Confocal Raman Spectroscopic Imaging for Evaluation of Distribution of Nano-Formulated Hydrophobic Active Cosmetic Ingredients in Hydrophilic Films
Abstract
:1. Introduction
2. Materials and Methods
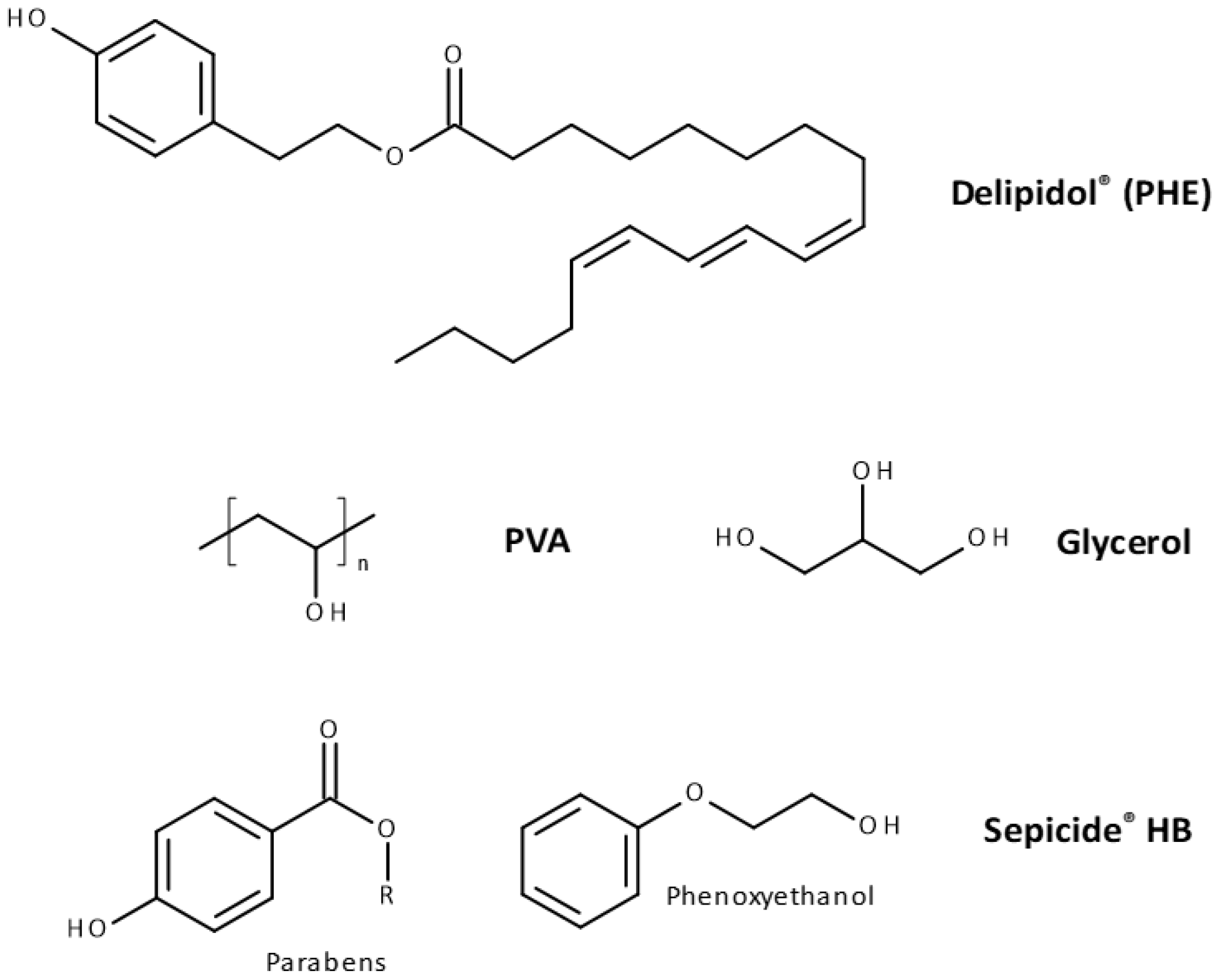
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Confocal Raman Imaging
3. Results and Discussion
3.1. Preparation and Characterisation of NanoPHE
3.2. Spectral Characterisation of NanoPHE
3.3. Spectral Characterisation of PVA-GS-NanoPHE Films
3.3.1. Reference Compounds
3.3.2. PVA-GS-NanoPHE Films
3.4. Distribution and Content of PHE in PVA-GS-Based Films
3.5. Absolute Quantification of PHE in PVA-GS Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Voss, G.T.; Gularte, M.S.; de Oliveira, R.L.; Luchese, C.; Fajardo, A.R.; Wilhelm, E.A. Biopolymeric Films as Delivery Vehicles for Controlled Release of Hydrocortisone: Promising Devices to Treat Chronic Skin Diseases. Mater. Sci. Eng. C 2020, 114, 111074. [Google Scholar] [CrossRef] [PubMed]
- Kathe, K.; Kathpalia, H. Film Forming Systems for Topical and Transdermal Drug Delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Pünnel, L.C.; Lunter, D.J. Film-Forming Systems for Dermal Drug Delivery. Pharmaceutics 2021, 13, 932. [Google Scholar] [CrossRef]
- Frederiksen, K.; Guy, R.H.; Petersson, K. The Potential of Polymeric Film-Forming Systems as Sustained Delivery Platforms for Topical Drugs. Expert Opin. Drug Deliv. 2016, 13, 349–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, P.R.; Diwan, P.V. Formulation and in Vitro Evaluation of Polymeric Films of Diltiazem Hydrochloride and Indomethacin for Transdermal Administration. Drug Dev. Ind. Pharm. 1998, 24, 327–336. [Google Scholar] [CrossRef]
- Zurdo Schroeder, I.; Franke, P.; Schaefer, U.F.; Lehr, C.-M. Development and Characterization of Film Forming Polymeric Solutions for Skin Drug Delivery. Eur. J. Pharm. Biopharm. 2007, 65, 111–121. [Google Scholar] [CrossRef]
- Frederiksen, K.; Guy, R.H.; Petersson, K. Formulation Considerations in the Design of Topical, Polymeric Film-Forming Systems for Sustained Drug Delivery to the Skin. Eur. J. Pharm. Biopharm. 2015, 91, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Mandapalli, P.K.; Labala, S.; Jose, A.; Bhatnagar, S.; Janupally, R.; Sriram, D.; Venuganti, V.V.K. Layer-by-Layer Thin Films for Co-Delivery of TGF-β SiRNA and Epidermal Growth Factor to Improve Excisional Wound Healing. AAPS PharmSciTech 2017, 18, 809–820. [Google Scholar] [CrossRef]
- Aisyah, Y.; Irwanda, L.P.; Haryani, S.; Safriani, N. Characterization of Corn Starch-Based Edible Film Incorporated with Nutmeg Oil Nanoemulsion. IOP Conf. Ser. Mater. Sci. Eng. 2018, 352, 012050. [Google Scholar] [CrossRef]
- Alkilani, A.; McCrudden, M.T.; Donnelly, R. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amnuaikit, C.; Ikeuchi, I.; Ogawara, K.; Higaki, K.; Kimura, T. Skin Permeation of Propranolol from Polymeric Film Containing Terpene Enhancers for Transdermal Use. Int. J. Pharm. 2005, 289, 167–178. [Google Scholar] [CrossRef] [PubMed]
- The Mystical Effects of Dermatological Vehicles-FullText-Dermatology 2005, Vol. 210, No. 2-Karger Publishers. Available online: https://www.karger.com/Article/FullText/82572 (accessed on 27 April 2021).
- Adrover, A.; di Muzio, L.; Trilli, J.; Brandelli, C.; Paolicelli, P.; Petralito, S.; Casadei, M.A. Enhanced Loading Efficiency and Mucoadhesion Properties of Gellan Gum Thin Films by Complexation with Hydroxypropyl-β-Cyclodextrin. Pharmaceutics 2020, 12, 819. [Google Scholar] [CrossRef] [PubMed]
- Munnier, E.; Al Assaad, A.; David, S.; Mahut, F.; Vayer, M.; Van Gheluwe, L.; Yvergnaux, F.; Sinturel, C.; Soucé, M.; Chourpa, I.; et al. Homogeneous Distribution of Fatty Ester-based Active Cosmetic Ingredients in Hydrophilic Thin Films by Means of Nanodispersion. Int. J. Cosmet. Sci. 2020, 42, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Heredia-Guerrero, J.A.; Perotto, G.; Valentini, P.; Pompa, P.P.; Spanò, R.; Goldoni, L.; Bertorelli, R.; Athanassiou, A.; Bayer, I.S. Transparent Ciprofloxacin-Povidone Antibiotic Films and Nanofiber Mats as Potential Skin and Wound Care Dressings. Eur. J. Pharm. Sci. 2017, 104, 133–144. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Preparation and Optimization of Chitosan-Gelatin Films for Sustained Delivery of Lupeol for Wound Healing. Int. J. Biol. Macromol. 2018, 107, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Gehrcke, M.; de Bastos Brum, T.; da Rosa, L.S.; Ilha, B.D.; Soares, F.Z.M.; Cruz, L. Incorporation of Nanocapsules into Gellan Gum Films: A Strategy to Improve the Stability and Prolong the Cutaneous Release of Silibinin. Mater. Sci. Eng. C 2021, 119, 111624. [Google Scholar] [CrossRef] [PubMed]
- Niamlang, P.; Tongrain, T.; Ekabutr, P.; Chuysinuan, P.; Supaphol, P. Preparation, Characterization and Biocompatibility of Poly(Vinyl Alcohol) Films Containing Tetracycline Hydrochloride-Loaded Quaternized Chitosan Nanoparticles. J. Drug Deliv. Sci. Technol. 2017, 38, 36–44. [Google Scholar] [CrossRef]
- Magalhães, T.M.; Guerra, R.C.; da San Gil, R.A.S.; Valente, A.P.; Simão, R.A.; Soares, B.G.; de Carvalho Mendes, T.; dos Santos Pyrrho, A.; de Sousa, V.P.; Rodrigues-Furtado, V.L. PAMAM Dendrimer Hydrogel Film—Biocompatible Material to an Efficient Dermal Delivery of Drugs. J. Nanopart. Res. 2017, 19, 277. [Google Scholar] [CrossRef]
- Garvie-Cook, H.; Frederiksen, K.; Petersson, K.; Guy, R.H.; Gordeev, S. Characterization of Topical Film-Forming Systems Using Atomic Force Microscopy and Raman Microspectroscopy. Mol. Pharm. 2015, 12, 751–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, U.; Hild, S.; Ibach, W.; Hollricher, O. Characterization of Thin Polymer Films on the Nanometer Scale with Confocal Raman AFM. Macromol. Symp. 2005, 230, 133–143. [Google Scholar] [CrossRef]
- Ghita, A.; Pascut, F.C.; Sottile, V.; Denning, C.; Notingher, I. Applications of Raman Micro-Spectroscopy to Stem Cell Technology: Label-Free Molecular Discrimination and Monitoring Cell Differentiation. EPJ Tech. Instrum. 2015, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rammal, H.; Al Assaad, A.; Dosio, F.; Stella, B.; Maksimenko, A.; Mura, S.; Van Gulick, L.; Callewaert, M.; Desmaële, D.; Couvreur, P.; et al. Investigation of Squalene-Doxorubicin Distribution and Interactions within Single Cancer Cell Using Raman Microspectroscopy. Nanomed. Nanotechnol. Biol. Med. 2021, 35, 102404. [Google Scholar] [CrossRef] [PubMed]
- Dorney, J.; Bonnier, F.; Garcia, A.; Casey, A.; Chambers, G.; Byrne, H.J. Identifying and Localizing Intracellular Nanoparticles Using Raman Spectroscopy. Analyst 2012, 137, 1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beleites, C.; Bonifacio, A.; Codrich, D.; Krafft, C.; Sergo, V. Raman Spectroscopy and Imaging: Promising Optical Diagnostic Tools in Pediatrics. CMC 2013, 20, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Miloudi, L.; Bonnier, F.; Tfayli, A.; Yvergnaux, F.; Byrne, H.J.; Chourpa, I.; Munnier, E. Confocal Raman Spectroscopic Imaging for in Vitro Monitoring of Active Ingredient Penetration and Distribution in Reconstructed Human Epidermis Model. J. Biophotonics 2018, 11, e201700221. [Google Scholar] [CrossRef] [Green Version]
- Paudel, A.; Raijada, D.; Rantanen, J. Raman Spectroscopy in Pharmaceutical Product Design. Adv. Drug Deliv. Rev. 2015, 89, 3–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waffo Tchounga, C.A.; Sacre, P.Y.; Ciza, P.; Ngono, R.; Ziemons, E.; Hubert, P.; Marini, R.D. Composition Analysis of Falsified Chloroquine Phosphate Samples Seized during the COVID-19 Pandemic. J. Pharm. Biomed. Anal. 2021, 194, 113761. [Google Scholar] [CrossRef]
- Miloudi, L.; Bonnier, F.; Bertrand, D.; Byrne, H.J.; Perse, X.; Chourpa, I.; Munnier, E. Quantitative Analysis of Curcumin-Loaded Alginate Nanocarriers in Hydrogels Using Raman and Attenuated Total Reflection Infrared Spectroscopy. Anal. Bioanal. Chem. 2017, 409, 4593–4605. [Google Scholar] [CrossRef]
- Mazurek, S.; Szostak, R. Quantitative Analysis of Topical Gels and Ointments by FT-Raman Spectroscopy. Vib. Spectrosc. 2016, 83, 1–7. [Google Scholar] [CrossRef]
- Strachan, C.J.; Rades, T.; Gordon, K.C.; Rantanen, J. Raman Spectroscopy for Quantitative Analysis of Pharmaceutical Solids. J. Pharm. Pharmacol. 2010, 59, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Hertrampf, A.; Sousa, R.M.; Menezes, J.C.; Herdling, T. Semi-Quantitative Prediction of a Multiple API Solid Dosage Form with a Combination of Vibrational Spectroscopy Methods. J. Pharm. Biomed. Anal. 2016, 124, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Bakkar, M.A.; Nawaz, H.; Majeed, M.I.; Naseem, A.; Ditta, A.; Rashid, N.; Ali, S.; Bajwa, J.; Bashir, S.; Ahmad, S.; et al. Raman Spectroscopy for the Qualitative and Quantitative Analysis of Solid Dosage Forms of Sitagliptin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 245, 118900. [Google Scholar] [CrossRef]
- Bajwa, J.; Nawaz, H.; Majeed, M.I.; Hussain, A.I.; Farooq, S.; Rashid, N.; Bakkar, M.A.; Ahmad, S.; Hyat, H.; Bashir, S.; et al. Quantitative Analysis of Solid Dosage Forms of Cefixime Using Raman Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 238, 118446. [Google Scholar] [CrossRef] [PubMed]
- Kwan, C.; Ayhan, B.; Chen, G.; Wang, J.; Ji, B.; Chang, C.-I. A Novel Approach for Spectral Unmixing, Classification, and Concentration Estimation of Chemical and Biological Agents. IEEE Trans. Geosci. Remote Sens. 2006, 44, 409–419. [Google Scholar] [CrossRef]
- Chiper, M.; Hervé Aubert, K.; Augé, A.; Fouquenet, J.-F.; Soucé, M.; Chourpa, I. Colloidal Stability and Thermo-Responsive Properties of Iron Oxide Nanoparticles Coated with Polymers: Advantages of Pluronic® F68–PEG Mixture. Nanotechnology 2013, 24, 395605. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gupta, S.; Jindal, N.; Jindal, A.; Bansal, R. Nanocarriers and Nanoparticles for Skin Care and Dermatological Treatments. Indian Dermatol. Online J. 2013, 4, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, H.; Wang, J.; Chen, J. Conformational Structure of Triblock Copolymers by FT-Raman and FTIR Spectroscopy. J. Colloid Interface Sci. 1999, 209, 368–373. [Google Scholar] [CrossRef]
- Beattie, J.R.; Maguire, C.; Gilchrist, S.; Barrett, L.J.; Cross, C.E.; Possmayer, F.; Ennis, M.; Elborn, J.S.; Curry, W.J.; McGarvey, J.J.; et al. The Use of Raman Microscopy to Determine and Localize Vitamin E in Biological Samples. FASEB J. 2007, 21, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, D.-W.; Wei, Q.; Pu, H. Quantification and Visualization of α-Tocopherol in Oil-in-Water Emulsion Based Delivery Systems by Raman Microspectroscopy. LWT 2018, 96, 66–74. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chiu, S.-J.; Lee, K.-T.; Chien, W.-C.; Lin, C.-T.; Huang, C.-A. Study of Poly(Vinyl Alcohol)/Titanium Oxide Composite Polymer Membranes and Their Application on Alkaline Direct Alcohol Fuel Cell. J. Power Sources 2008, 184, 44–51. [Google Scholar] [CrossRef]
- Mudalige, A.; Pemberton, J.E. Raman Spectroscopy of Glycerol/D2O Solutions. Vib. Spectrosc. 2007, 45, 27–35. [Google Scholar] [CrossRef]
- Badawi, H.M. Vibrational Spectra and Assignments of 2-Phenylethanol and 2-Phenoxyethanol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ansari, Z.; Bhattacharya, T.S.; Saha, A.; Sen, K. Block Copolymer Mediated Generation of Bimetallic Ni-Pd Nanoparticles: Raman Sensors of Ethyl Paraben and Ciprofloxacin. React. Funct. Polym. 2018, 124, 1–11. [Google Scholar] [CrossRef]
- Elderderi, S.; Wils, L.; Leman-Loubière, C.; Henry, S.; Byrne, H.J.; Chourpa, I.; Munnier, E.; Elbashir, A.A.; Boudesocque-Delaye, L.; Bonnier, F. Comparison of Raman and Attenuated Total Reflectance (ATR) Infrared Spectroscopy for Water Quantification in Natural Deep Eutectic Solvent. Anal. Bioanal. Chem. 2021, 413, 4785–4799. [Google Scholar] [CrossRef] [PubMed]
- Elderderi, S.; Wils, L.; Leman-Loubière, C.; Byrne, H.J.; Chourpa, I.; Enguehard-Gueiffier, C.; Munnier, E.; Elbashir, A.A.; Boudesocque-Delaye, L.; Bonnier, F. In Situ Water Quantification in Natural Deep Eutectic Solvents Using Portable Raman Spectroscopy. Molecules 2021, 26, 5488. [Google Scholar] [CrossRef]
- Makki, A.A.; Elderderi, S.; Massot, V.; Respaud, R.; Byrne, H.J.; Tauber, C.; Bertrand, D.; Mohammed, E.; Chourpa, I.; Bonnier, F. In Situ Analytical Quality Control of Chemotherapeutic Solutions in Infusion Bags by Raman Spectroscopy. Talanta 2021, 228, 122137. [Google Scholar] [CrossRef] [PubMed]
- Makki, A.A.; Massot, V.; Byrne, H.J.; Respaud, R.; Bertrand, D.; Mohammed, E.; Chourpa, I.; Bonnier, F. Vibrational Spectroscopy for Discrimination and Quantification of Clinical Chemotherapeutic Preparations. Vib. Spectrosc. 2021, 113, 103200. [Google Scholar] [CrossRef]








| N° | PVA Solution (g) | Glycerol (g) | AMA (g) | NanoPHE (g) |
|---|---|---|---|---|
| C0 | 8.8065 | 1.0572 | 0.1006 | - (0.1162 g of water) |
| C1 | 8.80850 | 1.0140 | 0.1027 | 0.11500 |
| C2 | 8.65000 | 0.9957 | 0.1023 | 0.26190 |
| C3 | 8.40000 | 1.0020 | 0.1010 | 0.53590 |
| C4 | 8.15000 | 1.0147 | 0.1017 | 0.74240 |
| C5 | 7.89930 | 1.0031 | 0.1020 | 1.02180 |
| N° | Nominal % of PHE (w/w) | Nominal % of PVA (w/w) | Nominal % of Glycerol (w/w) | Nominal % of AMA (w/w) |
|---|---|---|---|---|
| C0 | 0 | 46.3 | 48.96 | 4.7 |
| C1 | 0.333 | 47.2 | 47.5 | 4.8 |
| C2 | 0.761 | 47.1 | 46.9 | 4.8 |
| C3 | 1.53 | 46.5 | 46.5 | 4.7 |
| C4 | 2.09 | 45.7 | 46.4 | 4.6 |
| C5 | 2.86 | 45.5 | 45.5 | 4.6 |
| Raman Shift (cm−1) | Shift Assignments |
|---|---|
| 1636–1639 | C=C stretching |
| 1440 | CH2 scissoring, asymmetric CH3 bending |
| 1294 | C-OH (enol group) bending, C-C-H bending (aromatic ring), CH2 twisting |
| 1257 | C-C stretching, =CH bending |
| 1202 | C-C stretching |
| 1169 | C-O stretching, C-C stretching |
| 1114 | C-O stretching, C-C stretching |
| 846 | C-C stretching |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Gheluwe, L.; Munnier, E.; Kichou, H.; Kemel, K.; Mahut, F.; Vayer, M.; Sinturel, C.; Byrne, H.J.; Yvergnaux, F.; Chourpa, I.; et al. Confocal Raman Spectroscopic Imaging for Evaluation of Distribution of Nano-Formulated Hydrophobic Active Cosmetic Ingredients in Hydrophilic Films. Molecules 2021, 26, 7440. https://doi.org/10.3390/molecules26247440
Van Gheluwe L, Munnier E, Kichou H, Kemel K, Mahut F, Vayer M, Sinturel C, Byrne HJ, Yvergnaux F, Chourpa I, et al. Confocal Raman Spectroscopic Imaging for Evaluation of Distribution of Nano-Formulated Hydrophobic Active Cosmetic Ingredients in Hydrophilic Films. Molecules. 2021; 26(24):7440. https://doi.org/10.3390/molecules26247440
Chicago/Turabian StyleVan Gheluwe, Louise, Emilie Munnier, Hichem Kichou, Kamilia Kemel, Frédéric Mahut, Marylène Vayer, Christophe Sinturel, Hugh J. Byrne, Florent Yvergnaux, Igor Chourpa, and et al. 2021. "Confocal Raman Spectroscopic Imaging for Evaluation of Distribution of Nano-Formulated Hydrophobic Active Cosmetic Ingredients in Hydrophilic Films" Molecules 26, no. 24: 7440. https://doi.org/10.3390/molecules26247440
APA StyleVan Gheluwe, L., Munnier, E., Kichou, H., Kemel, K., Mahut, F., Vayer, M., Sinturel, C., Byrne, H. J., Yvergnaux, F., Chourpa, I., & Bonnier, F. (2021). Confocal Raman Spectroscopic Imaging for Evaluation of Distribution of Nano-Formulated Hydrophobic Active Cosmetic Ingredients in Hydrophilic Films. Molecules, 26(24), 7440. https://doi.org/10.3390/molecules26247440







