Boron Hydrogen Compounds: Hydrogen Storage and Battery Applications
Abstract
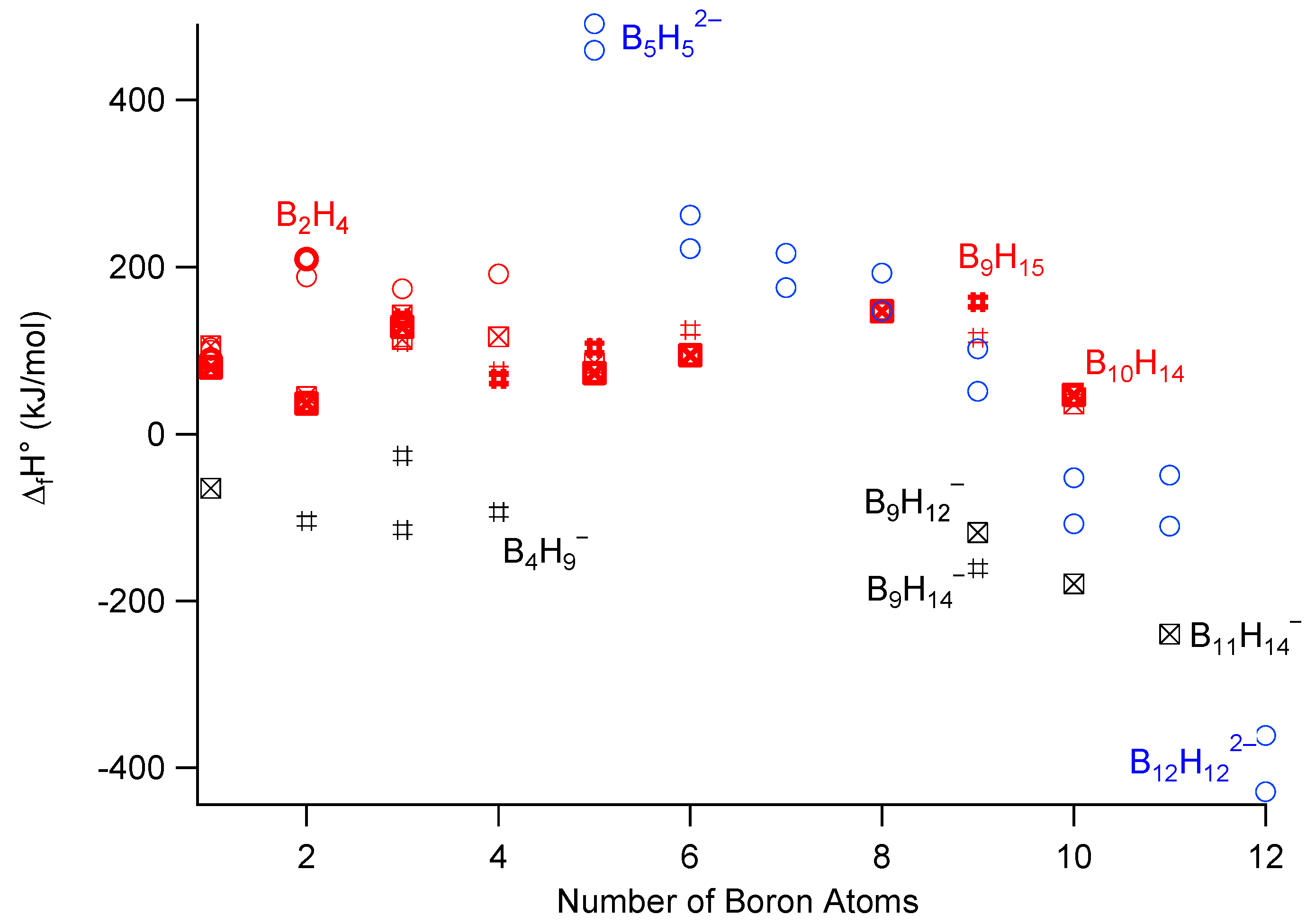
:1. Introduction
2. Magnesium Borohydride
3. DFT Calculations
4. Formation and Reactions of B3H8−
5. Closoborates and Related Species as Solid Ionic Conductors
- -
- “open structure” with a low coordination number of the mobile ion;
- -
- The presence of structural phase transitions at low pressure. In the case of AgI, the ambient pressure wurtzite structure (space group P63mc) transforms at 3 kbar and 315 K into a NaCl structure (space group Fm-3m), thus going from a rather covalent network with coordination number 4 to a rather ionic structure with coordination number 6. The associated charge fluctuations between ions can potentially be coupled to vibrational motions and thus dynamically favor ionic conduction.
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stock, A. The Hydrides of Boron and Silicon; Cornell University Press: New York, NY, USA, 1933. [Google Scholar]
- Martin, D.R. The Development of Borane Fuels. J. Chem. Educ. 1959, 36, 208–214. [Google Scholar] [CrossRef]
- Baier, M.J.; Veeraraghavan Ramachandran, P.; Son, S.F. Characterization of the Hypergolic Ignition Delay of Ammonia Borane. J. Propuls. Power 2019, 35, 182–189. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Wang, B.; Zhang, J. Boron based hypergolic ionic liquids: A review. Green Energy Environ. 2020, 6, 794–822. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, S.; Dewhurst, R.D.; Ignat’ev, N.V.; Finze, M.; Braunschweig, H. Boron: Its Role in Energy-Related Processes and Applications. Angew. Chem. Int. Ed. 2020, 59, 8800–8816. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Z.; Chen, H.; Wang, Z.; Zhou, X.; Zhang, H. Progress in three-dimensional aromatic-like closo-dodecaborate. Coord. Chem. Rev. 2021, 444, 214042. [Google Scholar] [CrossRef]
- Stauber, J.M.; Schwan, J.; Zhang, X.; Axtell, J.C.; Jung, D.; McNicholas, B.J.; Oyala, P.H.; Martinolich, A.J.; Winkler, J.R.; See, K.A.; et al. A Super-Oxidized Radical Cationic Icosahedral Boron Cluster. J. Am. Chem. Soc. 2020, 142, 12948–12953. [Google Scholar] [CrossRef]
- Tu, D.; Yan, H.; Poater, J.; Solà, M. The nido-Cage···π Bond: A Non-covalent Interaction between Boron Clusters and Aromatic Rings and Its Applications. Angew. Chemie Int. Ed. 2020, 59, 9018–9025. [Google Scholar] [CrossRef] [PubMed]
- Alamón, C.; Dávila, B.; García, M.F.; Sánchez, C.; Kovacs, M.; Trias, E.; Barbeito, L.; Gabay, M.; Zeineh, N.; Gavish, M.; et al. Sunitinib-Containing Carborane Pharmacophore with the Ability to Inhibit Tyrosine Kinases Receptors FLT3, KIT and PDGFR-β, Exhibits Powerful In Vivo Anti-Glioblastoma Activity. Cancers 2020, 12, 3423. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; S Hosmane, N.; Zhu, Y. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828. [Google Scholar] [CrossRef] [Green Version]
- Stockmann, P.; Gozzi, M.; Kuhnert, R.; Sárosi, M.B.; Hey-Hawkins, E. New keys for old locks: Carborane-containing drugs as platforms for mechanism-based therapies. Chem. Soc. Rev. 2019, 48, 3497–3512. [Google Scholar] [CrossRef] [Green Version]
- Bogdanovic, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloys Compd. 1997, 253–254, 1–9. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C.; Siegel, D.J. High capacity hydrogen storage materials: Attributes for automotive applications and techniques for materials discovery. Chem. Soc. Rev. 2010, 39, 656–675. [Google Scholar] [CrossRef] [Green Version]
- Bellosta von Colbe, J.; Ares, J.-R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Schneemann, A.; White, J.L.; Kang, S.; Jeong, S.; Wan, L.F.; Cho, E.S.; Heo, T.W.; Prendergast, D.; Urban, J.J.; Wood, B.C.; et al. Nanostructured Metal Hydrides for Hydrogen Storage. Chem. Rev. 2018, 118, 10775–10839. [Google Scholar] [CrossRef]
- Ohno, S.; Banik, A.; Dewald, G.F.; Kraft, M.A.; Krauskopf, T.; Minafra, N.; Till, P.; Weiss, M.; Zeier, W.G. Materials design of ionic conductors for solid state batteries. Prog. Energy 2020, 2, 022001. [Google Scholar] [CrossRef]
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Černý, R.; Ravnsbæk, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacher, F.; Jensen, T.R. Metal borohydrides and derivatives–synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef]
- Černý, R.; Schouwink, P. The crystal chemistry of inorganic metal borohydrides and their relation to metal oxides. Acta Cryst. 2015, B71, 619–640. [Google Scholar] [CrossRef]
- Moussa, G.; Moury, R.; Demirci, U.B.; Sener, T.; Miele, P. Boron-based Hydrides for Chemical Hydrogen Storage. Int. J. Energy Res. 2013, 37, 825–842. [Google Scholar] [CrossRef]
- Suárez-Alcántara, K.; Tena-Garcia, J.R.; Ricardo Guerrero-Ortiz, R. Alanates, a Comprehensive Review. Materials 2019, 12, 2724. [Google Scholar] [CrossRef] [Green Version]
- Dobbins, T.A. Overview of the Structure–Dynamics–Function Relationships in Borohydrides for Use as Solid-State Electrolytes in Battery Applications. Molecules 2021, 26, 3239. [Google Scholar] [CrossRef] [PubMed]
- Zavorotynska, O.; El-Kharbachi, A.; Deledda, S.; Hauback, B.C. Recent progress in magnesium borohydride Mg(BH4)2: Fundamentals and applications for energy storage. Int. J. Hydrogen Energy 2016, 41, 14387–14403. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Majzoub, E.; Ozoliņš, V.; Wolverton, C. Theoretical Prediction of Metastable Intermediates in the Decomposition of Mg(BH4)2. J. Phys. Chem. C 2012, 116, 10522–10528. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Bowman, R.C.; Reiter, J.W.; Rijssenbeek, J.; Soloveichik, G.L.; Zhao, J.-C.; Kabbour, H.; Ahn, C.C. NMR confirmation for formation of [B12H12]2− complexes during hydrogen desorption from metal borohydrides. J. Phys. Chem. C 2008, 112, 3164–3169. [Google Scholar] [CrossRef]
- Golub, I.E.; Filippov, O.A.; Belkova, N.V.; Epstein, L.M.; Shubina, E.S. The Reaction of Hydrogen Halides with Tetrahydroborate Anion and Hexahydro-closo-hexaborate Dianion. Molecules 2021, 26, 3754. [Google Scholar] [CrossRef]
- Voinova, V.V.; Selivanov, N.A.; Plyushchenko, I.V.; Vokuev, M.F.; Bykov, A.Y.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Grigoriev, M.S.; Rodin, I.A.; et al. Fused 1,2-Diboraoxazoles Based on closo-Decaborate Anion–Novel Members of Diboroheterocycle Class. Molecules 2021, 26, 248. [Google Scholar] [CrossRef]
- Andreichuk, E.P.; Anufriev, S.A.; Suponitsky, K.Y.; Sivaev, I.B. The First Nickelacarborane with closo-nido Structure. Molecules 2020, 25, 6009. [Google Scholar] [CrossRef]
- Klyukin, I.N.; Vlasova, Y.S.; Novikov, A.S.; Zhdanov, A.P.; Hagemann, H.R.; Zhizhin, K.Y.; Kuznetsov, N.T. B-F bonding and reactivity analysis of mono- and perfluoro-substituted derivatives of closo-borate anions (6, 10, 12): A computational study. Polyhedron 2022, 211, 115559. [Google Scholar] [CrossRef]
- Udovic, T.J.; Matsuo, M.; Unemoto, A.; Verdal, N.; Stavila, V.; Skripov, A.V.; Rush, J.J.; Takamura, H.; Orimo, S. Sodium Superionic Conduction in Na2B12H12. Chem. Commun. 2014, 50, 3750–3752. [Google Scholar] [CrossRef]
- Duchêne, L.; Remhof, A.; Hagemann, H.; Battaglia, C. Status and prospects of hydroborate electrolytes for all-solid-state batteries. Energy Storage Mat. 2020, 26, 543–549. [Google Scholar] [CrossRef]
- He, L.; Li, H.-W.; Hwang, S.-J.; Akiba, E. Facile Solvent-Free Synthesis of anhydrous alkali metal dodecaborate M2B12H12 (M = Li, Na, K). J. Phys. Chem. C 2014, 118, 6084–6089. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, X.; Huang, Z.; Li, H.-W.; Gao, M.; Pan, H.; Liu, Y. Recent Development of Lithium Borohydride-Based Materials for Hydrogen Storage. Adv. Energy Sustain. Res. 2021, 2, 2100073. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Richter, B.; Jensen, T.R.; Dmitriev, V.; Chernyshov, D.; Hagemann, H. Porous and Dense Magnesium Borohydride Frameworks: Synthesis, Stability, and Reversible Absorption of Guest Species. Angew. Chem. Int. Ed. 2011, 50, 11162–11166. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Zheng, J.; Song, P.; Li, X. Decomposition pathway of Mg(BH4)2 under pressure: Metastable phases and thermodynamic parameters. Scr. Mater. 2011, 64, 225–228. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Bordiga, S.; Baricco, M. Spectroscopic and Structural Characterization of Thermal Decomposition of γ-Mg(BH4)2: Dynamic Vacuum versus H2 Atmosphere. J. Phys. Chem. C 2015, 119, 25340–25351. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, X.; Zheng, J.; Yao, Z.; Zhang, M.; Huang, X.; Chen, L. Insights into magnesium borohydride dehydrogenation mechanism from its partial reversibility under moderate conditions. Mater. Today Energy 2020, 18, 100552. [Google Scholar] [CrossRef]
- Crociani, L.; Rossetto, G.; Kaciulis, S.; Mezzi, A.; El-Habra, N.; Palmieri, V. Study of Magnesium Boride Films Obtained From Mg(BH4)2 by CVD. Chem. Vap. Depos. 2007, 13, 414–419. [Google Scholar] [CrossRef]
- Kim, D.Y.; Yang, Y.; Abelson, J.R.; Girolami, G.S. Volatile magnesium octahydrotriborate complexes as potential CVD Precursors to MgB2. Synthesis and Characterization of Mg(B3H8)2 and its etherates. Inorg. Chem. 2007, 46, 9060–9066. [Google Scholar] [CrossRef]
- Pistidda, C.; Garroni, S.; Dolci, F.; Gil Bardají, E.; Khandelwal, A.; Nolis, P.; Dornheim, M.; Gosalawit, R.; Jensen, T.; Cere-nius, Y.; et al. Synthesis of amorphous Mg(BH4)2 from MgB2 and H2 at room temperature. J. Alloys Comp. 2010, 508, 212–215. [Google Scholar] [CrossRef]
- Severa, G.; Rönnebro, E.; Jensen, C.M. Direct hydrogenation of magnesium boride to magnesium borohydride: Demonstration of >11 weight percent reversible hydrogen storage. Chem. Commun. 2010, 46, 421–423. [Google Scholar] [CrossRef]
- Sugai, C.; Kim, S.; Severa, G.; White, J.L.; Leick, N.; Martinez, M.B.; Gennett, T.; Stavila, V.; Jensen, C. Kinetic Enhancement of Direct Hydrogenation of MgB2 to Mg(BH4)2 upon Mechanical Milling with THF, MgH2, and/or Mg. ChemPhysChem 2019, 20, 1301–1304. [Google Scholar] [CrossRef]
- Pistidda, C.; Santhosh, A.; Jerabek, P.; Shang, Y.; Girella, A.; Milanese, C.; Dore, M.; Garroni, S.; Bordignon, S.; Chierotti, M.R.; et al. Hydrogenation via a low energy mechanochemical approach: The MgB2 case. J. Phys. Energy 2021, 3, 044001. [Google Scholar] [CrossRef]
- Ray, K.G.; Klebanoff, L.E.; Lee, J.R.I.; Stavila, V.; Wook Heo, T.; Shea, P.; Baker, A.A.; Kang, S.; Bagge-Hansen, M.; Liu, Y.-S.; et al. Elucidating the mechanism of MgB2 initial hydrogenation via a combined experimental–theoretical study. Phys. Chem. Chem. Phys. 2017, 19, 22646–22658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, M.; Matsuo, M.; Orimo, S.; Autrey, T.; Jensen, C.M. Selective Reversible Hydrogenation of Mg(B3H8)2/MgH2 to Mg(BH4)2: Pathway to Reversible Borane-Based Hydrogen Storage? Inorg. Chem. 2015, 54, 4120–4125. [Google Scholar] [CrossRef]
- Yue, Y.; Chen, L.; Peng, J. Thermal Behaviors and Their Correlations of Mg(BH4)2-Contained Explosives. J. Energetic Mater. 2018, 36, 82–92. [Google Scholar] [CrossRef]
- Hagemann, H. Estimation of Thermodynamic Properties of Metal Hydroborates. ChemistrySelect 2019, 4, 8989–8992. [Google Scholar] [CrossRef]
- Chase, M.W. NIST-JANAF Thermochemical Tables. J. Phys. Chem. Ref. Data Monogr. 1998, 2. [Google Scholar] [CrossRef]
- Hagemann, H.; D’Anna, V.; Rapin, J.-P.; Yvon, K. Deuterium-Hydrogen Exchange in Solid Mg(BH4)2. J. Phys. Chem. C 2010, 114, 10045–10047. [Google Scholar] [CrossRef]
- Sharma, M.; Sethio, D.; D’Anna, V.; Fallas, J.C.; Schouwink, P.; Cerný, R.; Hagemann, H. Isotope Exchange Reactions in Ca(BH4)2. J. Phys. Chem. C 2015, 119, 29–32. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Y.; Wang, D.; Yang, F.; Wu, Z.; Wua, L.; Zhang, Z. Role of native defects and the effects of metal additives on the kinetics of magnesium borohydride. Phys. Chem. Chem. Phys. 2019, 21, 11226–11233. [Google Scholar] [CrossRef]
- Heere, M.; Zavorotynska, O.; Deledda, S.; Sørby, M.H.; Book, D.; Steriotis, T.; Hauback, B.C. Effect of additives, ball milling and isotopic exchange in porous magnesium borohydride. RSC Adv. 2018, 8, 27645–27653. [Google Scholar] [CrossRef] [Green Version]
- Li, H.W.; Kikuchi, K.; Nakamori, Y.; Miwa, K.; Towata, S.; Orimo, S. Effects of ball milling and additives on dehydriding behaviors of well-crystallized Mg(BH4)2. Scr. Mater. 2007, 57, 679–682. [Google Scholar] [CrossRef]
- Saldan, I.; Frommen, C.; Llamas-Jansa, I.; Kalantzopoulos, G.N.; Hino, S.; Arstad, B.; Heyn, R.H.; Zavorotynska, O.; Deledda, S.; Sørby, M.H.; et al. Hydrogen storage properties of gamma-Mg(BH4)2 modified by MoO3 and TiO2. Int. J. Hydrogen Energy 2015, 40, 12286–12293. [Google Scholar] [CrossRef]
- Chong, M.; Autrey, T.; Jensen, C.M. Lewis Base Complexes of Magnesium Borohydride: Enhanced Kinetics and Product Selectivity upon Hydrogen Release. Inorganics 2017, 5, 89. [Google Scholar] [CrossRef] [Green Version]
- Dimitrievska, M.; Chong, M.; Bowden, M.E.; Wu, H.; Zhou, W.; Nayyar, I.; Ginovska, B.; Gennett, T.; Autrey, T.; Jensen, C.M.; et al. Structural and reorientational dynamics of tetrahydroborate (BH4−) and tetrahydrofuran (THF) in a Mg(BH4)2·3THF adduct: Neutron-scattering characterization. Phys. Chem. Chem. Phys. 2020, 22, 368–378. [Google Scholar] [CrossRef]
- Tran, B.L.; Allen, T.N.; Bowden, M.E.; Autrey, T.; Jensen, C.M. Effects of Glymes on the Distribution of Mg(B10H10) and Mg(B12H12) from the Thermolysis of Mg(BH4)2. Inorganics 2021, 9, 41. [Google Scholar] [CrossRef]
- Bell, R.T.; Strange, N.A.; Leick, N.; Stavila, V.; Bowden, M.E.; Autrey, T.S.; Gennett, T. Mg(BH4)2-Based Hybrid Metal–Organic Borohydride System Exhibiting Enhanced Chemical Stability in Melt. ACS Appl. Energy Mater. 2021, 4, 1704–1713. [Google Scholar] [CrossRef]
- Wegner, W.; Jaroń, T.; Dobrowolski, M.A.; Dobrzycki, Ł.; Cyrańskic, M.K.; Grochala, W. Organic derivatives of Mg(BH4)2 as precursors towards MgB2 and novel inorganic mixed-cation borohydrides. Dalton Trans. 2016, 45, 14370–14377. [Google Scholar] [CrossRef]
- Moury, R.; Gigante, A.; Remhof, A.; Roedern, E.; Hagemann, H. Experimental investigation of Mg(B3H8)2 dimensionality, materials for energy storage applications. Dalton Trans. 2020, 49, 12168–12173. [Google Scholar] [CrossRef]
- Gigante, A.; Leick, N.; Lipton, A.S.; Tran, B.; Strange, N.A.; Bowden, M.; Martinez, M.B.; Moury, R.; Gennett, T.; Hagemann, H.; et al. Thermal Conversion of Unsolvated Mg(B3H8)2 to BH4− in the Presence of MgH2. ACS Appl. Energy Mater. 2021, 4, 3737–3747. [Google Scholar] [CrossRef]
- Newhouse, R.J.; Stavila, V.; Hwang, S.-J.; Klebanoff, L.E.; Zhang, J.Z. Reversibility and Improved Hydrogen Release of Magnesium Borohydride. J. Phys. Chem. C 2010, 114, 5224–5232. [Google Scholar] [CrossRef] [Green Version]
- Beall, H.; Gaines, D.F. Mechanistic aspects of boron hydride reactions. Inorg. Chim. Acta 1999, 289, 1–10. [Google Scholar] [CrossRef]
- Kurbonbekov, A.; Alikhanova, T.K.; Badalov, A.; Murufi, V.K.; Mirsaidov, U. Solubility in the lanthanum borohydride -potassium borohydride -tetrahydrofuran system at 25 °C and some thermodynamic characteristics of lanthanum borohydride. Dokl. Akad. Nauk Tadzh. SSR 1990, 33, 393–395. [Google Scholar]
- Huang, Z.; Chen, X.; Yisgedu, T.; Meyers, E.A.; Shore, S.G.; Zhao, J.-C. Ammonium Octahydrotriborate (NH4B3H8): New Synthesis, Structure, and Hydrolytic Hydrogen Release. Inorg. Chem. 2011, 50, 3738–3742. [Google Scholar] [CrossRef] [Green Version]
- Good, W.D.; Mansson, M. The Thermochemistry of Boron and Some of Its Compounds. The Enthalpies of Formation of Orthoboric Acid, Trimethylamineborane, and Diammoniumdecaborane. J. Phys. Chem. 1966, 70, 97–104. [Google Scholar] [CrossRef]
- Hanumantha Rao, M.; Muralidharan, K. closo-Dodecaborate (B12H12)2− salts with nitrogen based cations and their energetic properties. Polyhedron 2016, 115, 105–110. [Google Scholar]
- Dematteis, E.M.; Jensen, S.R.; Jensen, T.R.; Baricco, M. Heat capacity and thermodynamic properties of alkali and alkali-earth borohydrides. J. Chem. Thermodyn. 2020, 143, 106055. [Google Scholar] [CrossRef]
- Pinatel, E.R.; Albanese, E.; Civalleri, B.; Baricco, M. Thermodynamic modelling of Mg(BH4)2. J. Alloys Comp. 2015, 645, S64–S68. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.T.; Matus, M.H.; Dixon, D.A. Heats of Formation of Boron Hydride Anions and Dianions and Their Ammonium Salts [BnHmy−][NH4+]y with y = 1−2. Inorg. Chem. 2007, 46, 7561–7570. [Google Scholar] [CrossRef]
- McKee, M.L. Estimation of Heats of Formation of Boron Hydrides from ab Initio Energies. J. Phys. Chem. 1990, 94, 435–440. [Google Scholar] [CrossRef]
- Kelley, S.P.; McCrary, P.D.; Flores, L.; Garner, E.B.; Dixon, D.A.; Rogers, R.D. Structural and Theoretical Study of Salts of the [B9H14]− Ion: Isolation of Multiple Isomers and Implications for Energy Storage. ChemPlusChem 2016, 81, 922–925. [Google Scholar] [CrossRef]
- Sethio, D.; Lawson Daku, L.M.; Hagemann, H.; Kraka, E. Quantitative Assessment of B–B–B, B–Hb–B, and B–Ht Bonds: From BH3 to B12H122−. ChemPhysChem 2019, 20, 1967–1977. [Google Scholar] [CrossRef]
- Maillard, R.; Sethio, D.; Hagemann, H.; Lawson Daku, L.M. Accurate Computational Thermodynamics Using Anharmonic Density Functional Theory Calculations: The Case Study of B−H Species. ACS Omega 2019, 4, 8786–8794. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.L.; Bauer, S.H. Thermochemistry of the boranes. J. Phys. Chem. Ref. Data 1998, 27, 807–835. [Google Scholar] [CrossRef]
- Lee, T.B.; Mc Kee, M.L. Redox Energetics of Hypercloso Boron Hydrides BnHn (n = 6–13) and B12X12 (X = F, Cl, OH, and CH3). Inorg. Chem. 2012, 51, 4205–4214. [Google Scholar] [CrossRef]
- Lee, T.B.; Mc Kee, M.L. Dissolution Thermochemistry of Alkali Metal Dianion Salts (M2X1, M = Li+, Na+, and K+ with X = CO32−, SO42−, C8H82−, and B12H122−). Inorg. Chem. 2011, 50, 11412–11422. [Google Scholar] [CrossRef]
- Yan, Y.; Remhof, A.; Rentsch, D.; Züttel, A. The role of MgB12H12 in the hydrogen desorption process of Mg(BH4)2. Chem. Commun. 2015, 51, 700–702. [Google Scholar]
- Yan, Y.; Remhof, A.; Rentsch, D.; Lee, Y.S.; Cho, Y.W.; Züttel, A. Is Y2(B12H12)3 the main intermediate in the decomposition process of Y(BH4)3? Chem. Commun. 2013, 49, 5234–5236. [Google Scholar] [CrossRef] [Green Version]
- Godfroid, R.A.; Hill, T.G.; Onak, T.P.; Shore, S.G. Formation of [BH3]2− and [B2H6]2− From the Homogeneous Reduction of B2H6. J. Am. Chem. Soc. 1994, 116, 12107–12108. [Google Scholar] [CrossRef]
- Hill, T.G.; Godfroid, R.A.; White III, J.P.; Shore, S.G. Reduction of borane THF by alkali metal (potassium, rubidium, cesium) and ytterbium mercury amalgams to form salts of octahydrotriborate(1-); a simple procedure for the synthesis of tetraborane(10). Inorg. Chem. 1991, 30, 2952–2954. [Google Scholar] [CrossRef]
- Gaines, D.F.; Schaeffer, R.; Tebbe, F. Convenient Preparations of Solutions Containing the Triborohydride Ion. Inorg. Chem. 1963, 2, 526–528. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.-R.; Wang, X.; Chen, X.-M.; Jing, Y.; Wie, D. A Safe and Efficient Synthetic Method of the Alkali Metal Octahydrotriborate, Unravelling a General Mechanism of Constructing the Delta B3 Unit of Polyhedral Boranes. Dalton Trans. 2021, 50, 13676–13679. [Google Scholar] [CrossRef]
- Chen, X.-M.; Ma, N.; Zhang, Q.-F.; Wang, J.; Feng, X.; Wei, C.; Wang, L.-S.; Zhang, J.; Chen, X. Elucidation of the Formation Mechanisms of the Octahydrotriborate Anion (B3H8−) through the Nucleophilicity of the B–H Bond. J. Amer. Chem. Soc. 2018, 140, 6718–6726. [Google Scholar] [CrossRef]
- Moury, R.; Gigante, A.; Hagemann, H. An alternative approach to the synthesis of NaB3H8 and Na2B12H12 for solid electrolyte applications. Int. J. Hydrogen Energy 2017, 42, 22417–22421. [Google Scholar] [CrossRef]
- Grinderslev, J.B.; Møller, K.T.; Yan, Y.; Chen, X.-M.; Li, Y.; Li, H.-W.; Zhou, W.; Skibsted, J.; Chen, X.; Jensen, T.R. Potassium octahydridotriborate: Diverse polymorphism in a potential hydrogen storage material and potassium ion conductor. Dalton Trans. 2019, 48, 8872–8881. [Google Scholar] [CrossRef]
- Gigante, A.; Duchêne, L.; Moury, R.; Pupier, M.; Remhof, A.; Hagemann, H. Direct solution–based synthesis of the Na4(B12H12)(B10H10) solid electrolyte. ChemSusChem 2019, 12, 4832–4837. [Google Scholar] [CrossRef]
- Aniya, M. A chemical approach for the microscopic mechanism of fast ion transport in solids. Solid State Ion. 1992, 50, 125–129. [Google Scholar] [CrossRef]
- Matsuo, M.; Nakamori, Y.; Orimo, S.; Maekawa, H.; Takamura, H. Lithium superionic conduction in lithium borohydride accompanied by structural transition. Appl. Phys. Lett. 2007, 91, 224103. [Google Scholar] [CrossRef]
- Muetterties, E.L.; Balthis, J.H.; Chia, Y.T.; Knoth, W.H.; Miller, H.C. Chemistry of Boranes. VIII. Salts and Acids of B10H10−2 and B12H12−2. Inorg. Chem. 1964, 3, 444–451. [Google Scholar] [CrossRef]
- Černý, R.; Brighi, M.; Murgia, F. The Crystal Chemistry of Inorganic Hydroborates. Chemistry 2020, 2, 805–826. [Google Scholar] [CrossRef]
- Wu, H.; Tang, W.S.; Stavila, V.; Zhou, W.; Rush, J.J.; Udovic, T.J. Structural Behavior of Li2B10H10. J. Phys. Chem. C 2015, 119, 6481–6487. [Google Scholar] [CrossRef]
- Her, J.-H.; Yousufuddin, M.; Zhou, W.; Jalisatgi, S.S.; Kulleck, J.G.; Zan, J.A.; Hwang, S.-J.; Bowman, R.C.; Udovic, T.J. Crystal structure of Li2B12H12: A possible intermediate species in the decomposition of LiBH4. Inorg. Chem. 2008, 47, 9757–9759. [Google Scholar] [CrossRef] [Green Version]
- Paskevicius, M.; Pitt, M.P.; Brown, D.H.; Sheppard, D.A.; Chumphongphan, S.; Buckley, C.E. First-order phase transition in the Li2B12H12 system. Phys. Chem. Chem. Phys. 2013, 15, 15825–15828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Tang, W.S.; Zhou, W.; Stavila, V.; Rush, J.J.; Udovic, T.J. The structure of monoclinic Na2B10H10: A combined diffraction, spectroscopy, and theoretical approach. Cryst. Eng. Comm. 2015, 17, 3533–3540. [Google Scholar] [CrossRef]
- Verdal, N.; Her, J.-H.; Stavila, V.; Soloninin, A.V.; Babanova, O.A.; Skripov, A.V.; Udovic, T.J.; Rush, J.J. Complex high-temperature phase transitions in Li2B12H12 and Na2B12H12. J. Solid State Chem. 2014, 212, 81–91. [Google Scholar] [CrossRef]
- Wunderlich, J.A.; Lipscomb, W.N. Structure of B12H12−2 ion. J. Am. Chem. Soc. 1960, 82, 4427–4428. [Google Scholar] [CrossRef]
- Hofmann, K.; Albert, B. Crystal structures of M2[B10H10] (M = Na, K, Rb) via real space simulated annealing powder techniques. Z. Kristall. 2005, 220, 142–146. [Google Scholar] [CrossRef]
- Bukovsky, E.V.; Peryshkov, D.V.; Wu, H.; Zhou, W.; Tang, W.S.; Jones, W.M.; Stavila, V.; Udovic, T.J.; Strauss, S.H. Comparison of the Coordination of B12F122−, B12Cl122−, and B12H122− to Na+ in the Solid State: Crystal Structures and Thermal Behavior of Na2B12F12,Na2(H2O)4B12F12, Na2B12Cl12, and Na2(H2O)6B12Cl12. Inorg. Chem. 2017, 56, 4369–4379. [Google Scholar] [CrossRef] [PubMed]
- Tiritiris, I.; Schleid, T. The Dodecahydro-closo-Dodecaborates M2[B12H12] of the Heavy Alkali Metals (M = K+, Rb+, NH4+, Cs+) and their Formal Iodide Adducts M3I [B12H12] (= MI·M2[B12H12]). Z. Anorg. Allg. Chem. 2003, 629, 1390–1402. [Google Scholar] [CrossRef]
- Guggenberger, L.J. Chemistry of boranes. XXXIII. The crystal structure of Rb2B9H9. Inorg. Chem. 1968, 7, 2260–2264. [Google Scholar] [CrossRef]
- Verdal, N.; Wu, H.; Udovic, T.J.; Stavila, V.; Zhou, W.; Rush, J.J. Evidence of a transition to reorientational disorder in the cubic alkali-metal dodecahydro-closo-dodecaborates. J. Solid State Chem. 2011, 184, 3110–3116. [Google Scholar] [CrossRef]
- Moury, R.; Lodziana, Z.; Remhof, A.; Duchêne, L.; Roedern, E.; Gigante, A.; Hagemann, H. Pressure-induced phase transitions in Na2B12H12, structural investigation on a candidate for solid-state electrolyte. Acta Cryst. B 2019, B75, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, H.; Matsuo, M.; Takamura, H.; Ando, M.; Noda, Y.; Karahashi, T.; Orimo, S. Halide-stabilized LiBH4, a room-temperature lithium fast-ion conductor. J. Am. Chem. Soc. 2009, 894–895. [Google Scholar] [CrossRef]
- Gulino, V.; Brighi, M.; Dematteis, E.M.; Murgia, F.; Nervi, C.; Cerny, R.; Baricco, M. Phase Stability and Fast Ion Conductivity in the Hexagonal LiBH4-LiBr-LiCl Solid Solution. Chem. Mater. 2019, 31, 5133–5144. [Google Scholar] [CrossRef] [Green Version]
- Sadikin, Y.; Schouwink, P.; Brighi, M.; Łodziana, Z.; Cerný, R. Modified anion packing of Na2B12H12 in close to room temperature superionic conductors. Inorg. Chem. 2017, 56, 5006–5016. [Google Scholar] [CrossRef]
- Kim, S.; Oguchi, H.; Toyama, N.; Sato, T.; Takagi, S.; Otomo, T.; Arunkumar, D.; Kuwata, N.; Kawamura, J.; Orimo, S. A complex hydride lithium superionic conductor for high-energy-density all-solid-state lithium metal batteries. Nat. Comm. 2019, 10, 1081. [Google Scholar] [CrossRef] [Green Version]
- Payandeh, S.H.; Rentsch, D.; Łodziana, Z.; Asakura, R.; Bigler, L.; Černý, R.; Battaglia, C.; Remhof, A. Nido-Hydroborate-Based Electrolytes for All-Solid-State Lithium Batteries. Adv. Funct. Mater. 2021, 31, 2010046. [Google Scholar] [CrossRef]
- Brighi, M.; Murgia, F.; Łodziana, Z.; Schouwink, P.; Wołczyk, A.; Černý, R. A mixed anion hydroborate/carba-hydroborate as a room temperature Na ion solid electrolyte. J. Power Sources 2019, 404, 7–12. [Google Scholar] [CrossRef]
- Duchêne, L.; Kühnel, R.-S.; Rentsch, D.; Remhof, A.; Hagemann, H.; Battaglia, C. A highly stable sodium solid-state electrolyte based on a dodeca/deca-borate equimolar mixture. Chem. Commun. 2017, 53, 4195–4198. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Sato, T.; Unemoto, A.; Matsuo, M.; Ikeshoji, T.; Udovic, T.J.; Orimo, S.I. Fast sodium ionic conduction in Na2B10H10-Na2B12H12 pseudo-binary complex hydride and application to a bulk-type all-solid-state battery. Appl. Phys. Lett. 2017, 110, 103901. [Google Scholar] [CrossRef] [Green Version]
- Payandeh, S.H.; Asakura, R.; Avramidou, P.; Rentsch, D.; Łodziana, Z.; Černý, R.; Remhof, A.; Battaglia, C. Nido-Borate/Closo-borate mixed-anion electrolytes for all-solid-state batteries. Chem. Mater. 2020, 32, 1101–1110. [Google Scholar] [CrossRef]
- Brighi, M.; Murgia, F.; Cerny, R. Closo-Hydroborate Sodium Salts as an Emerging Class of Room-Temperature Solid Electrolytes. Cell Rep. Phys. Sci. 2020, 1, 100217. [Google Scholar] [CrossRef]
- Skripov, A.V.; Soloninin, A.V.; Babanova, O.A.; Skoryunov, R.V. Anion and Cation Dynamics in Polyhydroborate Salts: NMR Studies. Molecules 2020, 25, 2940. [Google Scholar] [CrossRef]
- Lohstroh, W.; Heere, M. Structure and Dynamics of Borohydrides Studied by Neutron Scattering Techniques: A Review. J. Phys. Soc. Japan. 2020, 89, 1–12. [Google Scholar] [CrossRef]
- Duchêne, L.; Lunghammer, S.; Burankova, T.; Liao, W.-C.; Embs, J.P.; Coperet, C.; Wilkening, H.M.R.; Remhof, A.; Hagemann, H.; Battaglia, C. Ionic conduction mechanism in the Na2(B12H12)0.5(B10H10)0.5 closo-borate solid-state electrolyte: Interplay of disorder and ion–ion interactions. Chem. Mater. 2019, 31, 3449–3460. [Google Scholar] [CrossRef]
- Asakura, R.; Duchêne, L.; Kühnel, R.-S.; Remhof, A.; Hagemann, H.; Battaglia, C. Electrochemical Oxidative Stability of Hydroborate-Based Solid-State Electrolytes. ACS Appl. Energy Mater. 2019, 2, 6924–6930. [Google Scholar] [CrossRef]
- Matsuo, M.; Orimo, S. Lithium Fast-Ionic Conduction in Complex Hydrides: Review and Prospects. Adv. Energy Mater. 2011, 1, 161–172. [Google Scholar] [CrossRef]
- Duchêne, L.; Kühnel, R.-S.; Stilp, E.; Reyes, E.C.; Remhof, A.; Hagemann, H.; Battaglia, C. A Stable 3 V all-solid-state sodium-ion battery based on a closo -borate electrolyte. Energy Environ. Sci. 2017, 10, 2609–2615. [Google Scholar] [CrossRef]
- Murgia, F.; Brighi, M.; Cerny, R. Room-temperature-operating Na-ion solid state-battery with complex hydride as electrolyte. Electrochem. Comm. 2019, 106, 106534. [Google Scholar] [CrossRef]
- Asakura, R.; Reber, D.; Duchêne, L.; Payandeh, S.; Remhof, A.; Hagemann, H.; Battaglia, C. 4 V room-temperature all-solid-state sodium battery enabled by a passivating cathode/hydroborate solid electrolyte interface. Energy Environ. Sci. 2020, 13, 5048–5058. [Google Scholar] [CrossRef]


| Compound | Temperature | Conductivity | Reference |
|---|---|---|---|
| 0.7 Li(CB9H10)–0.3 Li(CB11H12) | 298 K | 6.7 mS/cm | [106] |
| Li2(B11H14)(CB11H12) | 298 K | 0.11 mS/cm | [107] |
| Li3(B11H14)(CB11H12)2 | 298 K | 1.1 mS/cm | [107] |
| Na3(CB11H12)(B12H12) | 298 K | 2 mS/cm | [108] |
| Na4(CB11H12)2(B12H12) | 298 K | 2 mS/cm | [108] |
| Na4(B10H10)(B12H12) | 298 K | 0.9 mS/cm | [109] |
| Na2(B10H10)−3 Na2(B12H12) | 298 K | 0.34 mS/cm | [110] |
| Nax+2y(B11H14)x(B12H12)y | 298 K | 3–4 mS/cm | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagemann, H. Boron Hydrogen Compounds: Hydrogen Storage and Battery Applications. Molecules 2021, 26, 7425. https://doi.org/10.3390/molecules26247425
Hagemann H. Boron Hydrogen Compounds: Hydrogen Storage and Battery Applications. Molecules. 2021; 26(24):7425. https://doi.org/10.3390/molecules26247425
Chicago/Turabian StyleHagemann, Hans. 2021. "Boron Hydrogen Compounds: Hydrogen Storage and Battery Applications" Molecules 26, no. 24: 7425. https://doi.org/10.3390/molecules26247425
APA StyleHagemann, H. (2021). Boron Hydrogen Compounds: Hydrogen Storage and Battery Applications. Molecules, 26(24), 7425. https://doi.org/10.3390/molecules26247425





