Hydrothermal Synthesis of Silicoaluminophosphate with AEL Structure Using a Residue of Fluorescent Lamps as Starting Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrothermal Synthesis of Materials
2.2. Physicochemical Characterization
3. Results and Discussion
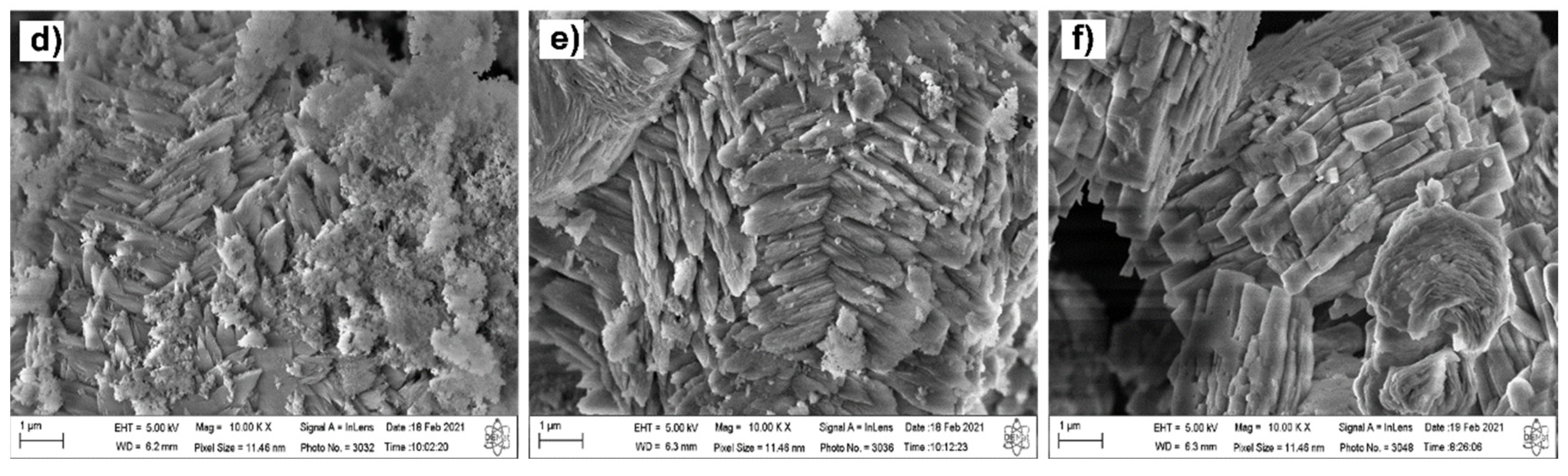
3.1. Characterization from XRF, XRD, and SEM
3.2. Specific Surface Area and Porositie
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A



References
- Wu, Y.; Yin, X.; Zhang, Q.; Wang, W.; Mu, X. The recycling of rare earths from waste tricolor phosphors in fluorescent lamps: A review of processes and technologies. Resour. Conserv. Recycl. 2014, 88, 21–31. [Google Scholar] [CrossRef]
- Apliquim. Apliquim Brasil Recicle. 2021. Available online: http://www.apliquimbrasilrecicle.com.br/noticias/288/virada-sustentavel-contara-com-recolhimento-de-lampadas-fluorescentes- (accessed on 20 September 2021).
- RCO. Join the Movement and Take Back the Light, RCO. 2021. Available online: www.takebackthelight.ca/recyclingRecolight (accessed on 20 September 2021).
- Recycling. Recycling Light. 2021. Available online: www.recolight.co.uk (accessed on 20 September 2021).
- Vicent, M.; Criado, M.; García-Ten, J. Alkali-activated materials obtained from asphalt fillers and fluorescent lamps wastes. J. Clean. Prod. 2019, 215, 343–353. [Google Scholar] [CrossRef]
- Xie, F.; Liu, L.; Li, J. Recycling of Leaded Glass: Scrap Cathode Ray Glass and Fluorescent Lamp Glass. Procedia Environ. Sci. 2012, 16, 585–589. [Google Scholar] [CrossRef]
- Durão, W.A.; de Castro, C.A.; Windmöller, C.C. Mercury reduction studies to facilitate the thermal decontamination of phosphor powder residues from spent fluorescent lamps. Waste Manag. 2008, 28, 2311–2319. [Google Scholar] [CrossRef]
- Kadam, A.; Nair, G.B.; Dhoble, S. Insights into the extraction of mercury from fluorescent lamps: A review. J. Environ. Chem. Eng. 2019, 7, 103279. [Google Scholar] [CrossRef]
- de Farias, C.V.; Paulino, J.F.; Barcelos, D.A.; Rodrigues, A.P.D.C.; Pontes, F.V.M. Is mercury in fluorescent lamps the only risk to human health? A study of environmental mobility of toxic metals and human health risk assessment. Chemosphere 2020, 261, 128107. [Google Scholar] [CrossRef]
- Park, H.S.; Rhee, S.-W. Estimation of retorted phosphor powder from spent fluorescent lamps by thermal process. Waste Manag. 2016, 50, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Raposo, C.; Windmöller, C.C.; Júnior, W.A.D. Mercury speciation in fluorescent lamps by thermal release analysis. Waste Manag. 2003, 23, 879–886. [Google Scholar] [CrossRef]
- Yurramendi, L.; Gijsemans, L.; Forte, F.; Aldana, J.L.; del Río, C.; Binnemans, K. Enhancing rare-earth recovery from lamp phosphor waste. Hydrometallurgy 2019, 187, 38–44. [Google Scholar] [CrossRef]
- Pavón, S.; Fortuny, A.; Coll, M.; Sastre, A.M. Rare earths separation from fluorescent lamp wastes using ionic liquids as extractant agents. Waste Manag. 2018, 82, 241–248. [Google Scholar] [CrossRef]
- Hirajima, T.; Sasaki, K.; Bissombolo, A.; Hirai, H.; Hamada, M.; Tsunekawa, M. Feasibility of an efficient recovery of rare earth-activated phosphors from waste fluorescent lamps through dense-medium centrifugation. Sep. Purif. Technol. 2005, 44, 197–204. [Google Scholar] [CrossRef]
- Hirajima, T.; Bissombolo, A.; Sasaki, K.; Nakayama, K.; Hirai, H.; Tsunekawa, M. Floatability of rare earth phosphors from waste fluorescent lamps. Int. J. Miner. Process. 2005, 77, 187–198. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Mierzwa, J. Investigation of activator (Mn, Sb) speciation in phosphors for fluorescent lamps. Mater. Chem. Phys. 1993, 34, 270–273. [Google Scholar] [CrossRef]
- Murthy, K.; Patel, Y.; Prasad, A.S.; Natarajan, V.; Page, A. Role of fluorescent lamp phosphors in accidental radiation monitoring. Radiat. Meas. 2003, 36, 483–485. [Google Scholar] [CrossRef]
- Ryan, F.M. Changing requirements of fluorescent lamp phosphors. J. Lumin. 1981, 24, 827–834. [Google Scholar] [CrossRef]
- Kang, K.; Huang, S.; Huang, X.; Zhuang, W.; You, F.; Zhang, S.; He, H. Preparation for a new green-emitting phosphor for cold cathode fluorescent lamp. J. Lumin. 2007, 122, 804–807. [Google Scholar] [CrossRef]
- Yang, F.; Kubota, F.; Baba, Y.; Kamiya, N.; Goto, M. Selective extraction and recovery of rare earth metals from phosphor powders in waste fluorescent lamps using an ionic liquid system. J. Hazard. Mater. 2013, 254–255, 79–88. [Google Scholar] [CrossRef]
- Čejka, J. Recent trends in the synthesis of molecular sieves. Stud. Surf. Sci. Catal. 2005, 157, 111–134. [Google Scholar] [CrossRef]
- Ateka, A.; Sánchez-Contador, M.; Ereña, J.; Aguayo, A.T.; Bilbao, J. Catalyst configuration for the direct synthesis of dimethyl ether from CO and CO2 hydrogenation on CuO–ZnO–MnO/SAPO-18 catalysts. React. Kinet. Mech. Catal. 2018, 124, 401–418. [Google Scholar] [CrossRef]
- Ateka, A.; Ereña, J.; Bilbao, J.; Aguayo, A.T. Strategies for the Intensification of CO2 Valorization in the One-Step Dimethyl Ether Synthesis Process. Ind. Eng. Chem. Res. 2019, 59, 713–722. [Google Scholar] [CrossRef]
- Sánchez-Contador, M.; Ateka, A.; Aguayo, A.; Bilbao, J. Direct synthesis of dimethyl ether from CO and CO2 over a core-shell structured CuO-ZnO-ZrO2@SAPO-11 catalyst. Fuel Process. Technol. 2018, 179, 258–268. [Google Scholar] [CrossRef]
- Numpilai, T.; Wattanakit, C.; Chareonpanich, M.; Limtrakul, J.; Witoon, T. Optimization of synthesis condition for CO2 hydrogenation to light olefins over In2O3 admixed with SAPO-34. Energy Convers. Manag. 2018, 180, 511–523. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Yin, H.; Hu, J.; Cheng, K.; Kang, J.; Zhang, Q.; Wang, Y. Tandem Catalysis for Hydrogenation of CO and CO2 to Lower Olefins with Bifunctional Catalysts Composed of Spinel Oxide and SAPO-34. ACS Catal. 2020, 10, 8303–8314. [Google Scholar] [CrossRef]
- Yao, L.; Shen, X.; Pan, Y.; Peng, Z. Unravelling Proximity-Driven Synergetic Effect within CIZO–SAPO Bifunctional Catalyst for CO2 Hydrogenation to DME. Energy Fuels 2020, 34, 8635–8643. [Google Scholar] [CrossRef]
- Raveendra, G.; Li, C.; Cheng, Y.; Meng, F.; Li, Z. Direct transformation of syngas to lower olefins synthesis over hybrid Zn–Al2O3/SAPO-34 catalysts. New J. Chem. 2018, 42, 4419–4431. [Google Scholar] [CrossRef]
- Li, Z.; Martínez-Triguero, J.; Concepción, P.; Yu, J.; Corma, A. Methanol to olefins: Activity and stability of nanosized SAPO-34 molecular sieves and control of selectivity by silicon distribution. Phys. Chem. Chem. Phys. 2013, 15, 14670–14680. [Google Scholar] [CrossRef]
- Liu, M.; Wu, W.; Kikhtyanin, O.; Xiao, L.; Toktarev, A.; Wang, G.; Zhao, A.; Smirnova, M.; Echevsky, G. Alkylation of naphthalene with methanol over SAPO-11 molecular sieve synthesized by different crystallization methods. Microporous Mesoporous Mater. 2013, 181, 132–140. [Google Scholar] [CrossRef]
- Bértolo, R.; Silva, J.M.; Ribeiro, F.; Maldonado-Hódar, F.J.; Fernandes, A.; Martins, A. Effects of oxidant acid treatments on carbon-templated hierarchical SAPO-11 materials: Synthesis, characterization and catalytic evaluation in n-decane hydroisomerization. Appl. Catal. A Gen. 2014, 485, 230–237. [Google Scholar] [CrossRef]
- Choi, M.; Srivastava, R.; Ryoo, R. Organosilane surfactant-directed synthesis of mesoporous aluminophosphates constructed with crystalline microporous frameworks. Chem. Commun. 2006, 42, 4380–4382. [Google Scholar] [CrossRef]
- Alfonzo, M.; Goldwasser, J.; López, C.; Machado, F.; Matjushin, M.; Méndez, B.; de Agudelo, M.R. Effect of the synthesis conditions on the crystallinity and surface acidity of SAPO-11. J. Mol. Catal. A Chem. 1995, 98, 35–48. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, W.; Chang, W.; Pan, S.; Tian, Z.; Meng, X.; Rigutto, M.; van der Made, A.; Zhao, L.; Zheng, X.; et al. Catalytically active and hierarchically porous SAPO-11 zeolite synthesized in the presence of polyhexamethylene biguanidine. J. Colloid Interface Sci. 2014, 418, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Lee, K.; Choi, M. Cooperative effects of secondary mesoporosity and acid site location in Pt/SAPO-11 on n-dodecane hydroisomerization selectivity. J. Catal. 2014, 319, 232–238. [Google Scholar] [CrossRef]
- Wu, Q.; Oduro, I.N.; Huang, Y.; Fang, Y. Synthesis of hierarchical SAPO-11 via seeded crystallization. Microporous Mesoporous Mater. 2015, 218, 24–32. [Google Scholar] [CrossRef]
- McCusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Lover, D.; Scardi, P. Rietveld refinement guidelines. J. Appl. Crystallogr. 1999, 32, 36–50. [Google Scholar] [CrossRef]
- Fan, Y.; Lei, D.; Shi, G.; Bao, X. Synthesis of ZSM-5/SAPO-11 composite and its application in FCC gasoline hydro-upgrading catalyst. Catal. Today 2006, 114, 388–396. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, Z.; Song, H.; Bai, P.; Xing, W.; Yan, Z.; Zhao, L.; Zhang, Z.; Gao, X. Synthesis of hierarchical SAPO-11 for hydroisomerization reaction in refinery processes. Appl. Petrochem. Res. 2014, 4, 351–358. [Google Scholar] [CrossRef][Green Version]
- Wilson, S.T.; Lok, B.M.; Messina, C.A.; Cannan, T.R.; Flanigen, E.M. Aluminophosphate molecular sieves: A new class of microporous crystalline inorganic solids. J. Am. Chem. Soc 1982, 104, 1146–1147. [Google Scholar] [CrossRef]
- Pastore, H.; Coluccia, S.; Marchese, L. Porous Aluminophosphates: From Molecular Sieves to Designed Acid Catalysts. Annu. Rev. Mater. Res. 2005, 35, 351–395. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, X.; Chang, P.; Geng, S. Crystal morphology control of AlPO4-11 molecular sieves by microwave irradiation. Mater. Chem. Phys. 2009, 113, 899–904. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Wei, X.; Guo, F.; Li, P.; Guo, S. Synthesis of 2,6-dimethylnaphthalene over SAPO-11, SAPO-5 and Mordenite Molecular Sieves. Braz. J. Chem. Eng. 2017, 34, 295–306. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Liu, Y.; Liu, C. Effect of silicon precursor on silicon incorporation in SAPO-11 and their catalytic performance for hydroisomerization of n-octane on Pt-based catalysts. J. Energy Chem. 2017, 26, 688–694. [Google Scholar] [CrossRef]
- Meriaudeau, P.; Tuan, V.A.; Nghiem, V.T.; Lai, S.Y.; Hung, L.N.; Naccache, C. SAPO-11, SAPO-31, and SAPO-41 Molecular Sieves: Synthesis, Characterization, and Catalytic Properties in n-Octane Hydroisomerization. J. Catal. 1997, 169, 55–66. [Google Scholar] [CrossRef]
- Yu, G.; Qiu, M.; Wang, T.; Ge, L.; Chen, X.; Wei, W. Optimization of the pore structure and acidity of SAPO-11 for highly efficient hydroisomerization on the long-chain alkane. Microporous Mesoporous Mater. 2021, 320, 111076. [Google Scholar] [CrossRef]
- Mapele, R.; Silva, A.; Souza, M.; Pedrosa, A.; Coriolano, A.; Fernandes, G.; Fernandes, V.; Araujo, A. Insight in the Crystallization Kinetics of AlPO4-11 Molecular Sieve Using Di-Isopropylamine as Template. Appl. Sci. 2021, 11, 6544. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, D.; Zhao, L.; Peng, P.; Wang, X.; Li, L.; Yu, S.; Liu, S.; Liu, X.; Yan, Z. Effect of fluoride ions on the stability of SAPO-11 molecular sieves. Microporous Mesoporous Mater. 2020, 306, 110461. [Google Scholar] [CrossRef]
- Sheng, N.; Xu, H.; Liu, X.; Chu, Y.; Han, S.; Meng, X.; Liu, Y.; Liu, C.; Xiao, F.-S. Self-formation of hierarchical SAPO-11 molecular sieves as an efficient hydroisomerization support. Catal. Today 2019, 350, 165–170. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Tiuliukova, I.; Rudina, N.; Lysikov, A.; Cherepanova, S.; Parkhomchuk, E. Screw-like morphology of silicoaluminophosphate-11 (SAPO-11) crystallized in ethanol medium. Mater. Lett. 2018, 228, 61–64. [Google Scholar] [CrossRef]
- Li, L.; Shen, K.; Huang, X.; Lin, Y.; Liu, Y. SAPO-11 with preferential growth along the a-direction as an improved active catalyst in long-alkane isomerization reaction. Microporous Mesoporous Mater. 2020, 313, 110827. [Google Scholar] [CrossRef]
- Sinha, A.K.; Seelan, S. Chacacterização of SAPO-11 and SAPO-31 synthesized from aqueous and non-aqueous media. Appl. Catal. A Gen. 2004, 270, 245–252. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, H.; Yue, Y.; Zhu, H.; Bao, X. Direct synthesis of hierarchical SAPO-11 molecular sieve with enhanced hydroisomerization performance. Fuel Process. Technol. 2018, 179, 72–85. [Google Scholar] [CrossRef]
- Graetsch, H. Two forms of aluminium phosphate tridymite from X-ray powder data. Acta Cryst. 2000, C56, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Grenev, I.V.; Klimkin, N.D.; Shamanaeva, I.A.; Shubin, A.A.; Chetyrin, I.A.; Gavrilov, V.Y. A novel adsorption-based method for revealing the Si distribution in SAPO molecular sieves: The case of SAPO-11. Microporous Mesoporous Mater. 2021, 328, 111503. [Google Scholar] [CrossRef]



| Sample | Al Source | Time (h) | Residue (g) |
|---|---|---|---|
| 6/SA | Al2O3 | 6 | None |
| 12/SA | Al2O3 | 12 | None |
| 24/SA | Al2O3 | 24 | None |
| 36/SA | Al2O3 | 36 | None |
| 24/SH | Al(OH)3 | 24 | None |
| 48/SH | Al(OH)3 | 48 | None |
| 24/SHR0.5 | Al(OH)3 | 24 | 0.5 |
| 48/SHR0.5 | Al(OH)3 | 48 | 0.5 |
| 72/SHR0.5 | Al(OH)3 | 72 | 0.5 |
| 72/SAR1 | Al2O3 | 72 | 1.0 |
| 72/SHR1 | Al(OH)3 | 72 | 1.0 |
| 24/SAR2 | Al2O3 | 24 | 2.0 |
| 48/SAR2 | Al2O3 | 48 | 2.0 |
| Composition | Quantities (%) |
|---|---|
| CaO | 48.71 |
| P2O5 | 27.7 |
| SiO2 | 9.98 |
| Al2O3 | 8.9 |
| MgO | 1.7 |
| Cl | 0.83 |
| Fe2O3 | 0.55 |
| K2O | 0.48 |
| SrO | 0.34 |
| Sb2O3 | 0.3 |
| MnO | 0.24 |
| TiO2 | 0.1 |
| V2O5 | 0.05 |
| PbO | 0.05 |
| Samples | Molar Ratio | Yield † (%) | |
|---|---|---|---|
| P2O5/SiO2 | Si/Al | ||
| Residue | 2.35 | 0.51 | - |
| 6/SA | 1.8 | 0.23 | - |
| 12/SA | 1.08 | 0.23 | 55 |
| 24/SA | 1.93 | 0.16 | 68 |
| 36/SA | 2.08 | 0.12 | 72 |
| 24/SH | 1.85 | 0.17 | 41 |
| 48/SH | 1.65 | 0.17 | 38 |
| 24/SHR0.5 | 3.8 | 0.11 | 63 |
| 48/SHR0.5 | 3.8 | 0.13 | 64 |
| 72/SHR0.5 | 3.6 | 0.14 | 75 |
| 72/SHR1 | 3.33 | 0.22 | n.d. |
| 24/SAR2 | 2.14 | 0.30 | n.d. |
| 48/SAR2 | 2.41 | 0.27 | n.d. |
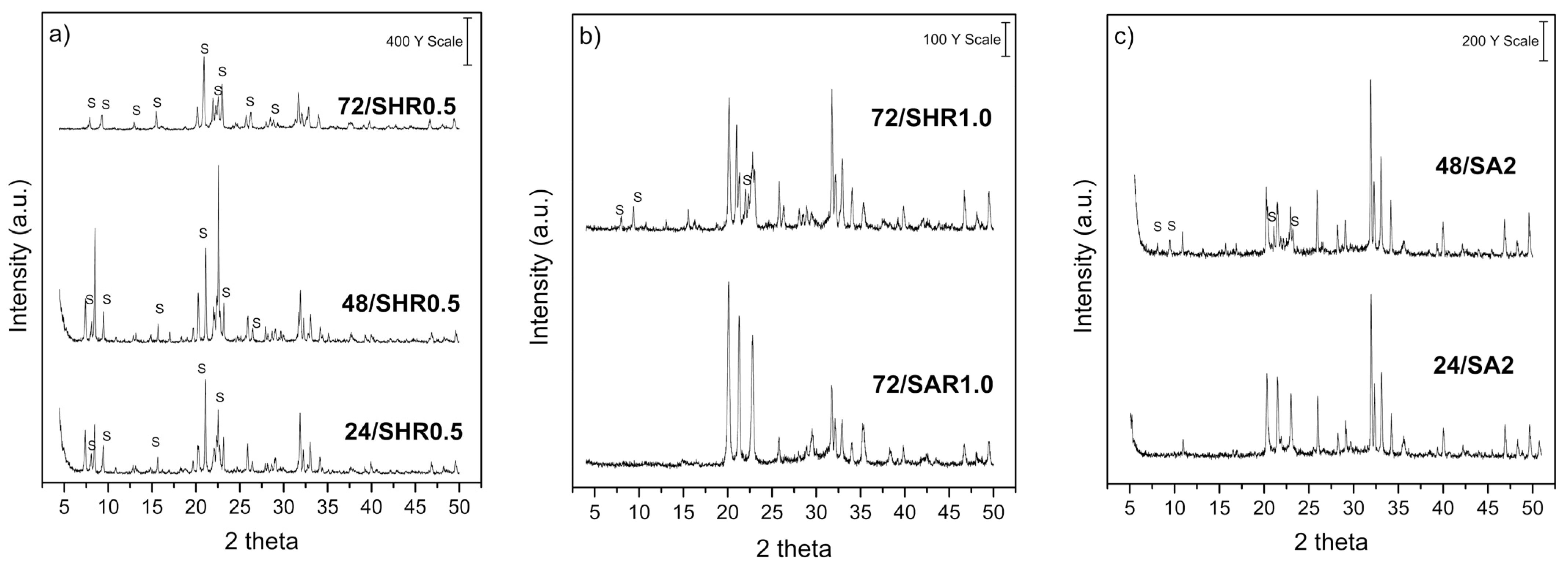
| Samples | Identified Phases (%) | ||||
|---|---|---|---|---|---|
| SAPO-11 | SAPO-31 | Calcium Phosphate (V) Fluoride Chloride | Aluminum Phosphate (V) | Aluminum Phosphate Tridymite | |
| 24/SA | 77.51 | n.d. | n.d. | 3.39 | 19.10 |
| 24/SH | 35.39 | n.d. | n.d. | 64.61 | n.d. |
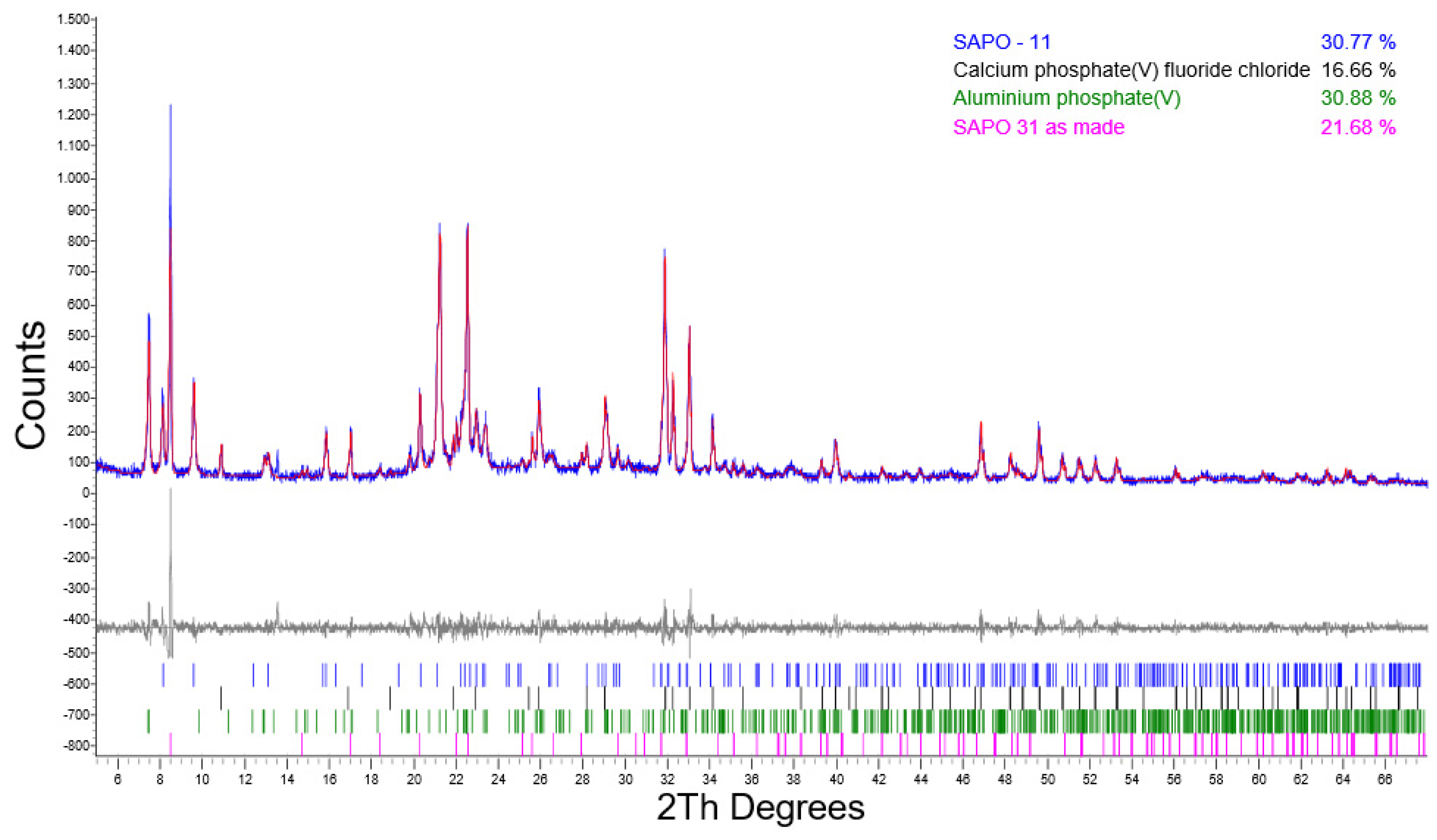
| 24/SHR0.5 | 30.77 | 21.68 | 16.66 | 30.88 | n.d. |
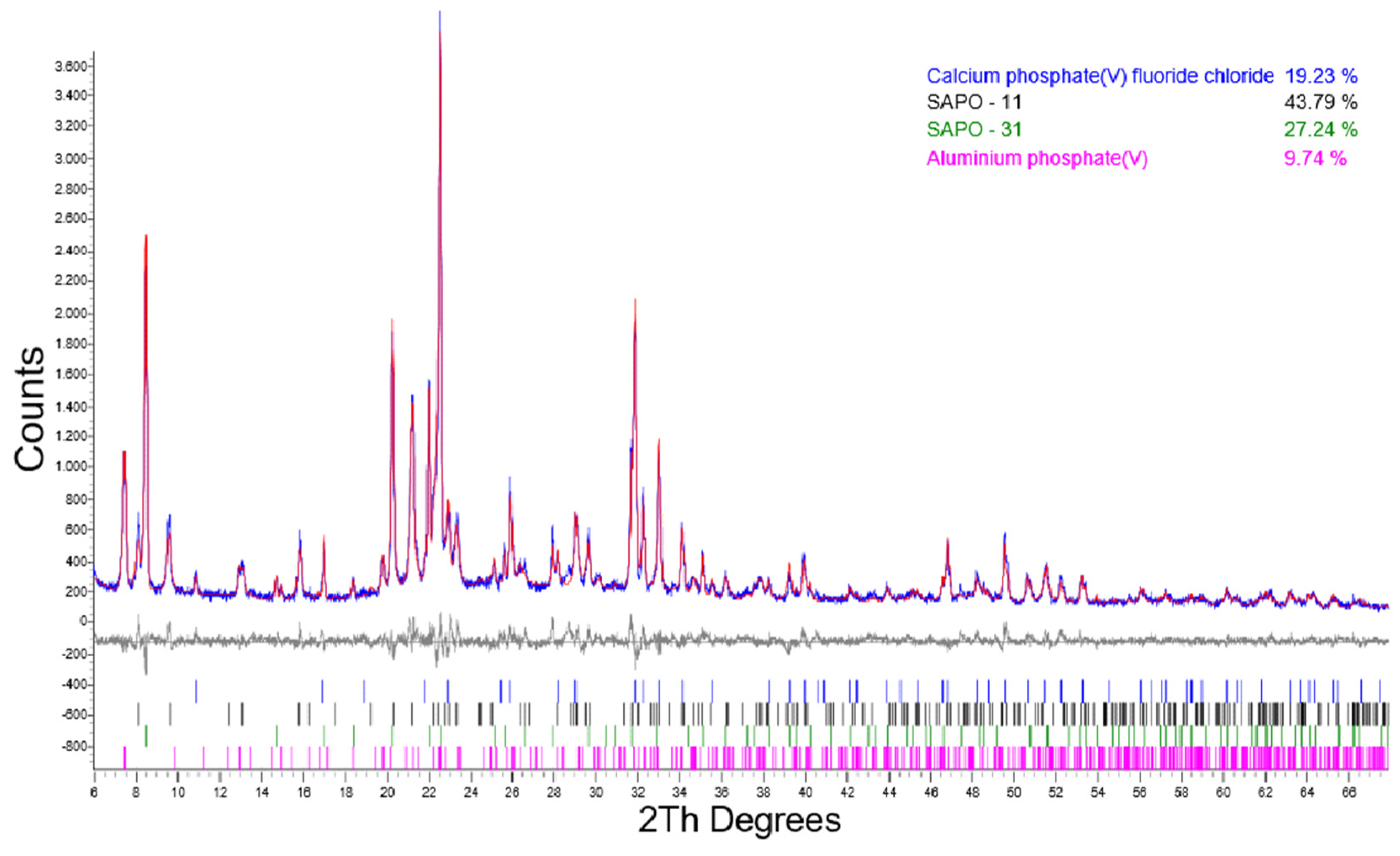
| 48/SHR0.5 | 43.79 | 27.24 | 19.23 | 9.74 | n.d. |
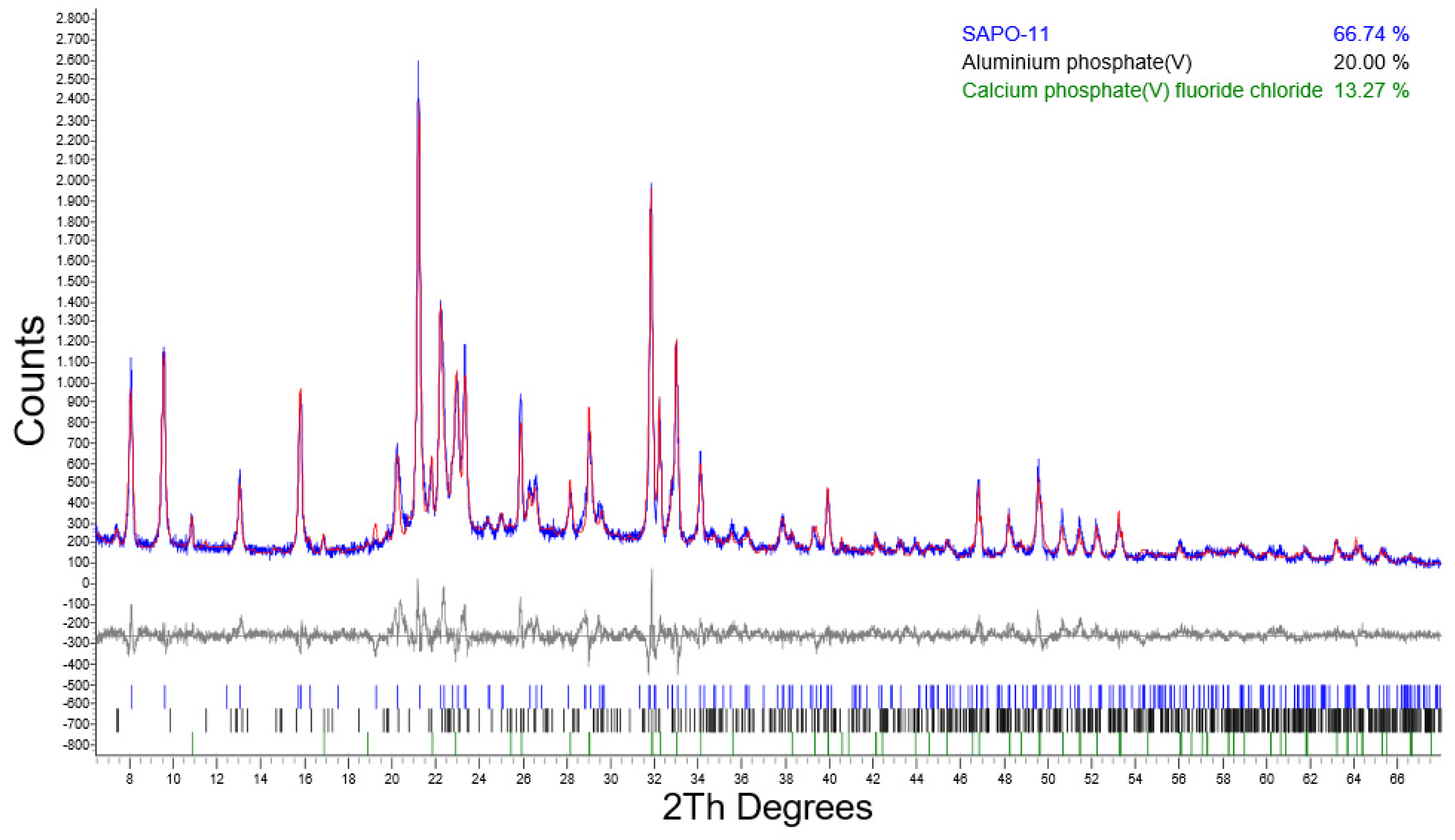
| 72/SHR0.5 | 66.74 | n.d. | 13.27 | 20.00 | n.d. |
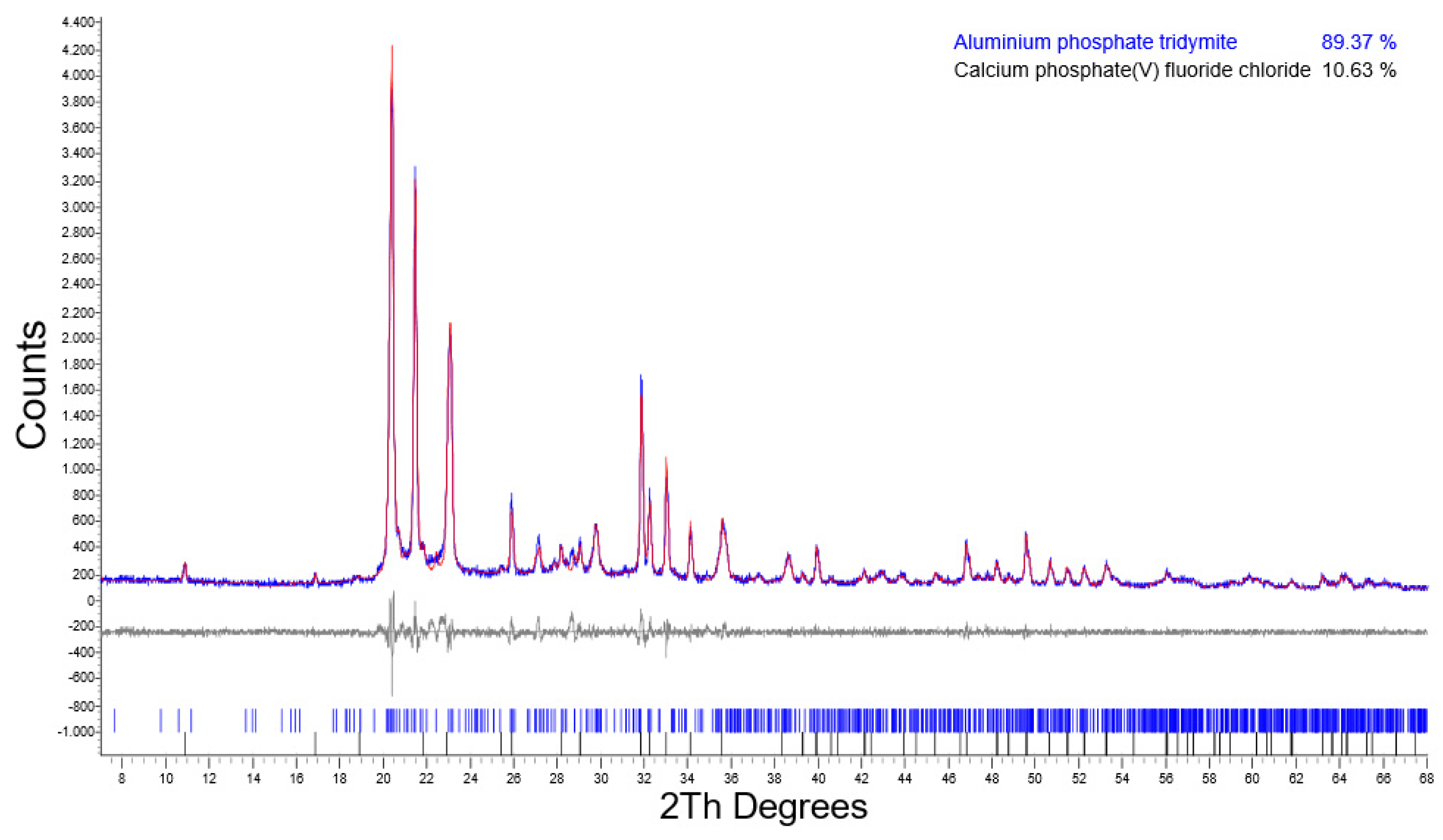
| 72/SHR1 | 37.36 | n.d. | 5.32 | n.d. | 57.32 |
| 72/SAR1 | n.d. | n.d. | 10.63 | n.d. | 89.37 |
| SAPO-11 | BET | t-Plot Harkins-Jura-de-Boer | Gurvich VTP | |
|---|---|---|---|---|
| Samples | SBET (m2/g) | Vo (cm3/g) | St (m2/g) | (cm3/g) |
| 24/SA | 100 | 0.02 | 50 | 0.15 |
| 24/SH | 187 | 0.05 | 56 | 0.13 |
| 24/SHR0.5 | 97 | 0.03 | 20 | 0.08 |
| 48/SHR0.5 | 87 | 0.02 | 31 | 0.06 |
| 72/SHR0.5 | 113 | 0.03 | 26 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, G.C.C.S.; Mello, M.I.S.; Bieseki, L.; Araujo, A.S.; Pergher, S.B.C. Hydrothermal Synthesis of Silicoaluminophosphate with AEL Structure Using a Residue of Fluorescent Lamps as Starting Material. Molecules 2021, 26, 7366. https://doi.org/10.3390/molecules26237366
Lima GCCS, Mello MIS, Bieseki L, Araujo AS, Pergher SBC. Hydrothermal Synthesis of Silicoaluminophosphate with AEL Structure Using a Residue of Fluorescent Lamps as Starting Material. Molecules. 2021; 26(23):7366. https://doi.org/10.3390/molecules26237366
Chicago/Turabian StyleLima, Gidiângela C. C. S., Mariele I. S. Mello, Lindiane Bieseki, Antonio S. Araujo, and Sibele B. C. Pergher. 2021. "Hydrothermal Synthesis of Silicoaluminophosphate with AEL Structure Using a Residue of Fluorescent Lamps as Starting Material" Molecules 26, no. 23: 7366. https://doi.org/10.3390/molecules26237366
APA StyleLima, G. C. C. S., Mello, M. I. S., Bieseki, L., Araujo, A. S., & Pergher, S. B. C. (2021). Hydrothermal Synthesis of Silicoaluminophosphate with AEL Structure Using a Residue of Fluorescent Lamps as Starting Material. Molecules, 26(23), 7366. https://doi.org/10.3390/molecules26237366