Imaging-Guided Delivery of a Hydrophilic Drug to Eukaryotic Cells Based on Its Hydrophobic Ion Pairing with Poly(hexamethylene guanidine) in a Maleated Chitosan Carrier
Abstract
:1. Introduction
2. Results and Discussion
2.1. Aggregation Studies in Poly(hexamethylene guanidine)–Ceftriaxone–Dye System
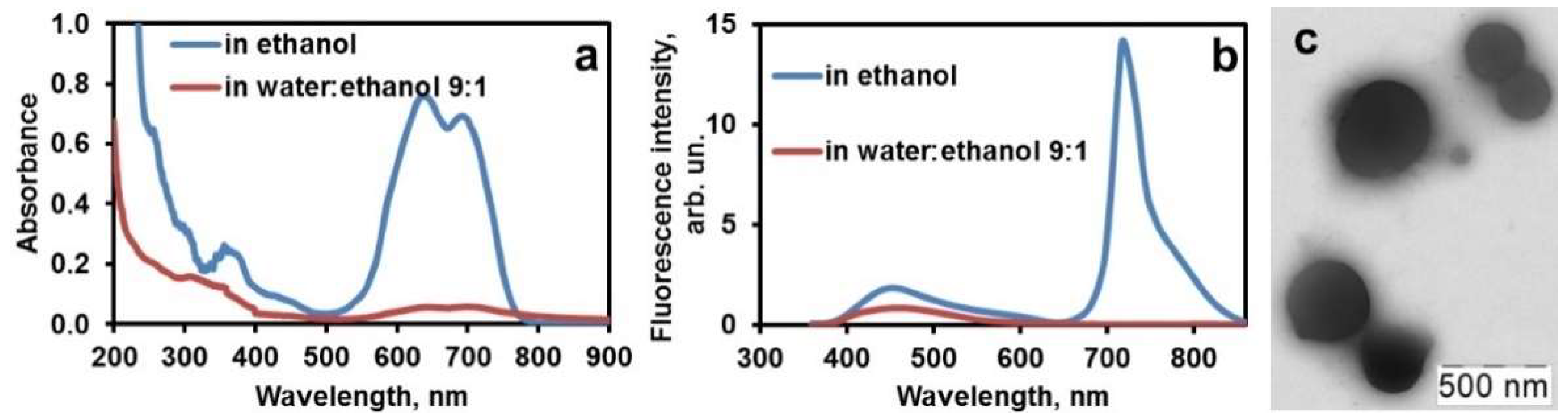
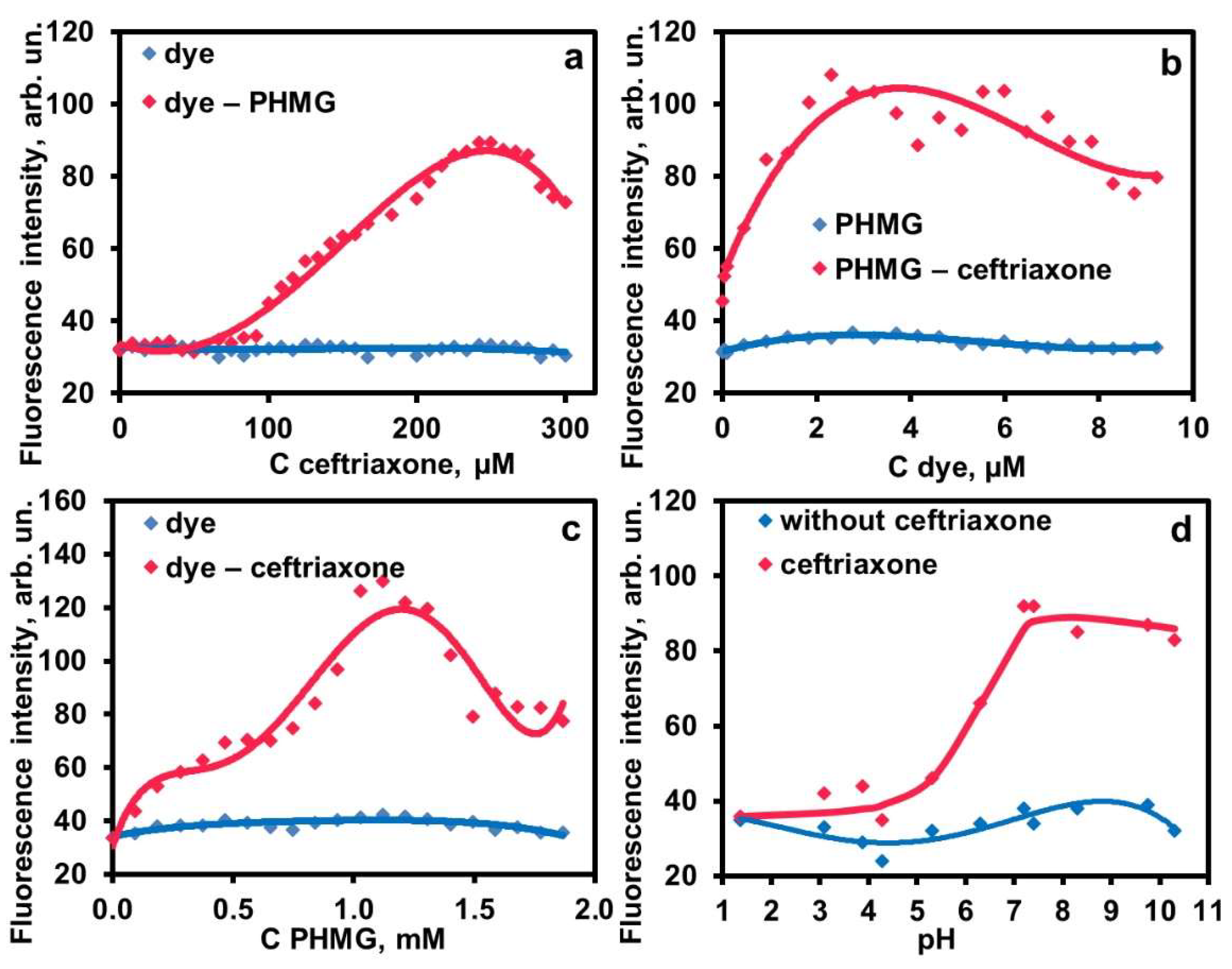
2.1.1. Quenching of the Dye Fluorescence in Water
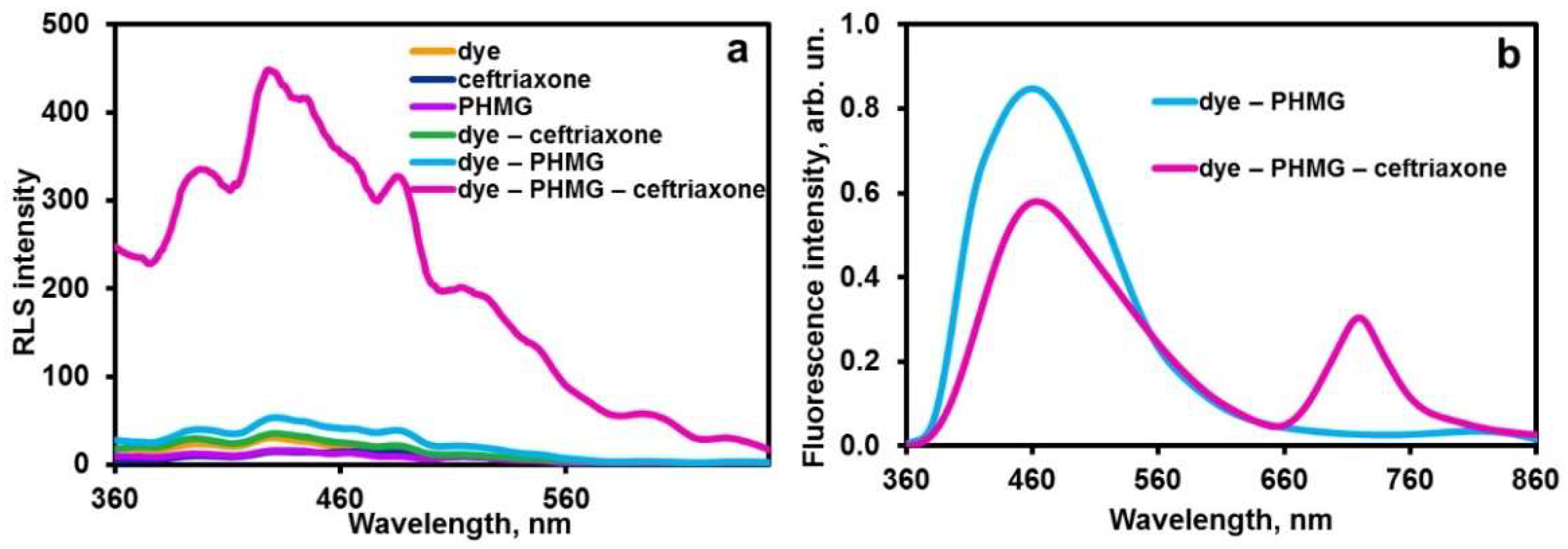
2.1.2. Aggregation in the Ternary System PHMG–Ceftriaxone–Dye
2.2. Encapsulation of Ternary Aggregates in Chitosan Containers
2.2.1. Anated Chitosans Used in Developing Containers
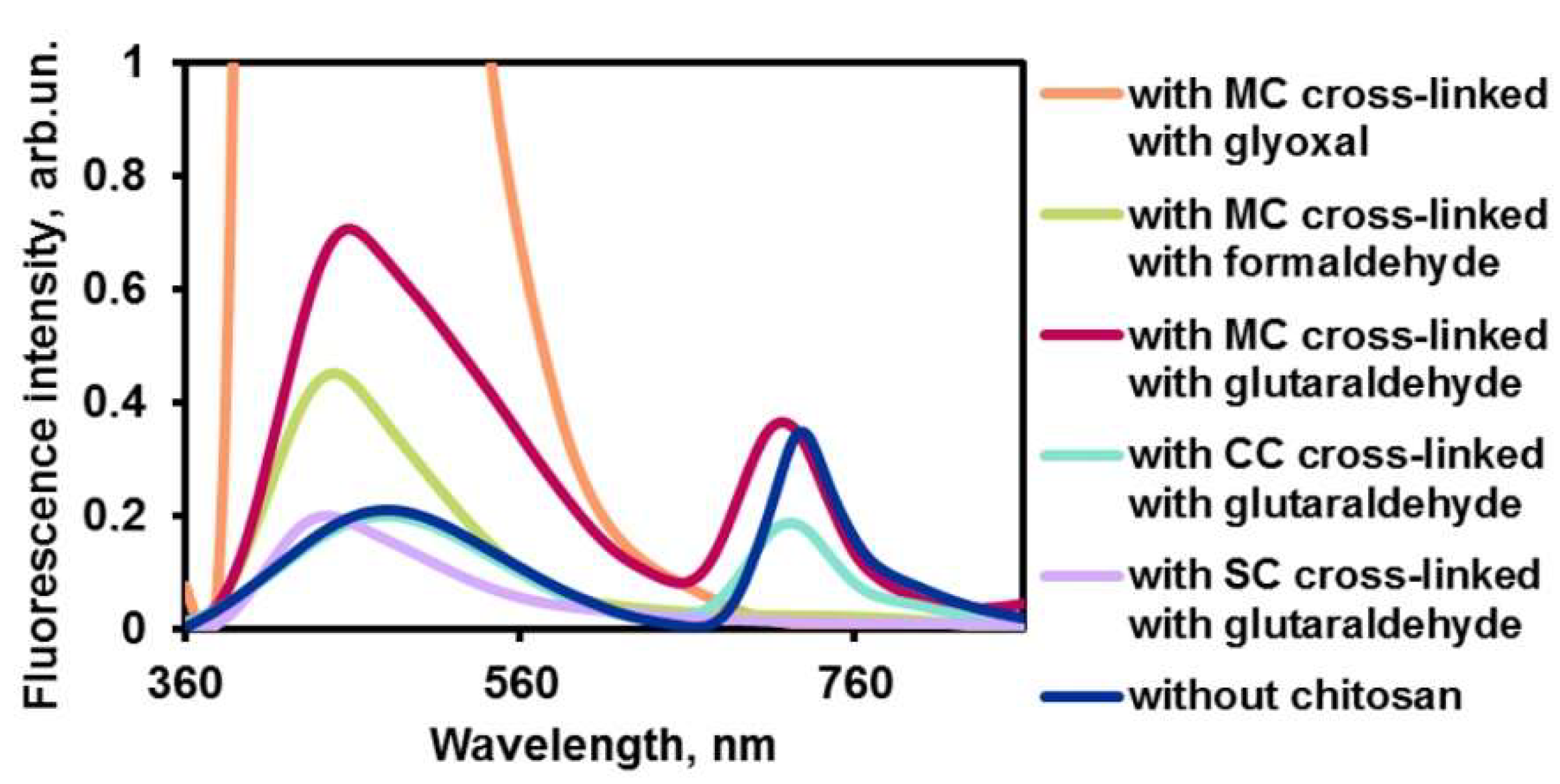
2.2.2. Chitosan Cross-Linking
2.3. Characterization of Poly(hexamethylene guanidine)–Ceftriaxone–Dye–Maleated Chitosan Containers
2.3.1. Morphology and Size of Container Particles
2.3.2. Zeta Potentials
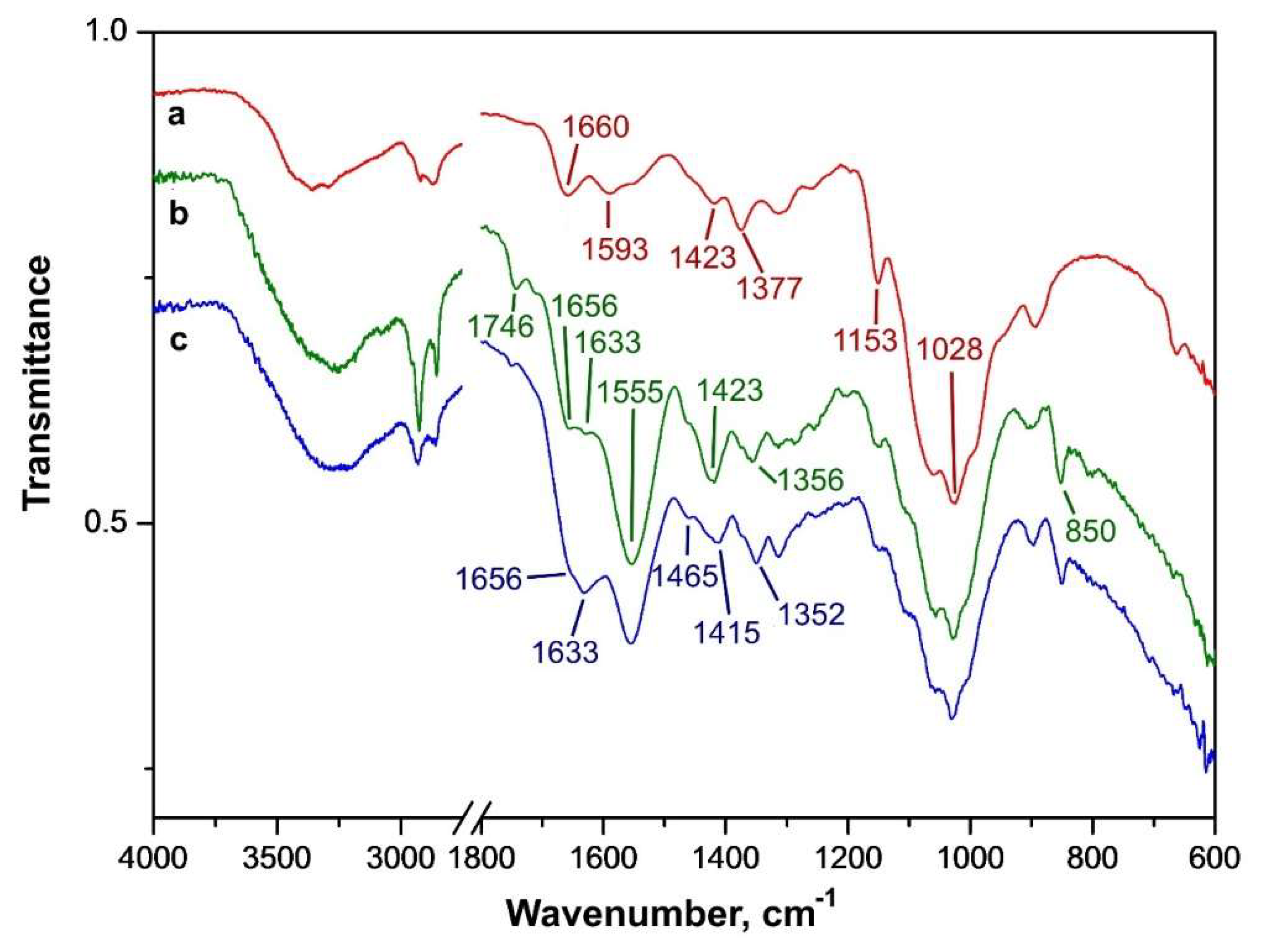
2.3.3. FT-IR Spectra
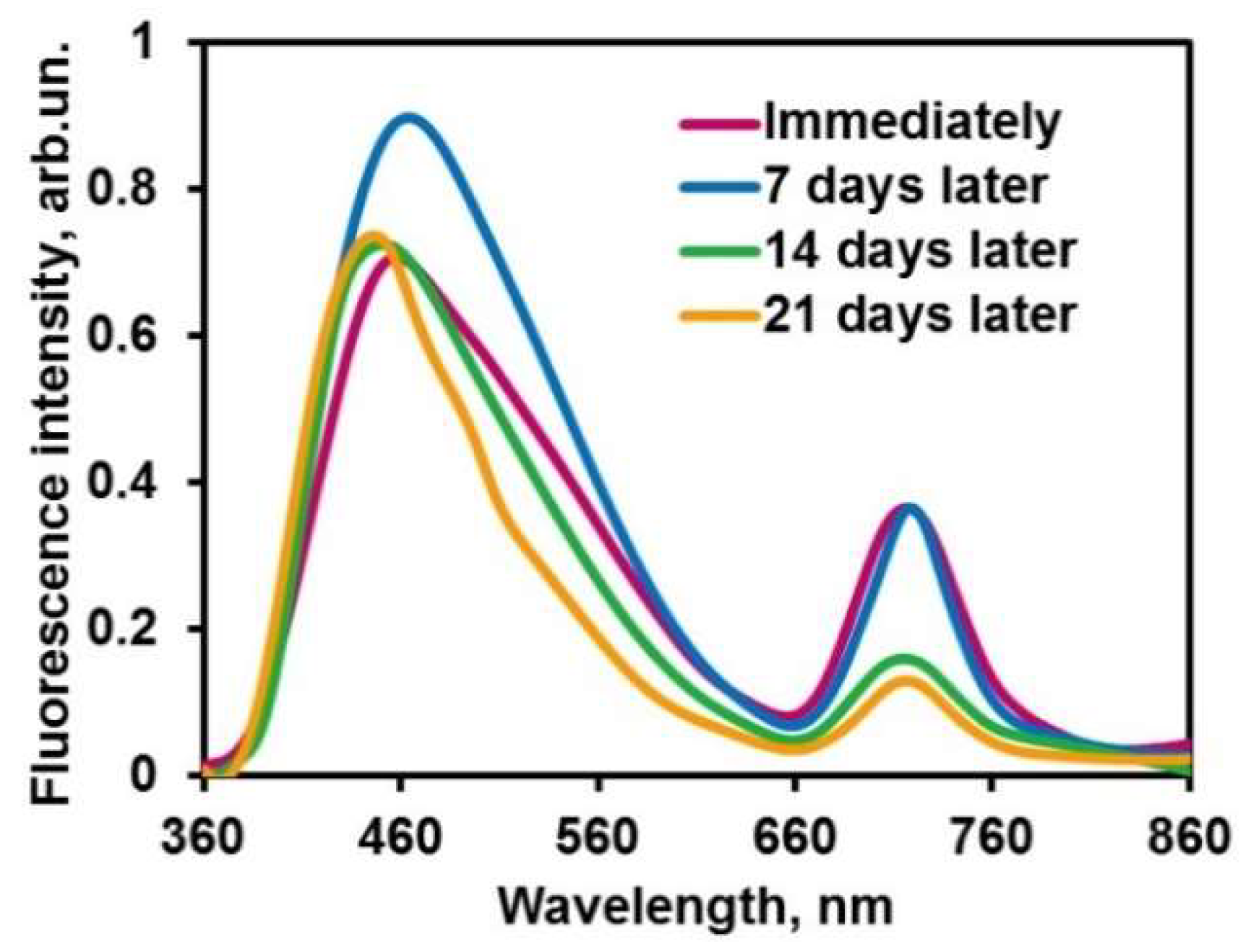
2.3.4. Kinetic Stability
2.3.5. Drug Loading Capacity
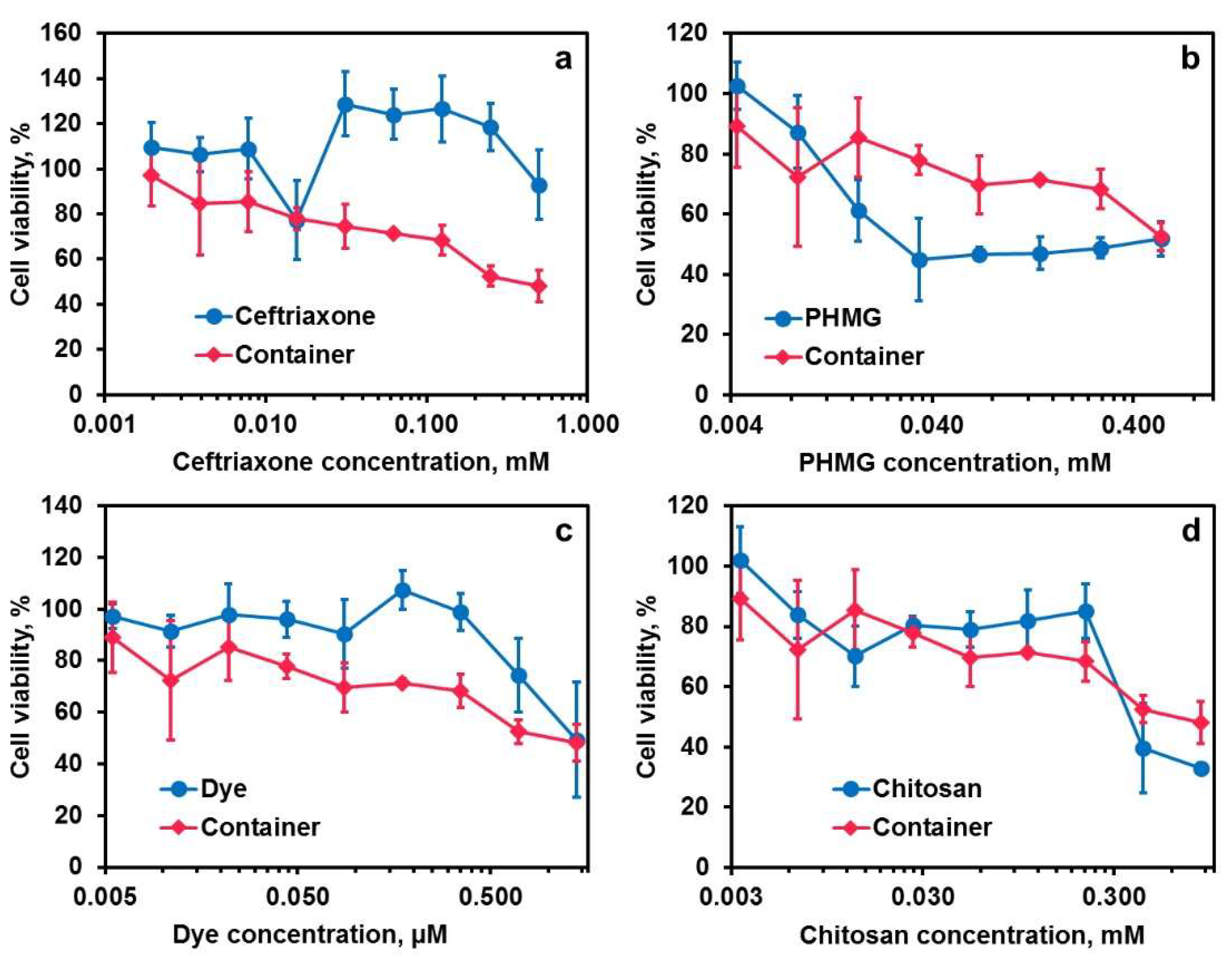
2.4. Cytotoxicity Measurements
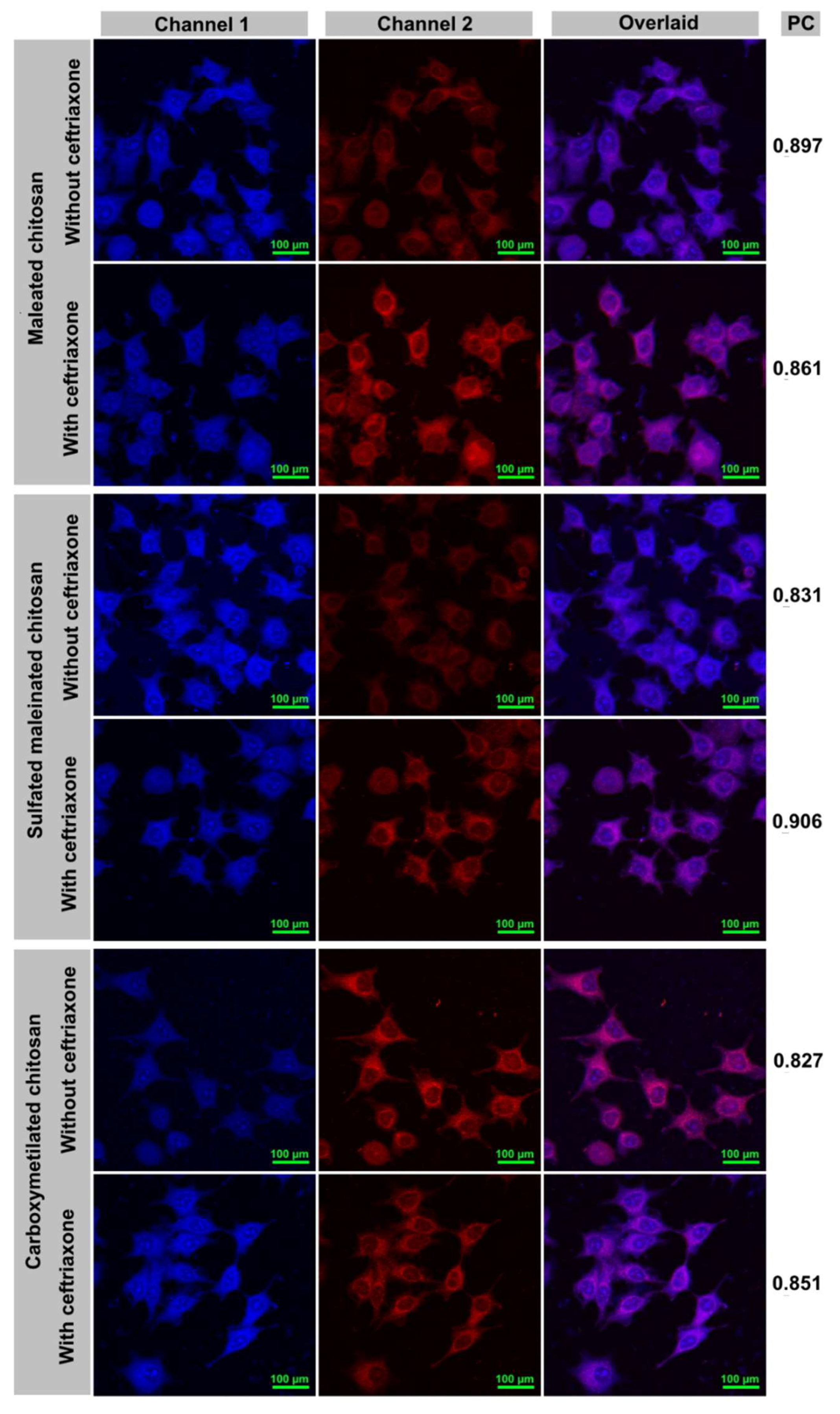
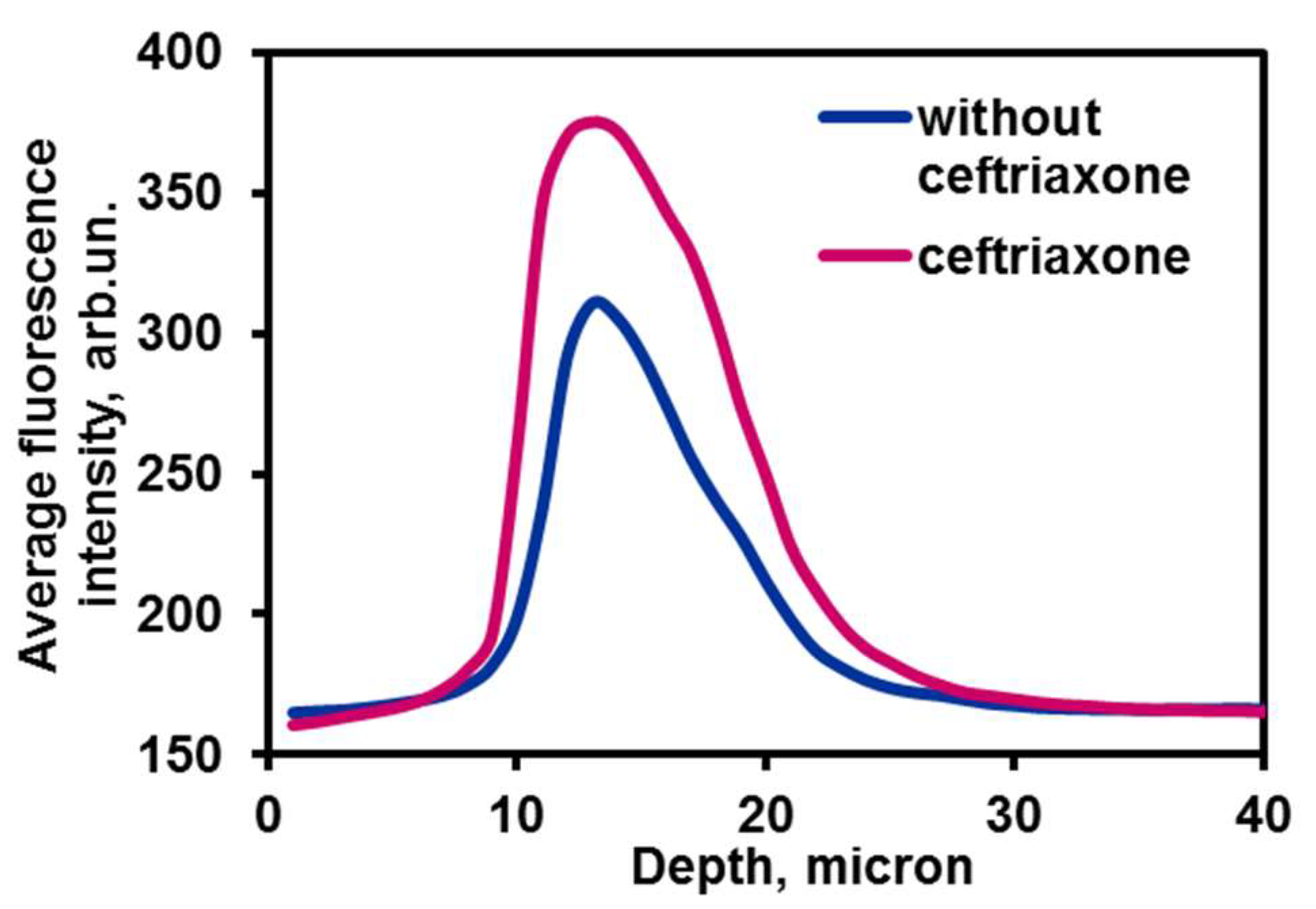
2.5. Chitosan Containers Uptake by Breast Adenocarcinoma Cells
3. Materials and Methods
3.1. Compounds
3.2. Instrumentation
3.3. Preparation of Maleated Chitosan
3.4. Preparation of Sulfated Maleated Chitosan
3.5. Preparation of Carboxymethylated Chitosan
3.6. Labeling of Chitosans with Rhodamine B
3.7. Preparation of Containers Dye–PHMG–Ceftriaxone–Chitosan
3.8. Cell Culture and Endocytosis
3.9. Cytotoxicity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A. Estimation of the Stability Constant of Aggregate Сeftriaxone–PHMG–Dye
References
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug. Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Xu, X.-D.; Zhao, L.; Qu, Q.; Wang, J.-G.; Shi, H.; Zhao, Y. Imaging-guided drug release from glutathione-responsive supramolecular porphysome nanovesicles. ACS Appl. Mater. Interfaces 2015, 7, 17371–17380. [Google Scholar] [CrossRef]
- Carmona, T.; Marcelo, G.; Rinaldi, L.; Martina, K.; Cravotto, G.; Mendicuti, F. Soluble cyanine dye/β-cyclodextrin derivatives: Potential carriers for drug delivery and optical imaging. Dyes Pigm. 2015, 114, 204–214. [Google Scholar] [CrossRef]
- Wu, J.B.; Shi, C.; Chu, G.C.; Xu, Q.; Zhang, Y.; Li, Q.; Yu, J.S.; Zhau, H.E.; Chung, L.W. Near-infrared fluorescence heptamethine carbocyanine dyes mediate imaging and targeted drug delivery for human brain tumor. Biomaterials 2015, 67, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hassan, U.A.; Hussein, M.Z.; Alitheen, N.B.; Yahya Ariff, S.A.; Masarudin, M.J. In vitro cellular localization and efficient accumulation of fluorescently tagged biomaterials from monodispersed chitosan nanoparticles for elucidation of controlled release pathways for drug delivery systems. Int. J. Nanomed. 2018, 13, 5075–5095. [Google Scholar] [CrossRef] [Green Version]
- Feng, T.; Ai, X.; An, G.; Yang, P.; Zhao, Y. Charge-convertible carbon dots for imaging-guided drug delivery with enhanced in vivo cancer therapeutic efficiency. ACS Nano 2016, 10, 4410–4420. [Google Scholar] [CrossRef]
- Cui, W.; Lu, X.; Cui, K.; Wu, J.; Wei, Y.; Lu, Q. Fluorescent nanoparticles of chitosan complex for real-time monitoring drug release. Langmuir 2011, 27, 8384–8390. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for hydrophobic drug delivery. classification, synthesis and applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Park, M.K.; Deng, S.; Advincula, R.C. Sustained release control via photo-cross-linking of polyelectrolyte layer-by-layer hollow capsules. Langmuir 2005, 21, 5272–5277. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mukherjee, S.; Yi, J.; Banerjee, P.; Chen, Q.; Zhou, S. Biocompatible chitosan-carbon dot hybrid nanogels for NIR-imaging-guided synergistic photothermal-chemo therapy. ACS Appl. Mater. Interfaces 2017, 9, 18639–18649. [Google Scholar] [CrossRef]
- Lim, E.K.; Sajomsang, W.; Choi, Y.; Jang, E.; Lee, H.; Kang, B.; Kim, E.; Haam, S.; Suh, J.S.; Chung, S.J.; et al. Chitosan-based intelligent theragnosis nanocomposites enable pH-sensitive drug release with MR-guided imaging for cancer therapy. Nanoscale Res. Lett. 2013, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.S.; Dong, Z.; Pauletti, G.M.; Zhang, J.; Xu, H.; Gu, H.; Wang, L.; Ewing, R.C.; Huth, C.; Wang, F.; et al. Fluorescent, superparamagnetic nanospheres for drug storage, targeting, and imaging: A multifunctional nanocarrier system for cancer diagnosis and treatment. ACS Nano 2010, 4, 5398–5404. [Google Scholar] [CrossRef]
- Kozuch, D.J.; Ristroph, K.; Prud’homme, R.K.; Debenedetti, P.G. Insights into hydrophobic ion pairing from molecular simulation and experiment. ACS Nano 2020, 14, 6097–6106. [Google Scholar] [CrossRef] [PubMed]
- Maha, A.H.; Al-Dujaili, E. Hydrophobic ion-paired drug delivery system: A review. Indian Drugs 2020, 57, 7–18. [Google Scholar] [CrossRef]
- Asfour, M.H.; Kassem, A.A.; Salama, A.; Abd El-Alim, S.H. Hydrophobic ion pair loaded self-emulsifying drug delivery system (SEDDS): A novel oral drug delivery approach of cromolyn sodium for management of bronchial asthma. Int. J. Pharm. 2020, 585, 119494. [Google Scholar] [CrossRef] [PubMed]
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A Review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, A.; Bernkop-Schnürch, A. SEDDS: A game changing approach for the oral administration of hydrophilic macromolecular drugs. Adv. Drug Deliv. Rev. 2019, 142, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-H.; Peng, C.-L.; Yang, S.-J.; Shieh, M.-J. Photothermal, targeting, theranostic near-infrared nanoagent with SN38 against colorectal cancer for chemothermal therapy. Mol. Pharm. 2017, 14, 2766–2780. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Zakharenkova, S.A.; Katkova, E.A.; Doroshenko, I.A.; Kriveleva, A.S.; Lebedeva, A.N.; Vidinchuk, T.A.; Shik, A.V.; Abramchuk, S.S.; Podrugina, T.A.; Beklemishev, M.K. Aggregation-based fluorescence amplification strategy: “turn-on” sensing of aminoglycosides using near-IR carbocyanine dyes and pre-micellar surfactants. Spectr. Acta A 2021, 247, 119109. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Hu, X.; Dong, X.; Lu, Y.; Lu, H.; Zhao, W.; Wu, W. Towards more accurate bioimaging of drug nanocarriers: Turning aggregation-caused quenching into a useful tool. Adv. Drug Deliv. Rev. 2019, 143, 206–225. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [Green Version]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Mater. Sci. Eng. C 2017, 77, 1349–1362. [Google Scholar] [CrossRef] [Green Version]
- Maji, P.; Gandhi, A.; Jana, S.; Maji, N. Preparation and characterization of maleic anhydride cross-linked chitosan-polyvinyl alcohol hydrogel matrix transdermal patch. J. Pharm. Sci. Tech. 2013, 2, 62–67. [Google Scholar]
- Wei, D.; Ma, Q.; Guan, Y.; Hu, F.; Zheng, A.; Zhang, X.; Teng, Z.; Jiang, H. Structural characterization and antibacterial activity of oligoguanidine (polyhexamethylene guanidine hydrochloride). Mater. Sci. Eng. C 2009, 29, 1776–1780. [Google Scholar] [CrossRef]
- Likhachev, K.V.; Ovcharenko, E.O.; Dityuk, A.I.; Abramchuk, S.S.; Efimov, K.M.; Beklemishev, M.K. Fluorescent determination of Poly(hexamethylene Guanidine) via the aggregates it forms with quantum dots and magnetic nanoparticles. Microchim. Acta 2016, 183, 1079–1087. [Google Scholar] [CrossRef]
- Asiedu-Gyekye, I.J.; Mahmood, S.A.; Awortwe, C.; Nyarko, A.K. A preliminary safety evaluation of polyhexamethylene guanidine hydrochloride. Int. J. Toxicol. 2014, 33, 523–531. [Google Scholar] [CrossRef]
- Yue, X.; Liu, L.; Wu, Y.; Liu, X.; Li, S.; Zhang, Z.; Han, S.; Wang, X.; Chang, Y.; Bai, H.; et al. Preparation and evaluation of chitosan-polyvinyl alcohol/polyhexamethylene guanidine hydrochloride antibacterial dressing to accelerate wound healing for infectious skin repair. Ann. Transl. Med. 2021, 9, 482. [Google Scholar] [CrossRef]
- Chen, J.; Wei, D.; Gong, W.; Zheng, A.; Guan, Y. Hydrogen-bond assembly of polyvinyl alcohol and polyhexamethylene guanidine for non-leaching and transparent antimicrobial films. ACS Appl. Mater. Interfaces 2018, 10, 37535–37543. [Google Scholar] [CrossRef]
- Ceftriaxone. Available online: https://go.drugbank.com/drugs/DB01212 (accessed on 8 October 2021).
- Kuntz, I.D., Jr.; Gasparro, F.P.; Johnston, M.D., Jr.; Taylor, R.P. Molecular interactions and the Benesi-Hildebrand equation. J. Am. Chem. Soc. 1968, 90, 4778–4781. [Google Scholar] [CrossRef]
- Radulović, D.; Vujić, Z.; Vasiljević, M. Spectrophotometric Investigation of the celiprolol-hydrochloride ion-pair. Spectrosc. Lett. 1994, 27, 503–513. [Google Scholar] [CrossRef]
- Singh, R.P.; Yeboah, Y.D.; Pambid, E.R.; Debayle, P. Stability constant of the calcium-citrate(3-) ion pair complex. J. Chem. Eng. Data 1991, 36, 52–54. [Google Scholar] [CrossRef]
- Ge, H.C.; Luo, D.K. Preparation of carboxymethyl chitosan in aqueous solution under microwave irradiation. Carbohydr. Res. 2005, 340, 1351–1356. [Google Scholar] [CrossRef]
- Gamzazade, A.I.; Nasibov, S.M. A Method for Obtaining Sulfated Chitosan. Russ. Patent No 2048475, 20 November 1995. Available online: https://patenton.ru/patent/RU2048475C1 (accessed on 8 October 2021).
- Kim, K.W.; Thomas, R.L.; Lee, C.; Park, H.J. Antimicrobial activity of native chitosan, degraded chitosan, and O-carboxymethylated chitosan. J. Food Prot. 2003, 66, 1495–1498. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.; Li, D.; Lang, J.; Zai, S.; Hao, J.; Wang, X. Inhibition of amphiphilic N-alkyl-O-carboxymethyl chitosan derivatives on Alternaria macrospora. Biomed. Res. Int. 2018, 2018, 5236324. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, H.; Kai, W.; Inoue, Y. Preparation and characterization of poly(ethylene glycol) crosslinked chitosan films. J. Appl. Polym. Sci. 2008, 107, 3823–3830. [Google Scholar] [CrossRef]
- Ding, Y.; Yin, H.; Shen, S.; Sun, K.; Liu, F. Chitosan-based magnetic/fluorescent nanocomposites for cell labelling and controlled drug release. New J. Chem. 2017, 41, 1736–1743. [Google Scholar] [CrossRef]
- Yasmeen, S.; Kabiraz, M.K.; Saha, B.; Qadir, M.; Gafur, M.; Masum, S.M. Chromium (VI) ions removal from tannery effluent using chitosan—Microcrystalline cellulose composite as adsorbent. Intern. Res. J. Pure Appl. Chem. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Ravishankar, K.; Shelly, K.M.; Desingh, R.P.; Subramaniyam, R.; Narayanan, A.; Dhamodharan, R. Green, solid-state synthesis of maleated chitosan and ionotropic gelation with chitosan. ACS Sustain. Chem. Eng. 2018, 6, 15191–15200. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Hanafiah, M.A.; Yong, S.S. Adsorption of humic acid from aqueous solutions on crosslinked chitosan-epichlorohydrin beads: Kinetics and isotherm studies. Colloids Surf. B 2008, 65, 18–24. [Google Scholar] [CrossRef]
- Da Trindade, M.T.; Salgado, H.R.N. Development and validation of a modern and stability-indicating method for the quantification of ceftriaxone sodium in powder for injection by infrared spectroscopy. Phys. Chem. 2017, 7, 55–62. [Google Scholar] [CrossRef]
- Cao, C.; Wu, K.; Yuan, W.; Zhang, Y.; Wang, H. Synthesis of non-water soluble polymeric guanidine derivatives and application in preparation of antimicrobial regenerated cellulose. Fibers Polym. 2017, 18, 1040–1047. [Google Scholar] [CrossRef]
- Mendes, A.C.; Gorzelanny, C.; Halter, N.; Schneider, S.W.; Chronakis, I.S. Hybridelectrospun chitosan-phospholipids nanofibers for transdermal drugdelivery. Int. J. Pharm. 2016, 510, 48–56. [Google Scholar] [CrossRef]
- Katas, H.; Raja, M.A.G.; Lam, K.L. Development of chitosan nanoparticles as astable drug delivery system for protein/siRNA. Int. J. Biomater. 2013, 2013, 1–9. [Google Scholar]
- Li, W.; Zhou, J.; Xu, Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.-N.; Zerin, T.; Podder, B.; Song, H.-Y.; Kim, Y.-S. Cytotoxicity and gene expression profiling of polyhexamethylene guanidine hydrochloride in human alveolar A549 cells. Toxicol. Vitr. 2014, 28, 684–692. [Google Scholar] [CrossRef]
- Wiesli, M.G.; Kaiser, J.P.; Gautier, E.; Wick, P.; Maniura-Weber, K.; Rottmar, M.; Wahl, P. Influence of ceftriaxone on human bone cell viability and in vitro mineralization potential is concentration- and time-dependent. Bone Joint Res. 2021, 10, 218–225. [Google Scholar] [CrossRef]
- Anitha, A.; Maya, S.; Deepa, N.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Curcumin-loaded N,O-carboxymethyl chitosan nanoparticles for cancer drug delivery. J. Biomater. Sci. Polym. Ed. 2012, 23, 1381–1400. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.K.; Mallick, S.K.; Santra, S.; Maiti, T.K.; Ghosh, S.K.; Pramanik, P. In vitro evaluation of folic acid modified carboxymethyl chitosan nanoparticles loaded with doxorubicin for targeted delivery. J. Mater. Sci. Mater. Med. 2010, 21, 1587–1597. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Das, M.; Mishra, D.; Banerjee, I.; Sahu, S.K.; Maiti, T.K.; Pramanik, P. Folate receptor targeted, carboxymethyl chitosan functionalized iron oxide nanoparticles: A novel ultradispersed nanoconjugates for bimodal imaging. Nanoscale 2011, 3, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Maya, S.; Deepa, N.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Efficient water soluble O-carboxymethyl chitosan nanocarrier for the delivery of curcumin to cancer cells. Carbohydr. Polym. 2011, 83, 452–461. [Google Scholar] [CrossRef]
- Snima, K.S.; Jayakumar, R.; Unnikrishnan, A.G.; Nair, S.V.; Lakshmanan, V.-K. O-Carboxymethyl chitosan nanoparticles for metformin delivery to pancreatic cancer cells. Carbohydr. Polym. 2012, 89, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Izco, J.M.; Torre, P.; Barcina, Y. Ripening of ossau-iraty cheese: Determination of free amino acids by RP-HPLC and of total free amino acids by the TNBS method. Food Control 2000, 11, 7–11. [Google Scholar] [CrossRef]




| Counter-Ion (P) | Concentration (or Its Range), mM, Used in Calculations | Regression Coefficients (±SD) for y =A + Bx | Number of Points | Stablitiy Constant K, ±SD | Coefficient of Determination for the Regression, R2 | |||
|---|---|---|---|---|---|---|---|---|
| Counter-Ion | Ceftriaxone | A * | B * | 1:1 | 1:2 ** | |||
| PHMG | 0.4–1.2 | 1.0 | (5.1 ± 0.8) × 10−3 | (4.3 ± 0.6) × 10−6 | 9 | (1.2 ± 0.2) × 106 | 0.93 | 0.86 |
| PHMG | 2.2 | 0.10–0.25 | (4.1 ± 0.3) × 10−3 | (1.76 ± 0.04) × 10−6 | 17 | (1.1 ± 0.1) × 106 | 0.99 | 0.97 |
| CTAB | 0.02–0.85 | 1.7 | (1.4 ± 0.2) × 10−3 | (2.4 ± 0.3) × 10−6 | 7 | (3.4 ± 0.5) × 105 | 0.96 | 0.80 |
| CTAB | 0.085 | 0.015–1.5 | (2.0 ± 0.1) × 10−2 | (3.6 ± 0.7) × 10−7 | 7 | (6.7 ± 0.7) × 105 | 0.97 | 0.89 |
| Scheme | Mean Particle Size, nm | ||
|---|---|---|---|
| by Intensity | by Volume | by Number | |
| Chitosan (maleated) | 548 | 712 | 66 |
| Chitosan–PHMG | 434 | 437 | 265 |
| Chitosan–PHMG–ceftriaxone | 1065 | 1267 | 253 |
| Chitosan–PHMG–dye | 1105 | 1238 | 207 |
| Chitosan–PHMG–ceftriaxone–dye | 1190 | 1426 | 319 |
| Excitation laser wavelength, nm | 515 | 635 |
| Registration wavelength range, nm | 540–590 (channel 1) | 655–755 (channel 2) |
| Species to be observed | Rhodamine-labeled chitosan | Dye |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharenkova, S.A.; Lebedeva, M.I.; Lebedeva, A.N.; Doroshenko, I.A.; Vlasova, K.Y.; Bartoshevich, A.A.; Senyavin, V.M.; Abramchuk, S.S.; Krivtsov, G.G.; Ezhov, A.A.; et al. Imaging-Guided Delivery of a Hydrophilic Drug to Eukaryotic Cells Based on Its Hydrophobic Ion Pairing with Poly(hexamethylene guanidine) in a Maleated Chitosan Carrier. Molecules 2021, 26, 7426. https://doi.org/10.3390/molecules26247426
Zakharenkova SA, Lebedeva MI, Lebedeva AN, Doroshenko IA, Vlasova KY, Bartoshevich AA, Senyavin VM, Abramchuk SS, Krivtsov GG, Ezhov AA, et al. Imaging-Guided Delivery of a Hydrophilic Drug to Eukaryotic Cells Based on Its Hydrophobic Ion Pairing with Poly(hexamethylene guanidine) in a Maleated Chitosan Carrier. Molecules. 2021; 26(24):7426. https://doi.org/10.3390/molecules26247426
Chicago/Turabian StyleZakharenkova, Sofia A., Marina I. Lebedeva, Alexandra N. Lebedeva, Irina A. Doroshenko, Ksenya Yu Vlasova, Anastasiya A. Bartoshevich, Vladimir M. Senyavin, Sergey S. Abramchuk, George G. Krivtsov, Alexander A. Ezhov, and et al. 2021. "Imaging-Guided Delivery of a Hydrophilic Drug to Eukaryotic Cells Based on Its Hydrophobic Ion Pairing with Poly(hexamethylene guanidine) in a Maleated Chitosan Carrier" Molecules 26, no. 24: 7426. https://doi.org/10.3390/molecules26247426
APA StyleZakharenkova, S. A., Lebedeva, M. I., Lebedeva, A. N., Doroshenko, I. A., Vlasova, K. Y., Bartoshevich, A. A., Senyavin, V. M., Abramchuk, S. S., Krivtsov, G. G., Ezhov, A. A., Podrugina, T. A., Klyachko, N. L., & Beklemishev, M. K. (2021). Imaging-Guided Delivery of a Hydrophilic Drug to Eukaryotic Cells Based on Its Hydrophobic Ion Pairing with Poly(hexamethylene guanidine) in a Maleated Chitosan Carrier. Molecules, 26(24), 7426. https://doi.org/10.3390/molecules26247426








