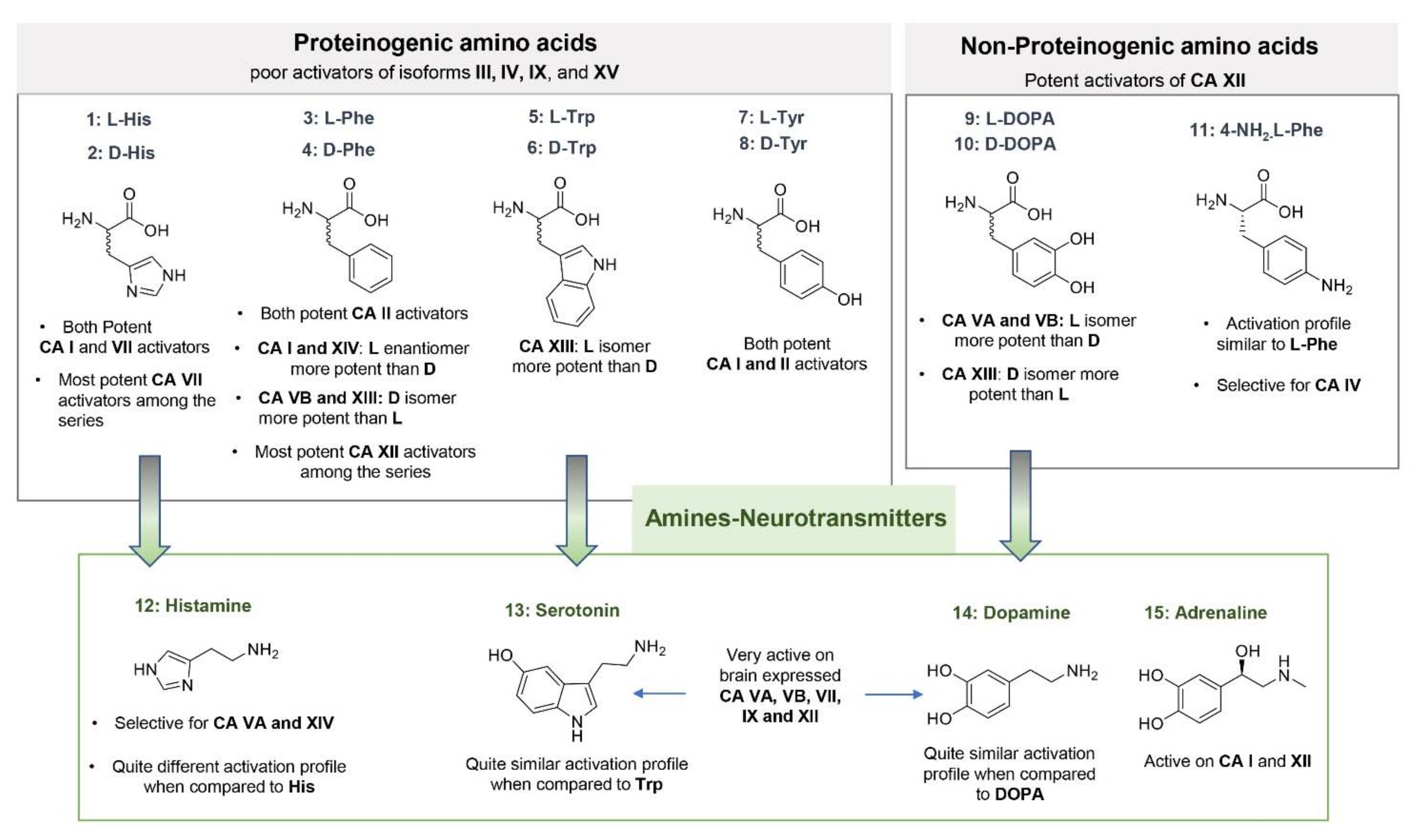
3.1. Natural and Synthetic Amino Acids and Amines
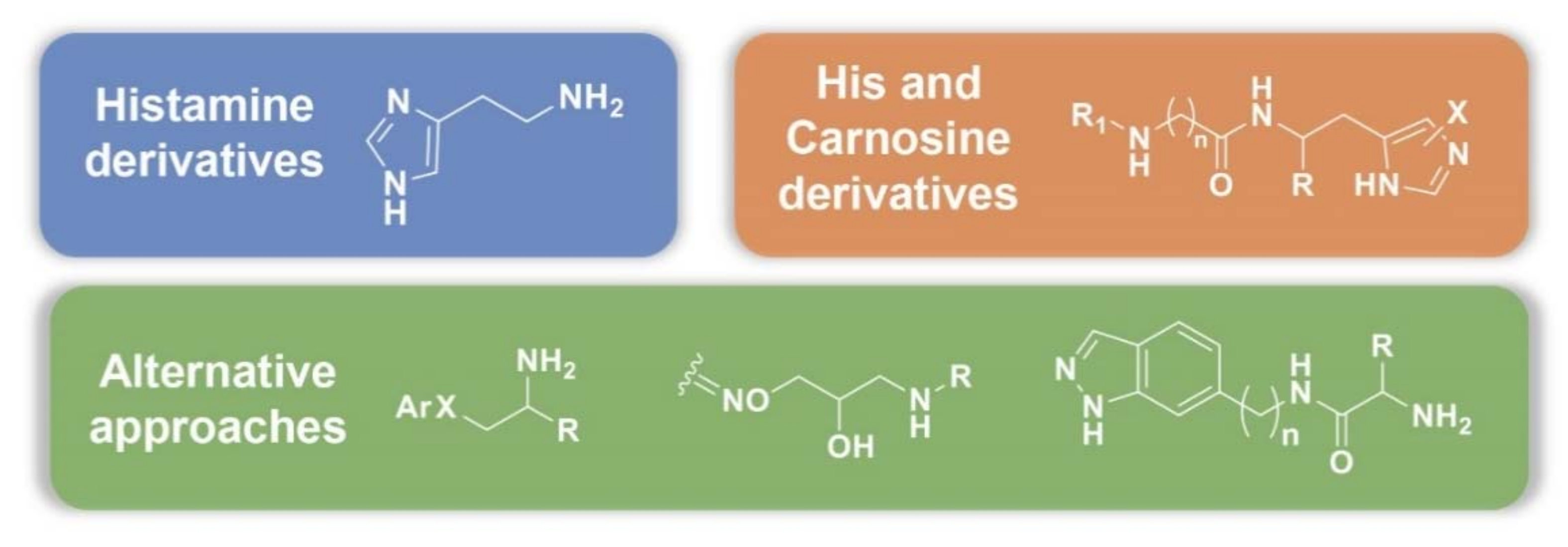
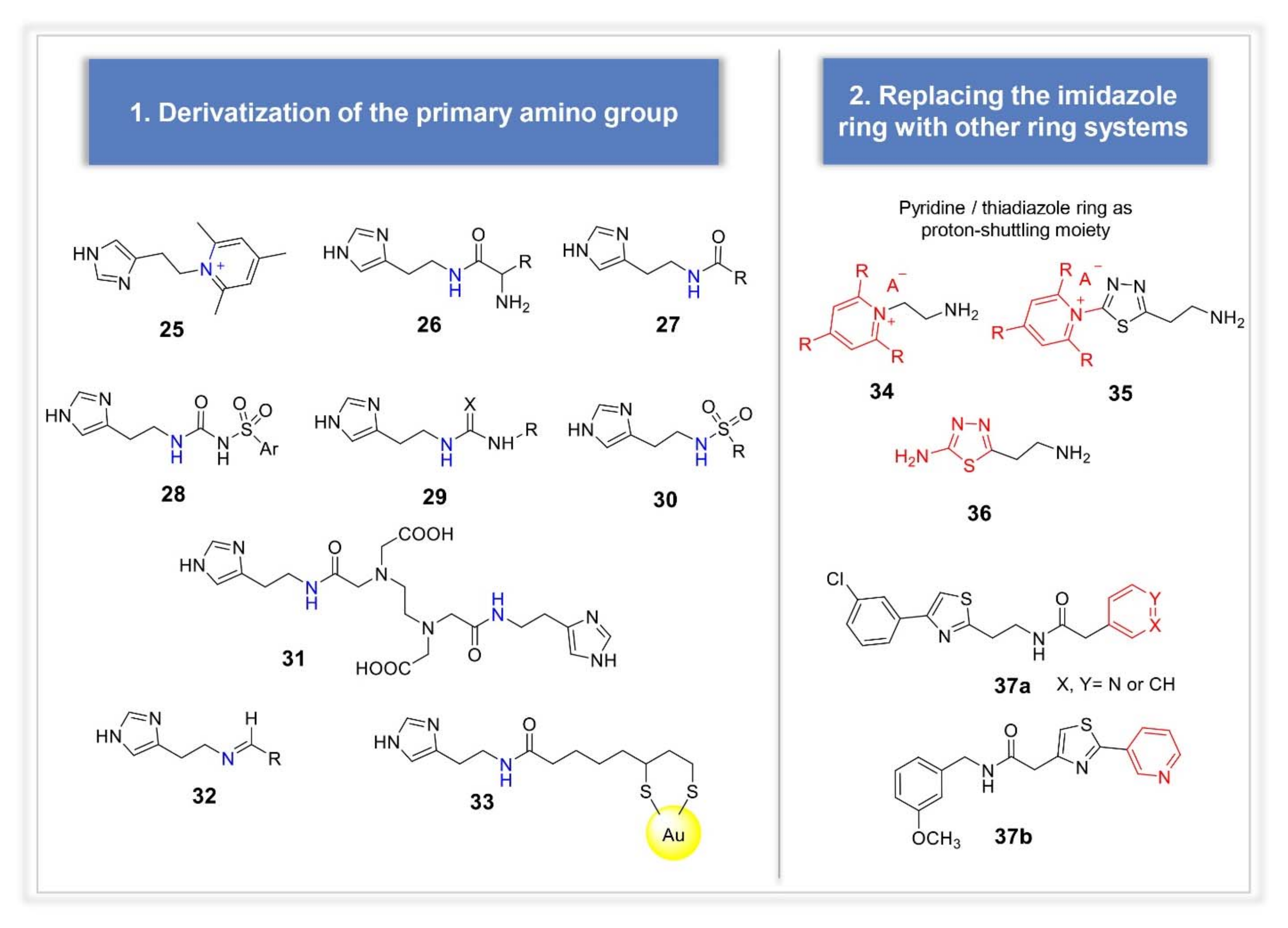
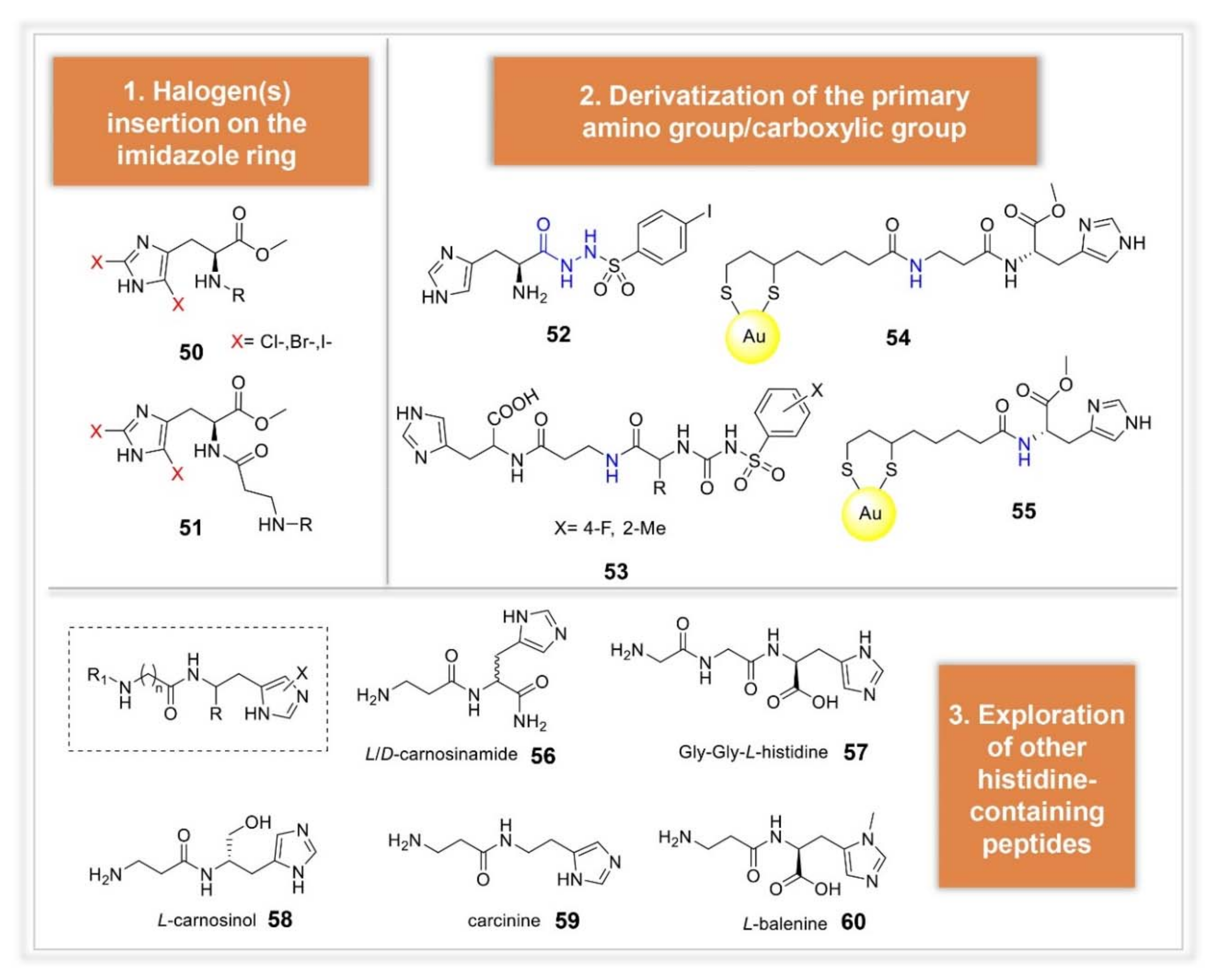
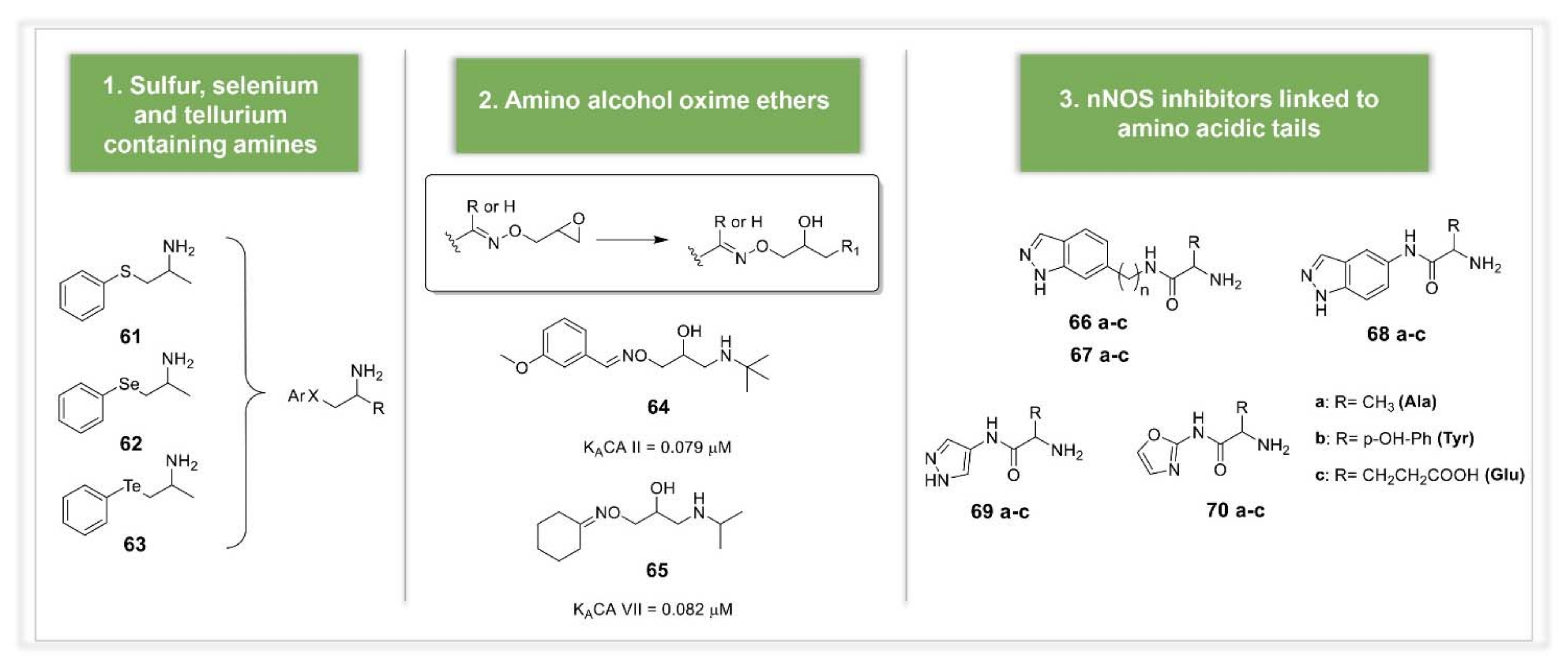
3.2. Synthetic Manipulations on Amines and Amino Acids
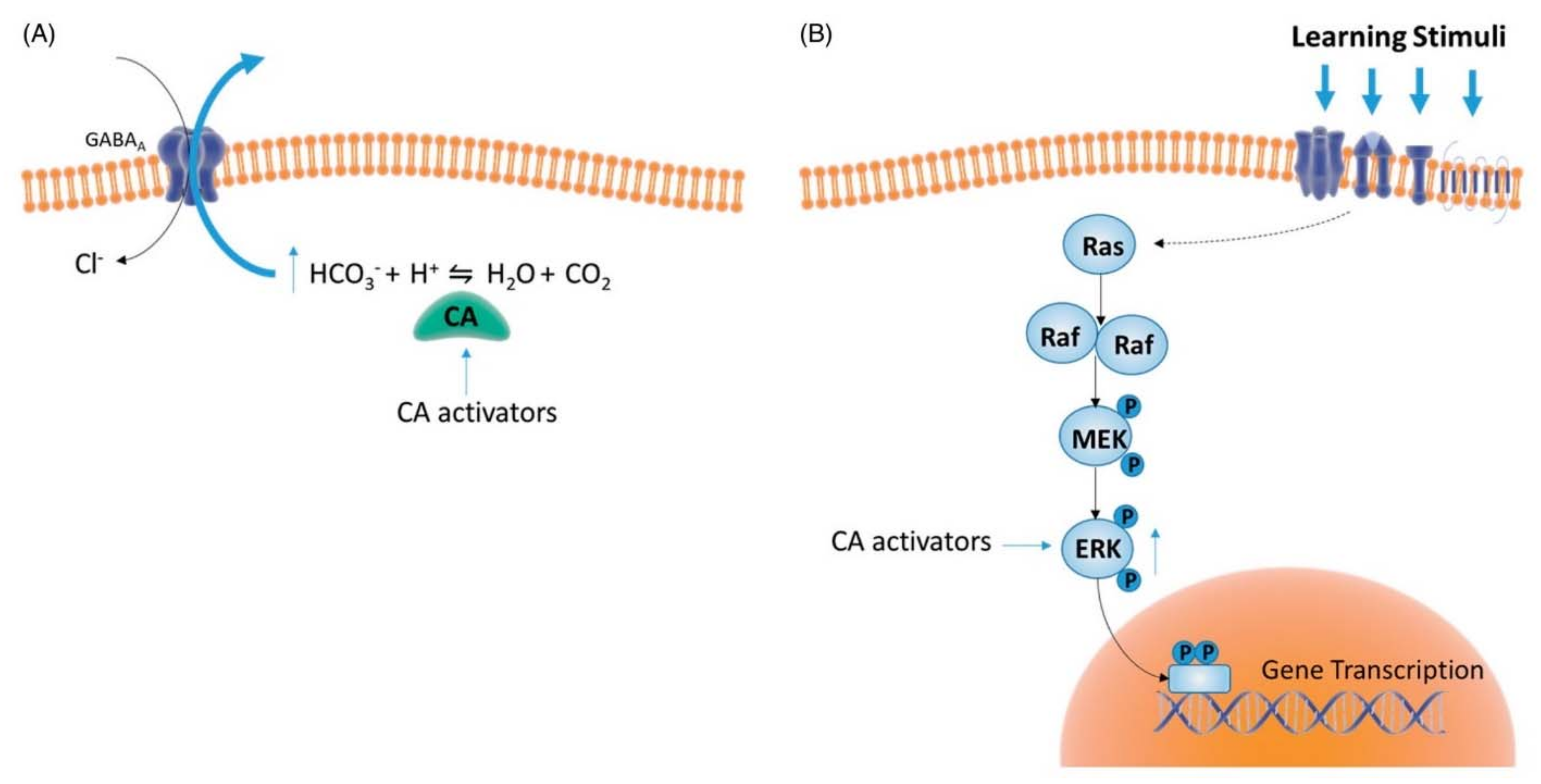
4. Therapeutic Applications for Human Health
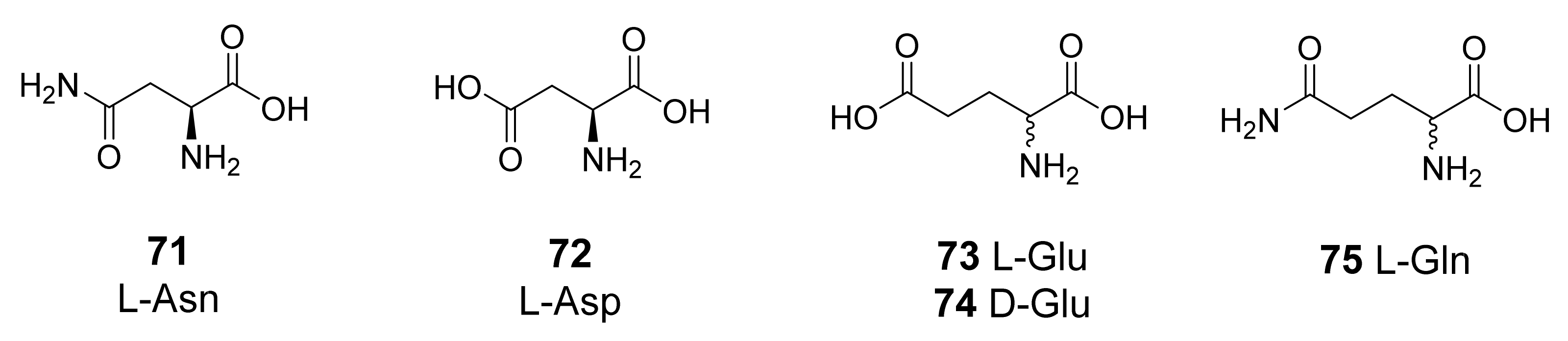
5. Natural and Synthetic Amino Acids and Amines Activating Non-Human CAs
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blaskovich, M.A.T. Unusual Amino Acids in Medicinal Chemistry. J. Med. Chem. 2016, 59, 10807–10836. [Google Scholar] [CrossRef] [PubMed]
- Akgül, Ö.; Angeli, A.; Vullo, D.; Carta, F.; Supuran, C.T. Unconventional Amino Acids in Medicinal Chemistry: First Report on Taurine Merged within Carbonic Anhydrase Inhibitors. Bioorg. Chem. 2020, 103, 104236. [Google Scholar] [CrossRef] [PubMed]
- Akocak, S.; Supuran, C.T. Activation of α-, β-, γ- δ-, ζ- and η- Class of Carbonic Anhydrases with Amines and Amino Acids: A Review. J. Enzym. Inhib. Med. Chem. 2019, 34, 1652–1659. [Google Scholar] [CrossRef] [Green Version]
- Bunch, L.; Krogsgaard-Larsen, P. Medicinal Chemistry of α-Amino Acids. In Amino Acids, Peptides and Proteins in Organic Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; Volume 4, pp. 149–174. [Google Scholar]
- Nuñez, M.; del Olmo, A.; Calzada, J. Biogenic Amines. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2015; pp. 416–423. ISBN 9780123849533. [Google Scholar]
- Supuran, C.T. Carbonic Anhydrases: Novel Therapeutic Applications for Inhibitors and Activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Supuran, C.T.; Capasso, C. An Overview on the Recently Discovered Iota-Carbonic Anhydrases. J. Enzym. Inhib. Med. Chem. 2021, 36, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Supuran, C.T.; De Simone, G. Multiple Binding Modes of Inhibitors to Carbonic Anhydrases: How to Design Specific Drugs Targeting 15 Different Isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef] [Green Version]
- Flaherty, D.P.; Seleem, M.N.; Supuran, C.T. Bacterial Carbonic Anhydrases: Underexploited Antibacterial Therapeutic Targets. Future Med. Chem. 2021, 13, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.D.; Pinard, M.; McKenna, R.; Silverman, D. Catalytic Mechanism of α-Class Carbonic Anhydrases: CO2 Hydration and Proton Transfer. Subcell. Biochem. 2014, 75, 31–52. [Google Scholar]
- Murray, A.B.; McKenna, R. β-Carbonic anhydrases. In Carbonic Anhydrases: Biochemistry and Pharmacology of an Evergreen Pharmaceutical Target; Elsevier: Amsterdam, The Netherlands, 2019; pp. 55–77. ISBN 9780128164761. [Google Scholar]
- Tripp, B.C.; Ferry, J.G. A Structure−Function Study of a Proton Transport Pathway in the γ-Class Carbonic Anhydrase from Methanosarcina Thermophila. Biochemistry 2000, 39, 9232–9240. [Google Scholar] [CrossRef] [PubMed]
- Kisker, C.; Schindelin, H.; Alber, B.E.; Ferry, J.G.; Rees, D.C. A Left-Hand Beta-Helix Revealed by the Crystal Structure of a Carbonic Anhydrase from the Archaeon Methanosarcina Thermophila. EMBO J. 1996, 15, 2323. [Google Scholar] [CrossRef] [PubMed]
- Alber, B.E.; Colangelo, C.M.; Dong, J.; Stålhandske, C.M.V.; Baird, T.T.; Tu, C.; Fierke, C.A.; Silverman, D.N.; Robert, A.; Scott, A.; et al. Kinetic and Spectroscopic Characterization of the Gamma-Carbonic Anhydrase from the Methanoarchaeon Methanosarcina Thermophila. Biochemistry 1999, 38, 13119–13128. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C. δ-Carbonic anhydrases. In Carbonic Anhydrases: Biochemistry and Pharmacology of an Evergreen Pharmaceutical Target; Academic Press: Cambridge, MA, USA, 2019; pp. 107–129. [Google Scholar]
- Lane, T.W.; Saito, M.A.; George, G.N.; Pickering, I.J.; Prince, R.C.; Morel, F.M. Biochemistry: A Cadmium Enzyme from a Marine Diatom. Nature 2005, 435, 42. [Google Scholar] [CrossRef]
- McGinn, P.J.; Morel, F.M. Expression and Regulation of Carbonic Anhydrases in the Marine Diatom Thalassiosira pseudonana and in Natural Phytoplankton Assemblages from Great Bay, New Jersey. Physiol. Plant. 2008, 133, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Feng, L.; Jeffrey, P.D.; Shi, Y.; Morel, F.M. Structure and Metal Exchange in the Cadmium Carbonic Anhydrase of Marine Diatoms. Nature 2008, 452, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Buonanno, M.; Donald, W.A.; Monti, S.M.; Supuran, C.T. The Zinc—But Not Cadmium-Containing ζ-Carbonic from the Diatom Thalassiosira Weissflogii Is Potently Activated by Amines and Amino Acids. Bioorg. Chem. 2018, 80, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Krungkrai, J.; Prapunwatana, P.; Wichitkul, C.; Reungprapavut, S.; Krungkrai, S.R.; Horii, T. Molecular Biology and Biochemistry of Malarial Parasite Pyrimidine Biosynthetic Pathway—PubMed. Southeast. Asian J. Trop. Med. Public Health 2003, 34, 32–43. [Google Scholar] [PubMed]
- Krungkrai, S.R.; Suraveratum, N.; Rochanakij, S.; Krungkrai, J. Characterisation of Carbonic Anhydrase in Plasmodium Falciparum. Int. J. Parasitol. 2001, 31, 661–668. [Google Scholar] [CrossRef]
- Del Prete, S.; Vullo, D.; De Luca, V.; Carginale, V.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Cloning, Expression, Purification and Sulfonamide Inhibition Profile of the Complete Domain of the η-Carbonic Anhydrase from Plasmodium Falciparum. Bioorg. Med. Chem. Lett. 2016, 26, 4184–4190. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; De Luca, V.; De Simone, G.; Supuran, C.T.; Capasso, C. Cloning, Expression and Purification of the Complete Domain of the η-Carbonic Anhydrase from Plasmodium Falciparum. J. Enzyme Inhib. Med. Chem. 2016, 31, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Hou, W.-C.; Chen, H.-J.; Yaw-Huei, L. Dioscorins from Different Dioscorea Species All Exhibit Both Carbonic Anhydrase and Trypsin Inhibitor Activities. Bot. Bull. Acad. Sin. 2000, 41, 191–196. [Google Scholar]
- Kikutani, S.; Nakajima, K.; Nagasato, C.; Tsuji, Y.; Miyatake, A.; Matsuda, Y. Thylakoid Luminal θ-Carbonic Anhydrase Critical for Growth and Photosynthesis in the Marine Diatom Phaeodactylum Tricornutum. Proc. Natl. Acad. Sci. USA 2016, 113, 9828–9833. [Google Scholar] [CrossRef] [Green Version]
- Jensen, E.L.; Clement, R.; Kosta, A.; Maberly, S.C.; Gontero, B. A New Widespread Subclass of Carbonic Anhydrase in Marine Phytoplankton. ISME J. 2019, 13, 2094–2106. [Google Scholar] [CrossRef] [Green Version]
- Del Prete, S.; Nocentini, A.; Supuran, C.T.; Capasso, C. Bacterial ι-Carbonic Anhydrase: A New Active Class of Carbonic Anhydrase Identified in the Genome of the Gram-Negative Bacterium Burkholderia Territorii. J. Enzyme Inhib. Med. Chem. 2020, 35, 1060–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, E.L.; Receveur-Brechot, V.; Hachemane, M.; Wils, L.; Barbier, P.; Parsiegla, G.; Gontero, B.; Launay, H.; Magyar, C. Structural Contour Map of the Iota Carbonic Anhydrase from the Diatom Thalassiosira Pseudonana Using a Multiprong Approach. Int. J. Mol. Sci. 2021, 22, 8723. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y.; Senda, M.; Fukuda, K.; Yang Yu, H.; Ishida, M.; Taira, M.; Kinbara, K.; Senda, T. Characterization of a Novel Type of Carbonic Anhydrase That Acts without Metal Cofactors. BMC Biol. 2021, 19, 105. [Google Scholar] [CrossRef] [PubMed]
- Leiner, M. Das Ferment Kohlensäureanhydrase Im Tierkörper. Naturwissenschaften 1940, 28, 316–317. [Google Scholar] [CrossRef]
- Leiner, M.; Leiner, G. Die Aktivatoren Der Kohlensäureanhydratase. Naturwissenschaften 1941, 29, 195–197. [Google Scholar] [CrossRef]
- Kiese, M. Die Aktivierung Der Kohlensäureanhydrase. Naturwissenschaften 1941, 29, 116–117. [Google Scholar] [CrossRef]
- Edna Main, B.R.; Locke, A. Carbonic Anhydrase, I. Factors Affecting Activity. J. Biol. Chem. 1941, 140, 909–918. [Google Scholar]
- Supuran, C.T. Carbonic Anhydrase Activators. Part 4. A General Mechanism of Action for Activators of Isozyme-I, Isozyme-II and Isozyme-III. Rev. Roum. Chim. 1992, 37, 411–421. [Google Scholar]
- Supuran, C.T.; Balaban, A.T. Carbonic Anhydrase Activators. Part 8. PKa–Activation Relationship in a Series of Amino Acid Derivatives Activators of Isozyme II. Rev. Roum. Chim. 1994, 39, 107–113. [Google Scholar]
- Tu, C.; Silverman, D.N.; Forsman, C.; Jonsson, B.H.; Lindskog, S. Role of Histidine 64 in the Catalytic Mechanism of Human Carbonic Anhydrase II Studied with a Site-Specific Mutant. Biochemistry 1989, 28, 7913–7918. [Google Scholar] [CrossRef]
- Briganti, F.; Mangani, S.; Orioli, P.; Scozzafava, A.; Vernaglione, G.; Supuran, C.T. Carbonic Anhydrase Activators: X-Ray Crystallographic and Spectroscopic Investigations for the Interaction of Isozymes I and II with Histamine. Biochemistry 1997, 36, 10384–10392. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic Anhydrase Activators. Future Med. Chem. 2018, 10, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Temperini, C.; Scozzafava, A.; Vullo, D.; Supuran, C.T. Carbonic Anhydrase Activators. Activation of Isozymes I, II, IV, VA, VII, and XIV with L- and D-Histidine and Crystallographic Analysis of Their Adducts with Isoform II: Engineering Proton-Transfer Processes within the Active Site of an Enzyme. Chem. A Eur. J. 2006, 12, 7057–7066. [Google Scholar] [CrossRef] [PubMed]
- Temperini, C.; Scozzafava, A.; Vullo, D.; Supuran, C.T. Carbonic Anhydrase Activators, Activation of Isoforms I, II, IV, VA, VII, and XIV with L- and D-Phenylalanine and Crystallographic Analysis of Their Adducts with Isozyme II: Stereospecific Recognition within the Active Site of an Enzyme and Its Consequenc. J. Med. Chem. 2006, 49, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Temperini, C.; Innocenti, A.; Scozzafava, A.; Mastrolorenzo, A.; Supuran, C.T. Carbonic Anhydrase Activators: L-Adrenaline Plugs the Active Site Entrance of Isozyme II, Activating Better Isoforms I, IV, VA, VII, and XIV. Bioorg. Med. Chem. Lett. 2007, 17, 628–635. [Google Scholar] [CrossRef]
- Temperini, C.; Innocenti, A.; Scozzafava, A.; Supuran, C.T. Carbonic Anhydrase Activators: Kinetic and X-Ray Crystallographic Study for the Interaction of d- and l-Tryptophan with the Mammalian Isoforms I-XIV. Bioorg. Med. Chem. 2008, 16, 8373–8378. [Google Scholar] [CrossRef] [PubMed]
- Petreni, A.; Osman, S.M.; Alasmary, F.A.; Almutairi, T.M.; Nocentini, A.; Supuran, C.T. Binding Site Comparison for Coumarin Inhibitors and Amine/Amino Acid Activators of Human Carbonic Anhydrases. Eur. J. Med. Chem. 2021, 226, 113875. [Google Scholar] [CrossRef]
- Supuran, C.T. Coumarin Carbonic Anhydrase Inhibitors from Natural Sources. J. Enzym. Inhib. Med. Chem. 2020, 35, 1462–1470. [Google Scholar] [CrossRef]
- Maresca, A.; Temperini, C.; Pochet, L.; Masereel, B.; Scozzafava, A.; Supuran, C.T. Deciphering the Mechanism of Carbonic Anhydrase Inhibition with Coumarins and Thiocoumarins. J. Med. Chem. 2010, 53, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Angeli, A.; Carta, F.; Nocentini, A.; Winum, J.Y.; Zalubovskis, R.; Onnis, V.; Eldehna, W.M.; Capasso, C.; Carradori, S.; Donald, W.A.; et al. Response to Perspectives on the Classical Enzyme Carbonic Anhydrase and the Search for Inhibitors. Biophys. J. 2021, 120, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, R. The Carbon Dioxide Hydration Activity of Carbonic Anhydrase. I. Stop-Flow Kinetic Studies on the Native Human Isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [CrossRef]
- Angeli, A.; Vaiano, F.; Mari, F.; Bertol, E.; Supuran, C.T. Psychoactive Substances Belonging to the Amphetamine Class Potently Activate Brain Carbonic Anhydrase Isoforms VA, VB, VII, and XII. J. Enzym. Inhib. Med. Chem. 2017, 32, 1253–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scozzafava, A.; Iorga, B.; Supuran, C.T. Carbonic Anhydrase Activators: Synthesis of High Affinity Isozymes I, II and IV Activators, Derivatives of 4-(4-Tosylureido-Amino Acyl)Ethyl-1H-Imidazole (Histamine Derivatives). J. Enzym. Inhib. 2000, 15, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Briganti, F.; Scozzafava, A.; Supuran, C.T. Novel Carbonic Anhydrase Isozymes I, II and IV Activators Incorporating Sulfonyl-Histamino Moieties. Bioorg. Med. Chem. Lett. 1999, 9, 2043–2048. [Google Scholar] [CrossRef]
- Supuran, C.T.; Scozzafava, A. Carbonic Anhydrase Activators: Amino Acyl/Dipeptidyl Histamine Derivatives Bind with High Affinity to Isozymes I, II and IV and Act as Efficient Activators. Bioorg. Med. Chem. 1999, 7, 2915–2923. [Google Scholar] [CrossRef]
- Scozzafava, A.; Supuran, C.T. Carbonic Anhydrase Activators: Part 24. High Affinity Isozymes I, II and IV Activators, Derivatives of 4-(4-Chlorophenylsulfonylureido-Amino Acyl)Ethyl-1H-Imidazole. Eur. J. Pharm. Sci. 2000, 10, 29–41. [Google Scholar] [CrossRef]
- Scozzafava, A.; Supuran, C.T. Carbonic Anhydrase Activators—Part 21. Novel Activators of Isozymes I, II and IV Incorporating Carboxamido and Ureido Histamine Moieties. Eur. J. Med. Chem. 2000, 35, 31–39. [Google Scholar] [CrossRef]
- Akocak, S.; Lolak, N.; Vullo, D.; Durgun, M.; Supuran, C.T. Synthesis and Biological Evaluation of Histamine Schiff Bases as Carbonic Anhydrase I, II, IV, VII, and IX Activators. J. Enzym. Inhib. Med. Chem. 2017, 32, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Dave, K.; Ilies, M.A.; Scozzafava, A.; Temperini, C.; Vullo, D.; Supuran, C.T. An Inhibitor-like Binding Mode of a Carbonic Anhydrase Activator within the Active Site of Isoform II. Bioorg. Med. Chem. Lett. 2011, 21, 2764–2768. [Google Scholar] [CrossRef] [PubMed]
- Saada, M.C.; Montero, J.L.; Vullo, D.; Scozzafava, A.; Winum, J.Y.; Supuran, C.T. Carbonic Anhydrase Activators: Gold Nanoparticles Coated with Derivatized Histamine, Histidine, and Carnosine Show Enhanced Activatory Effects on Several Mammalian Isoforms. J. Med. Chem. 2011, 54, 1170–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supuran, C.T.; Barboiu, M.; Luca, C.; Pop, E.; Brewster, M.E.; Dinculescu, A. Carbonic Anhydrase Activators. Part 14. Syntheses of Mono and Bis Pyridinium Salt Derivatives of 2-Amino-5-(2-Aminoethyl)- and 2-Amino-5-(3-Aminopropyl)-1,3,4-Thiadiazole and Their Interaction with Isozyme II. Eur. J. Med. Chem. 1996, 31, 597–606. [Google Scholar] [CrossRef]
- Rami, M.; Winum, J.-Y.; Supuran, C.T.; Melnyk, P.; Yous, S. (Hetero)Aryl Substituted Thiazol-2,4-Yl Scaffold as Human Carbonic Anhydrase I, II, VII and XIV Activators. J. Enzym. Inhib. Med. Chem. 2019, 34, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saada, M.C.; Vullo, D.; Montero, J.L.; Scozzafava, A.; Winum, J.Y.; Supuran, C.T. Carbonic Anhydrase I and II Activation with Mono- and Dihalogenated Histamine Derivatives. Bioorg. Med. Chem. Lett. 2011, 21, 4884–4887. [Google Scholar] [CrossRef]
- Draghici, B.; Vullo, D.; Akocak, S.; Walker, E.A.; Supuran, C.T.; Ilies, M.A. Ethylene Bis-Imidazoles Are Highly Potent and Selective Activators for Isozymes VA and VII of Carbonic Anhydrase, with a Potential Nootropic Effect. Chem. Commun. 2014, 50, 5980–5983. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, M.; Kondeti, B.; Tu, C.; Maupin, C.M.; Silverman, D.N.; McKenna, R. Structural Insight into Activity Enhancement and Inhibition of H64A Carbonic Anhydrase II by Imidazoles. IUCrJ 2014, 1, 129. [Google Scholar] [CrossRef]
- Akocak, S.; Lolak, N.; Bua, S.; Nocentini, A.; Supuran, C.T. Activation of Human α-Carbonic Anhydrase Isoforms I, II, IV and VII with Bis-Histamine Schiff Bases and Bis-Spinaceamine Substituted Derivatives. J. Enzym. Inhib. Med. Chem. 2019, 34, 1193–1198. [Google Scholar] [CrossRef] [Green Version]
- Akocak, S.; Lolak, N.; Bua, S.; Nocentini, A.; Karakoc, G.; Supuran, C.T. α-Carbonic Anhydrases Are Strongly Activated by Spinaceamine Derivatives. Bioorg. Med. Chem. 2019, 27, 800–804. [Google Scholar] [CrossRef]
- Ghiasi, M.; Shahabi, P.; Supuran, C.T. Quantum Mechanical Study on the Activation Mechanism of Human Carbonic Anhydrase VII Cluster Model with Bis-Histamine Schiff Bases and Bis-Spinaceamine Derivatives. Bioorg. Med. Chem. 2021, 44, 116276. [Google Scholar] [CrossRef]
- Mollica, A.; Macedonio, G.; Stefanucci, A.; Carradori, S.; Akdemir, A.; Angeli, A.; Supuran, C. Five- and Six-Membered Nitrogen-Containing Compounds as Selective Carbonic Anhydrase Activators. Molecules 2017, 22, 2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provensi, G.; Nocentini, A.; Passani, M.B.; Blandina, P.; Supuran, C.T. Activation of Carbonic Anhydrase Isoforms Involved in Modulation of Emotional Memory and Cognitive Disorders with Histamine Agonists, Antagonists and Derivatives. J. Enzym. Inhib. Med. Chem. 2021, 36, 719–726. [Google Scholar] [CrossRef]
- Scozzafava, A.; Supuran, C.T. Carbonic Anhydrase Activators: High Affinity Isozymes I, II, and IV Activators, Incorporating a β-Alanyl-Histidine Scaffold. J. Med. Chem. 2002, 45, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Abdo, M.R.; Vullo, D.; Saada, M.C.; Montero, J.L.; Scozzafava, A.; Winum, J.Y.; Supuran, C.T. Carbonic Anhydrase Activators: Activation of Human Isozymes I, II and IX with Phenylsulfonylhydrazido l-Histidine Derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 2440–2443. [Google Scholar] [CrossRef]
- Saada, M.C.; Vullo, D.; Montero, J.L.; Scozzafava, A.; Supuran, C.T.; Winum, J.Y. Mono- and Di-Halogenated Histamine, Histidine and Carnosine Derivatives Are Potent Carbonic Anhydrase I, II, VII, XII and XIV Activators. Bioorg. Med. Chem. 2014, 22, 4752–4758. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; Aldini, G.; Fumagalli, L.; Dallanoce, C.; Angeli, A.; Supuran, C.T. Activation Effects of Carnosine- and Histidine-Containing Dipeptides on Human Carbonic Anhydrases: A Comprehensive Study. Int. J. Mol. Sci. 2020, 21, 1761–1771. [Google Scholar] [CrossRef] [Green Version]
- Tanini, D.; Capperucci, A.; Supuran, C.T.; Angeli, A. Sulfur, Selenium and Tellurium Containing Amines Act as Effective Carbonic Anhydrase Activators. Bioorg. Chem. 2019, 87, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Cuffaro, D.; Ciccone, L.; Orlandini, E.; Nencetti, S.; Nuti, E.; Rossello, A.; Supuran, C.T. Activation of Carbonic Anhydrases from Human Brain by Amino Alcohol Oxime Ethers: Towards Human Carbonic Anhydrase VII Selective Activators. J. Enzym. Inhib. Med. Chem. 2020, 36, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, A.; Ikeda, H.; Tsukamoto, H.; Kihira, K.; Ishioka, M.; Hirose, J.; Hata, T.; Fujioka, H.; Ono, Y. Timolol Activates the Enzyme Activities of Human Carbonic Anhydrase I and II. Biol. Pharm. Bull. 2010, 33, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccallini, C.; Di Matteo, M.; Vullo, D.; Ammazzalorso, A.; Carradori, S.; De Filippis, B.; Fantacuzzi, M.; Giampietro, L.; Pandolfi, A.; Supuran, C.T.; et al. Indazole, Pyrazole, and Oxazole Derivatives Targeting Nitric Oxide Synthases and Carbonic Anhydrases. ChemMedChem 2016, 11, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-K.; Alkon, D.L. Pharmacological Enhancement of Synaptic Efficacy, Spatial Learning, and Memory through Carbonic Anhydrase Activation in Rats. J. Pharmacol. Exp. Ther. 2001, 297. [Google Scholar]
- Sun, M.K.; Alkon, D.L. Carbonic Anhydrase Gating of Attention: Memory Therapy and Enhancement. Trends Pharmacol. Sci. 2002, 23, 83–89. [Google Scholar] [CrossRef]
- Meier-Ruge, W.; Iwangoff, P.; Reichlmeier, K. Neurochemical Enzyme Changes in Alzheimer’s and Pick’s Disease. Arch. Gerontol. Geriatr. 1984, 3, 161–165. [Google Scholar] [CrossRef]
- Canto de Souza, L.; Provensi, G.; Vullo, D.; Carta, F.; Scozzafava, A.; Costa, A.; Schmidt, S.D.; Passani, M.B.; Supuran, C.T.; Blandina, P. Carbonic Anhydrase Activation Enhances Object Recognition Memory in Mice through Phosphorylation of the Extracellular Signal-Regulated Kinase in the Cortex and the Hippocampus. Neuropharmacology 2017, 118, 148–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, S.D.; Costa, A.; Rani, B.; Nachtigall, E.G.; Passani, M.B.; Carta, F.; Nocentini, A.; De Carvalho Myskiw, J.; Furini, C.R.G.; Supuran, C.T.; et al. The Role of Carbonic Anhydrases in Extinction of Contextual Fear Memory. Proc. Natl. Acad. Sci. USA 2020, 117, 16000–16008. [Google Scholar] [CrossRef]
- Blandina, P.; Provensi, G.; Passsani, M.B.; Capasso, C.; Supuran, C.T. Carbonic Anhydrase Modulation of Emotional Memory. Implications for the Treatment of Cognitive Disorders. J. Enzym. Inhib. Med. Chem. 2020, 35, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.G.; Efoudebe, M.; Passani, M.B.; Baldi, E.; Bucherelli, C.; Giachi, F.; Corradetti, R.; Blandina, P. Improvement in Fear Memory by Histamine-Elicited ERK2 Activation in Hippocampal CA3 Cells. J. Neurosci. 2003, 23, 9016–9023. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Schröder, H.C.; Schlossmacher, U.; Neufurth, M.; Feng, Q.; Diehl-Seifert, B.; Müller, W.E.G. Modulation of the Initial Mineralization Process of SaOS-2 Cells by Carbonic Anhydrase Activators and Polyphosphate. Calcif. Tissue Int. 2014, 94, 495–509. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Schröder, H.C.; Schlossmacher, U.; Grebenjuk, V.A.; Ushijima, H.; Wang, X. Induction of Carbonic Anhydrase in SaOS-2 Cells, Exposed to Bicarbonate and Consequences for Calcium Phosphate Crystal Formation. Biomaterials 2013, 34, 8671–8680. [Google Scholar] [CrossRef]
- Vullo, D.; De Luca, V.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. The First Activation Study of a Bacterial Carbonic Anhydrase (CA). the Thermostable α-CA from Sulfurihydrogenibium Yellowstonense YO3AOP1 Is Highly Activated by Amino Acids and Amines. Bioorg. Med. Chem. Lett. 2012, 22, 6324–6327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeli, A.; Del Prete, S.; Osman, S.M.; Alasmary, F.A.S.; AlOthman, Z.; Donald, W.A.; Capasso, C.; Supuran, C.T. Activation Studies of the α- and β-Carbonic Anhydrases from the Pathogenic Bacterium Vibrio Cholerae with Amines and Amino Acids. J. Enzym. Inhib. Med. Chem. 2018, 33, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Angeli, A.; Del Prete, S.; Donald, W.A.; Capasso, C.; Supuran, C.T. The γ-Carbonic Anhydrase from the Pathogenic Bacterium Vibrio Cholerae Is Potently Activated by Amines and Amino Acids. Bioorg. Chem. 2018, 77, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Stefanucci, A.; Angeli, A.; Dimmito, M.P.; Luisi, G.; Del Prete, S.; Capasso, C.; Donald, W.A.; Mollica, A.; Supuran, C.T. Activation of β- and γ-Carbonic Anhydrases from Pathogenic Bacteria with Tripeptides. J. Enzyme Inhib. Med. Chem. 2018, 33, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Del Prete, S.; Osman, S.M.; Alasmary, F.A.S.; AlOthman, Z.; Donald, W.A.; Capasso, C.; Supuran, C.T. Activation Studies with Amines and Amino Acids of the β-Carbonic Anhydrase Encoded by the Rv3273 Gene from the Pathogenic Bacterium Mycobacterium Tuberculosis. J. Enzym. Inhib. Med. Chem. 2018, 33, 364–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innocenti, A.; Zimmerman, S.A.; Scozzafava, A.; Ferry, J.G.; Supuran, C.T. Carbonic Anhydrase Activators: Activation of the Archaeal β-Class (Cab) and γ-Class (Cam) Carbonic Anhydrases with Amino Acids and Amines. Bioorg. Med. Chem. Lett. 2008, 18, 6194–6198. [Google Scholar] [CrossRef]
- Nocentini, A.; Del Prete, S.; Mastrolorenzo, M.D.; Donald, W.A.; Capasso, C.; Supuran, C.T. Activation Studies of the β-Carbonic Anhydrases from Escherichia Coli with Amino Acids and Amines. J. Enzyme Inhib. Med. Chem. 2020, 35, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Del Prete, S.; Pinteala, M.; Maier, S.S.; Donald, W.A.; Simionescu, B.C.; Capasso, C.; Supuran, C.T. The First Activation Study of the β-Carbonic Anhydrases from the Pathogenic Bacteria Brucella Suis and Francisella Tularensis with Amines and Amino Acids. J. Enzym. Inhib. Med. Chem. 2019, 34, 1178–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vullo, D.; Del Prete, S.; Osman, S.M.; AlOthman, Z.; Capasso, C.; Donald, W.A.; Supuran, C.T. Burkholderia Pseudomallei γ-Carbonic Anhydrase Is Strongly Activated by Amino Acids and Amines. Bioorg. Med. Chem. Lett. 2017, 27, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Del Prete, S.; Osman, S.M.; AlOthman, Z.; Donald, W.A.; Capasso, C.; Supuran, C.T. Activation Studies of the γ-Carbonic Anhydrases from the Antarctic Marine Bacteria Pseudoalteromonas Haloplanktis and Colwellia Psychrerythraea with Amino Acids and Amines. Mar. Drugs 2019, 17, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luca, V.; Petreni, A.; Carginale, V.; Scaloni, A.; Supuran, C.T.; Capasso, C. Effect of Amino Acids and Amines on the Activity of the Recombinant ι-Carbonic Anhydrase from the Gram-Negative Bacterium Burkholderia territorii. J. Enzyme Inhib. Med. Chem. 2021, 36, 1000–1006. [Google Scholar] [CrossRef]
- Isik, S.; Kockar, F.; Aydin, M.; Arslan, O.; Guler, O.O.; Innocenti, A.; Scozzafava, A.; Supuran, C.T. Carbonic Anhydrase Activators: Activation of the β-Carbonic Anhydrase Nce103 from the Yeast Saccharomyces Cerevisiae with Amines and Amino Acids. Bioorg. Med. Chem. Lett. 2009, 19, 1662–1665. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Hall, R.A.; Scozzafava, A.; Mühlschlegel, F.A.; Supuran, C.T. Carbonic Anhydrase Activators: Activation of the β-Carbonic Anhydrases from the Pathogenic Fungi Candida Albicans and Cryptococcus Neoformans with Amines and Amino Acids. Bioorg. Med. Chem. 2010, 18, 1034–1037. [Google Scholar] [CrossRef]
- Innocenti, A.; Leewattanapasuk, W.; Manole, G.; Scozzafava, A.; Mühlschlegel, F.A.; Supuran, C.T. Carbonic Anhydrase Activators: Activation of the β-Carbonic Anhydrase from the Pathogenic Yeast Candida Glabrata with Amines and Amino Acids. Bioorg. Med. Chem. Lett. 2010, 20, 1701–1704. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Del Prete, S.; Capasso, C.; Supuran, C.T. Carbonic Anhydrase Activators: Activation of the β-Carbonic Anhydrase from Malassezia Globosa with Amines and Amino Acids. Bioorg. Med. Chem. Lett. 2016, 26, 1381–1385. [Google Scholar] [CrossRef]
- Angeli, A.; Prete, S. Del; Ghobril, C.; Hitce, J.; Clavaud, C.; Marrat, X.; Donald, W.A.; Capasso, C.; Supuran, C.T. Activation Studies of the β-Carbonic Anhydrases from Malassezia Restricta with Amines and Amino Acids. J. Enzyme Inhib. Med. Chem. 2020, 35, 824–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeli, A.; Kuuslahti, M.; Parkkila, S.; Supuran, C.T. Activation Studies with Amines and Amino Acids of the α-Carbonic Anhydrase from the Pathogenic Protozoan Trypanosoma Cruzi. Bioorg. Med. Chem. 2018, 26, 4187–4190. [Google Scholar] [CrossRef]
- Angeli, A.; Donald, W.A.; Parkkila, S.; Supuran, C.T. Activation Studies with Amines and Amino Acids of the β-Carbonic Anhydrase from the Pathogenic Protozoan Leishmania Donovani Chagasi. Bioorg. Chem. 2018, 78, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Bua, S.; Haapanen, S.; Kuuslahti, M.; Parkkila, S.; Supuran, C.T. Activation Studies of the β-Carbonic Anhydrase from the Pathogenic Protozoan Entamoeba Histolytica with Amino Acids and Amines. Metabolites 2019, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Angeli, A.; Urbański, L.J.; Hytönen, V.P.; Parkkila, S.; Supuran, C.T. Activation of the β-Carbonic Anhydrase from the Protozoan Pathogen Trichomonas Vaginalis with Amines and Amino Acids. J. Enzyme. Inhib. Med. Chem. 2021, 36, 758–763. [Google Scholar] [CrossRef]
- Angeli, A.; Del Prete, S.; Alasmary, F.A.S.; Alqahtani, L.S.; AlOthman, Z.; Donald, W.A.; Capasso, C.; Supuran, C.T. The First Activation Studies of the η-Carbonic Anhydrase from the Malaria Parasite Plasmodium Falciparum with Amines and Amino Acids. Bioorg. Chem. 2018, 80, 94–98. [Google Scholar] [CrossRef]
- Angeli, A.; Alasmary, F.A.S.; Del Prete, S.; Osman, S.M.; AlOthman, Z.; Donald, W.A.; Capasso, C.; Supuran, C.T. The First Activation Study of a δ-Carbonic Anhydrase: TweCAδ from the Diatom Thalassiosira Weissflogii Is Effectively Activated by Amines and Amino Acids. J. Enzyme Inhib. Med. Chem. 2018, 33, 680–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Prete, S.; Vullo, D.; Zoccola, D.; Tambutté, S.; Supuran, C.T.; Capasso, C. Activation Profile Analysis of CruCA4, an α-Carbonic Anhydrase Involved in Skeleton Formation of the Mediterranean Red Coral, Corallium Rubrum. Molecules 2017, 23, 66. [Google Scholar] [CrossRef] [Green Version]
- Bertucci, A.; Zoccola, D.; Tambutté, S.; Vullo, D.; Supuran, C.T. Carbonic Anhydrase Activators. The First Activation Study of a Coral Secretory Isoform with Amino Acids and Amines. Bioorg. Med. Chem. 2010, 18, 2300–2303. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhang, Y.; Ulrich, S.; Barboiu, M. Constitutional Dynamic Inhibition/Activation of Carbonic Anhydrases. Chempluschem 2021, 86, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; Langella, E.; Buonanno, M.; Esposito, D.; Nocentini, A.; Berrino, E.; Bua, S.; Polentarutti, M.; Supuran, C.T.; Monti, S.M.; et al. Zeta-Carbonic Anhydrases Show CS2 Hydrolase Activity: A New Metabolic Carbon Acquisition Pathway in Diatoms? Comput. Struct. Biotechnol. J. 2021, 19, 3427–3436. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, C.; Dudhagara, P.; Tank, S. Trends, application and future prospectives of microbial carbonic anhydrase mediated carbonation process for CCUS. J. Appl. Microbiol. 2018, 124, 316–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| cmpd | KA (μM) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hCA I | hCA II | hCA III | hCA IV | hCA VA | hCA VB | hCA VI | hCA VII | hCA IX | hCA XII | mCA XIII | hCA IV | mCA XV | |
| 1 | 0.03 | 10.9 | 35.9 | 7.3 | 1.34 | 0.97 | 32 | 0.92 | 9.71 | 37.5 | 0.13 | 0.9 | 32.1 |
| 2 | 0.09 | 43 | 1.13 | 12.3 | 0.12 | 4.38 | 13 | 0.71 | 12.5 | 24.7 | 0.09 | 2.37 | 14.1 |
| 3 | 0.07 | 0.013 | 34.7 | 36.3 | 9.81 | 10.45 | 1.23 | 10.93 | 16.3 | 1.38 | 1.02 | 0.24 | 33.4 |
| 4 | 86 | 0.035 | 15.4 | 49.3 | 4.63 | 0.072 | 16 | 9.74 | 9.3 | 0.37 | 0.051 | 7.21 | 9.5 |
| 5 | 44 | 27 | 20.5 | 37.1 | 1.13 | 0.89 | - | 57.5 | 37.5 | 23 | 16 | 16.5 | 13.5 |
| 6 | 41 | 12 | 19 | 39.6 | 1.24 | 1.35 | - | 39.6 | 43.6 | 28.1 | 0.81 | 18 | 8.7 |
| 7 | 0.02 | 0.011 | 34.1 | 25.1 | 2.45 | 0.044 | - | 20.3 | 25.3 | 25.8 | - | 21.8 | 8.9 |
| 8 | 0.04 | 0.013 | - | - | - | - | - | - | - | - | - | - | - |
| 9 | 3.1 | 11.4 | 13.5 | 15.3 | 0.036 | 0.063 | - | 58.3 | 51.3 | 1.67 | 43 | 12.1 | 6.5 |
| 10 | 4.9 | 7.8 | 28.7 | 34.7 | 4.59 | 3.71 | - | 34.7 | 54.7 | 0.89 | 0.73 | 36.8 | 4 |
| 11 | 0.24 | 0.15 | 43.2 | 0.079 | 2.76 | 2.17 | - | 18.7 | 48.7 | 1.09 | - | 2.9 | 16.3 |
| 12 | 2.1 | 125 | 36.9 | 25.3 | 0.01 | 3.52 | 6.5 | 37.5 | 35.1 | 27.9 | 4.6 | 0.01 | 18.5 |
| 13 | 45 | 50 | 0.78 | 3.14 | 6.33 | 0.11 | - | 0.93 | 33.1 | 0.3 | 0.51 | 6.5 | 7.5 |
| 14 | 13.5 | 9.2 | 33.2 | 30.9 | 0.13 | 7.85 | 21.1 | 0.89 | 0.92 | 0.67 | 27 | 14.6 | 7.1 |
| 15 | 0.09 | 96 | 36.4 | 45 | - | - | - | - | 60 | 0.87 | - | 36.1 | 6.9 |
| 16 | 26 | 34 | 1.03 | 5.19 | 23.56 | 0.24 | - | 43.7 | 1.07 | 41.5 | 3.8 | 21.7 | 11.6 |
| 17 | 13 | 15 | 1.1 | 7.13 | 7.62 | 0.094 | - | 27.8 | 0.013 | 0.69 | 46 | 6.9 | 11.9 |
| 18 | 7.4 | 2.3 | 0.32 | 24.9 | 6.04 | 0.91 | 9.54 | 32.5 | 0.009 | 48.3 | 54 | 18.3 | 10.4 |
| 19 | 0.14 | 0.19 | 0.091 | 1.3 | 0.089 | 1.15 | 42 | 64.3 | 0.43 | 0.24 | 0.013 | 5.4 | 9.3 |
| 20 | >150 | >150 | - | 0.094 | 0.81 | 2.56 | >150 | 0.91 | >150 | 0.64 | 24.1 | 9.15 | - |
| 21 | >150 | >150 | - | 0.074 | 0.53 | 0.62 | >150 | 0.89 | 34.6 | 3.24 | 54.2 | 12.7 | - |
| 22 | >150 | >150 | - | 0.051 | 0.92 | 0.78 | >150 | 0.93 | >150 | 0.8 | 25.6 | 7.38 | - |
| 23 | >150 | >150 | - | 1.03 | 0.37 | 0.24 | >150 | 0.64 | 25.8 | 6.12 | 48.3 | 18.1 | - |
| 24 | >150 | >150 | - | 0.055 | 0.31 | 0.75 | >150 | 0.098 | 34.1 | 0.97 | 79.5 | 6.81 | - |
| Species | CA Family (Acronym) | Ref. |
|---|---|---|
| Gram-Positive and Gram-Negative Bacteria | ||
| Sulfurihydrogenibium yellowstonense | α (SSpCA) | [84] |
| Sulfurihydrogenibium azorense | α (SazCA) | |
| Vibrio cholerae | α (VchCAα), β (VchCAβ), and γ (VchCAγ) | [85,86,87] |
| Mycobacterium tuberculosis | β (mtCA3 or Rv3273 CA) | [87,88] |
| Methanobacterium thermoautotrophicum | β (Cab) | [89] |
| Escherichia coli | β (EcoCAβ) | [90] |
| Brucella suis | β (BsuCA1) | [91] |
| Francisella tularensis | β (FtuCA) | [91] |
| Burkholderia pseudomallei | β (BpsCAβ) | |
| Methanosarcina thermophila | γ (Cam) | [89] |
| Burkholderia pseudomallei | γ (BpsγCA) | [87,92] |
| Pseudoalteromonas haloplanktis | γ (PhaCA) | [93] |
| Colwellia psychrerythraea | γ (CpsCA) | [93] |
| Burkholderia territorii | ι (BteCAi) | [94] |
| Fungi and Yeasts | ||
| Saccharomyces cerevisiae | β (scCA) | [95] |
| Candida albicans | β (CaNce103) | [96] |
| Cryptococcus neoformans | β (Can2) | [96] |
| Candida glabrata | β (CgCA) | [97] |
| Malassezia globosa | β (MgCA) | [98] |
| Malassezia restricta | β (MreCA) | [99] |
| Protozoa | ||
| Trypanosoma cruzi | α (TcCA) | [100] |
| Leishmania donovani chagasi | β (LdcCA) | [101] |
| Entamoeba histolytica | β (EhiCA) | [102] |
| Trichomonas vaginalis | β (TvaCA1) | [103] |
| Plasmodium falciparum | η (PfACA) | [104] |
| Microalgae | ||
| Thalassiosira weissflogii | δ (TweCAδ) and ζ (TweCAζ) | [19,105] |
| Corals | ||
| Corallium rubrum | α (CruCA4) | [106] |
| Stylophora pystillata | α (STPCA) | [107] |
| Compound | KA (μM) | |||||
|---|---|---|---|---|---|---|
| STPCA | CruCA4 | VchCAα | SspCA | TcCA | SazCA | |
| 1 | 28.0 | 36.9 | 43.2 | 0.11 | 11.3 | 0.071 |
| 2 | 26.0 | 0.098 | 22.7 | 0.012 | 7.5 | 0.090 |
| 3 | 34.0 | 15.4 | 53.6 | 0.008 | 12.1 | 0.062 |
| 4 | 21.0 | 1.0 | 34.5 | 5.1 | 6.4 | 0.009 |
| 5 | 3.2 | 9.5 | 4.1 | 0.007 | 2.5 | 0.004 |
| 6 | 19.0 | 8.3 | 38.0 | 0.002 | 1.8 | 0.89 |
| 7 | 31.0 | 0.73 | 8.2 | 0.01 | 4.9 | 0.023 |
| 8 | nd | 18.9 | 37.8 | 0.83 | 2.8 | 0.003 |
| 9 | 15.0 | 13.7 | 23.1 | 0.09 | 0.83 | 0.052 |
| 10 | 0.18 | 0.93 | 19.4 | 0.43 | 0.38 | 0.11 |
| 11 | 10.1 | 0.074 | 41.6 | 0.97 | 0.75 | 0.09 |
| 12 | >100 | 0.007 | 9.1 | 0.08 | 2.7 | 0.10 |
| 13 | 56.0 | 0.006 | 11.7 | 0.021 | 2.0 | 0.011 |
| 14 | 89.0 | 0.005 | 35.2 | 0.037 | >100 | 0.007 |
| 15 | 47.0 | 0.009 | 18.2 | 0.68 | >100 | 0.081 |
| 16 | >100 | 0.41 | 68.3 | 0.10 | >100 | 0.34 |
| 17 | >100 | 0.26 | 71.9 | 0.33 | >100 | 0.076 |
| 18 | 11.5 | 0.004 | 57.3 | 0.09 | >100 | 1.15 |
| 19 | 64.0 | 0.15 | 12.0 | 0.10 | 0.14 | 0.074 |
| Compound | KA (μM) | |||||
|---|---|---|---|---|---|---|
| scCA | CaNce103 | CgCA | Can2 | MgCA | MreCA | |
| 1 | 82.0 | 24.1 | 37.0 | 45.0 | 29.3 | 12.8 |
| 2 | 85.0 | 19.5 | 21.2 | 47.2 | 18.1 | 1.8 |
| 3 | 86.0 | 15.5 | 24.1 | 44.1 | 34.1 | 3.0 |
| 4 | 86.0 | 8.4 | 15.7 | 45.2 | 10.7 | 0.76 |
| 5 | 91.0 | 19.2 | 22.8 | 28.7 | 10.1 | 0.32 |
| 6 | 90.0 | 43.0 | 12.1 | 42.1 | 12.5 | 0.89 |
| 7 | 85.0 | 46.1 | 9.5 | 29.5 | 15.7 | 4.1 |
| 8 | 84.0 | nd | 7.1 | nd | 25.1 | 7.8 |
| 9 | 90.0 | 0.96 | 23.3 | 43.3 | 8.31 | 0.87 |
| 10 | 89.0 | 2.5 | 15.1 | 35.1 | 13.7 | 0.70 |
| 11 | 21.3 | 23.7 | 31.6 | 30.4 | 13.4 | 0.61 |
| 12 | 20.4 | 18.4 | 27.4 | 33.2 | 10.9 | 0.90 |
| 13 | 15.0 | 28.6 | 16.7 | 46.7 | 14.2 | 0.82 |
| 14 | 13.1 | 18.5 | 27.6 | 34.6 | 9.43 | 2.7 |
| 15 | 0.95 | 13.2 | 10.8 | 32.8 | 0.72 | 0.015 |
| 16 | 16.2 | 29.1 | 15.0 | 47.0 | 6.1 | 0.34 |
| 17 | 11.2 | 30.2 | 16.3 | 46.3 | 7.3 | 2.1 |
| 18 | 9.3 | 17.3 | 14.9 | 44.9 | 0.81 | 0.25 |
| 19 | 10.2 | 25.4 | 10.1 | 40.1 | 5.8 | 0.33 |
| Compound | KA (µM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cab | mtCA3 | VchCAβ | EcoCAβ | BsuCA1 | FtuCA | LdcCA | EhiCA | TvaCA1 | BpsCAβ | |
| 1 | 69.0 | 18.2 | 20.3 | 36.0 | 1.8 | 40.7 | 8.2 | 78.7 | 20.1 | 31.6 |
| 2 | 57.0 | 32.5 | 18.0 | 23.7 | 12.3 | 78.3 | 4.1 | 9.8 | 24.5 | 0.98 |
| 3 | 70.0 | 30.6 | 15.4 | 12.0 | 1.2 | 69.1 | 9.2 | 16.5 | 23.6 | 3.42 |
| 4 | 10.3 | 44.1 | 5.1 | 15.4 | 1.1 | 75.0 | 3.9 | 10.1 | 16.3 | 0.075 |
| 5 | 16.9 | 8.9 | 4.2 | 18.3 | 1.2 | 34.1 | 4.0 | 5.2 | 5.1 | 0.009 |
| 6 | 41.0 | 43.7 | 5.9 | 11.5 | 13.7 | 30.5 | 6.2 | 4.9 | 3.6 | 0.007 |
| 7 | 10.5 | 28.9 | 6.1 | 9.9 | 1.4 | ˃100 | 8.1 | 4.5 | 4.9 | 0.002 |
| 8 | 19.2 | 17.6 | 0.94 | 17.9 | 0.95 | ˃100 | 1.3 | 1.1 | 3.0 | 0.001 |
| 9 | 11.4 | 30.0 | 8.4 | 10.7 | 2.1 | ˃100 | 1.6 | 16.6 | 12.1 | 0.003 |
| 10 | 15.6 | 9.7 | 6.3 | 3.1 | 2.3 | 44.8 | 5.5 | 4.1 | 11.0 | 1.89 |
| 11 | 89.0 | 40.5 | 7.2 | 7.3 | 1.2 | ˃100 | 15.9 | 8.1 | 3.5 | 0.0009 |
| 12 | 76.0 | 34.2 | 9.5 | 18.5 | 3.7 | ˃100 | 0.74 | 7.4 | 8.4 | 0.012 |
| 13 | 62.0 | 10.3 | 1.4 | 2.8 | 4.3 | ˃100 | 0.62 | 4.9 | 9.1 | 0.006 |
| 14 | 51.0 | 12.1 | 1.2 | 11.3 | 1.5 | ˃100 | 0.81 | 30.8 | 12.6 | 0.027 |
| 15 | 11.5 | 52.2 | 8.7 | 9.1 | 0.70 | ˃100 | 4.9 | 25.6 | 8.3 | 0.016 |
| 16 | 18.7 | 43.3 | 0.18 | 48.7 | 1.6 | 46.3 | 0.23 | ˃100 | 9.5 | 0.94 |
| 17 | 40.0 | 45.9 | 1.0 | 17.2 | 5.2 | ˃100 | 0.012 | ˃100 | 12.0 | 0.004 |
| 18 | 13.8 | 50.3 | 0.24 | 14.1 | 43.1 | 51.8 | 0.009 | 43.8 | 11.8 | 0.073 |
| 19 | 18.5 | 52.0 | 12.8 | 17.4 | 9.6 | ˃100 | 0.94 | ˃100 | 14.5 | 0.002 |
| Compound | KA (µM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| γ-CAs | δ-CAs | ζ-CAs | η-CAs | ι-CAs | ||||||
| Zn-Cam | Co-Cam | VchCAγ | BpsγCA | PhaCA | CpsCA | TweCAδ | Zn-TweCAζ | PfaCA | BteCAi | |
| 1 | 68.0 | ˃100 | 1.0 | 24.7 | 12.6 | 47.5 | 0.75 | 0.81 | 1.1 | 8.6 |
| 2 | 46.0 | 73.0 | 14.2 | 0.086 | 9.4 | 35.9 | 4.9 | 7.2 | 2.2 | 6.2 |
| 3 | 68.0 | 70.0 | 0.73 | 1.7 | 15.8 | ˃100 | 2.2 | 15.4 | 0.43 | 36.5 |
| 4 | 42.0 | 24.0 | 0.24 | 0.13 | 3.2 | 15.4 | 1.2 | 9.6 | 0.75 | 9.4 |
| 5 | 38.0 | 47.0 | 0.008 | 0.43 | 7.1 | 21.3 | 0.93 | 8.5 | 5.2 | 10.2 |
| 6 | 33.0 | 68.0 | 0.40 | 0.052 | 13.9 | 36.8 | 0.69 | 1.8 | 8.5 | 6.1 |
| 7 | 24.0 | 53.0 | 0.12 | 0.20 | 1.0 | 19.5 | 1.5 | 0.98 | 1.0 | 8.0 |
| 8 | nd | nd | 0.10 | 32.8 | 7.4 | 18.4 | 0.051 | 0.62 | 8.6 | 7.3 |
| 9 | 39.0 | 38.0 | 0.19 | 0.072 | 1.1 | 4.8 | 2.1 | 3.2 | 0.12 | 4.3 |
| 10 | 37.0 | 41.0 | 0.13 | 0.98 | 0.72 | 11.2 | 6.2 | 2.9 | 0.39 | 11.7 |
| 11 | 72.0 | 22.0 | 0.69 | 0.009 | 3.3 | 17.2 | 18.9 | 7.9 | 1.0 | 6.9 |
| 12 | 63.0 | 9.2 | 0.31 | 0.12 | 6.5 | 20.6 | 1.3 | 1.3 | 9.9 | 6.0 |
| 13 | 38.0 | 0.97 | 0.17 | 0.10 | 9.1 | 34.8 | 0.90 | 3.1 | 7.2 | 13.3 |
| 14 | 54.0 | 18.4 | 0.45 | 0.014 | 8.7 | 32.1 | 0.51 | 10.1 | 10.0 | 8.7 |
| 15 | 39.0 | 8.9 | 0.11 | 0.019 | 17.5 | 79.8 | 2.4 | 0.092 | 2.4 | 9.7 |
| 16 | 11.4 | 8.7 | 0.14 | 2.4 | 2.4 | 21.5 | 5.3 | 0.88 | 3.7 | 24.1 |
| 17 | 24.0 | 18.5 | 0.26 | 0.034 | 18.7 | 38.2 | 8.2 | 0.85 | 6.8 | 21.5 |
| 18 | 10.1 | 16.1 | 0.071 | 0.018 | 15.1 | 33.0 | 4.4 | 0.12 | 0.71 | 3.9 |
| 19 | 45.0 | 38.0 | 0.054 | 0.015 | 10.1 | 34.2 | 7.4 | 0.15 | 5.3 | 12.0 |
| Compound | NH2-Xaa1-Xaa2-Xaa3-NH2 | KA (µM) | |||||
|---|---|---|---|---|---|---|---|
| Xaa1 | Xaa2 | Xaa3 | VchCAβ | mtCA3 | VchCAγ | BpsCAγ | |
| 76 | Tyr | Phe | Asp | 3.5 | 8.4 | 14.7 | 10.1 |
| 77 | His | Phe | Glu | 1.2 | 6.3 | 5.8 | 1.6 |
| 78 | Glu | Ile | Thr | 1.1 | 4.3 | 11.9 | 3.7 |
| 79 | Gln | Asp | Ser | 0.21 | 15.8 | 12.9 | 6.2 |
| 80 | Asn | Asp | Ser | 7.2 | 18.1 | 10.6 | 0.95 |
| 81 | Glu | Phe | Glu | 4.2 | 9.4 | 2.7 | 5.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angeli, A.; Berrino, E.; Carradori, S.; Supuran, C.T.; Cirri, M.; Carta, F.; Costantino, G. Amine- and Amino Acid-Based Compounds as Carbonic Anhydrase Activators. Molecules 2021, 26, 7331. https://doi.org/10.3390/molecules26237331
Angeli A, Berrino E, Carradori S, Supuran CT, Cirri M, Carta F, Costantino G. Amine- and Amino Acid-Based Compounds as Carbonic Anhydrase Activators. Molecules. 2021; 26(23):7331. https://doi.org/10.3390/molecules26237331
Chicago/Turabian StyleAngeli, Andrea, Emanuela Berrino, Simone Carradori, Claudiu T. Supuran, Marzia Cirri, Fabrizio Carta, and Gabriele Costantino. 2021. "Amine- and Amino Acid-Based Compounds as Carbonic Anhydrase Activators" Molecules 26, no. 23: 7331. https://doi.org/10.3390/molecules26237331
APA StyleAngeli, A., Berrino, E., Carradori, S., Supuran, C. T., Cirri, M., Carta, F., & Costantino, G. (2021). Amine- and Amino Acid-Based Compounds as Carbonic Anhydrase Activators. Molecules, 26(23), 7331. https://doi.org/10.3390/molecules26237331










