New Monoterpenoid Indoles with Osteoclast Activities from Gelsemium elegans
Abstract
:1. Introduction
2. Results and Discussion
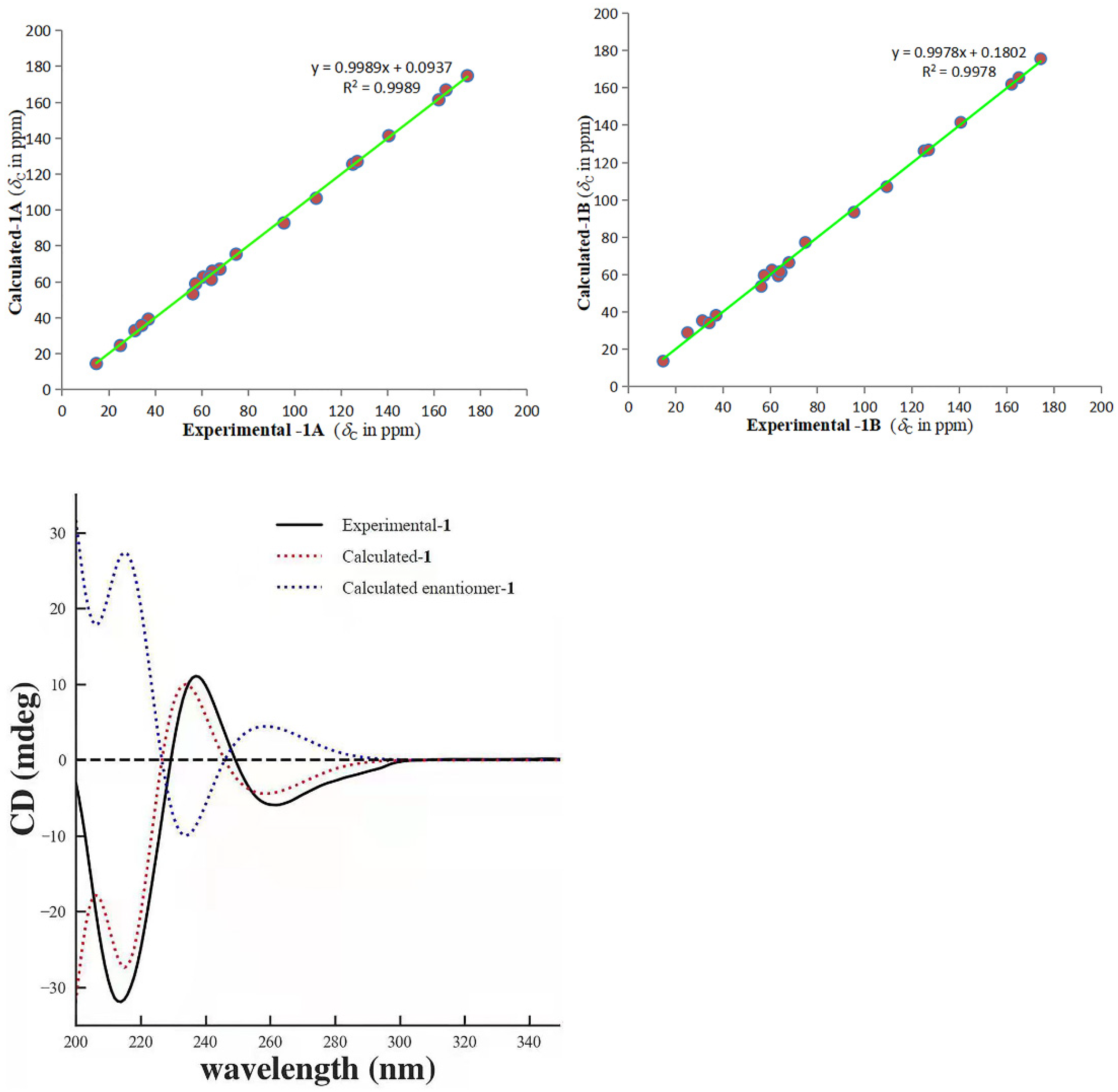
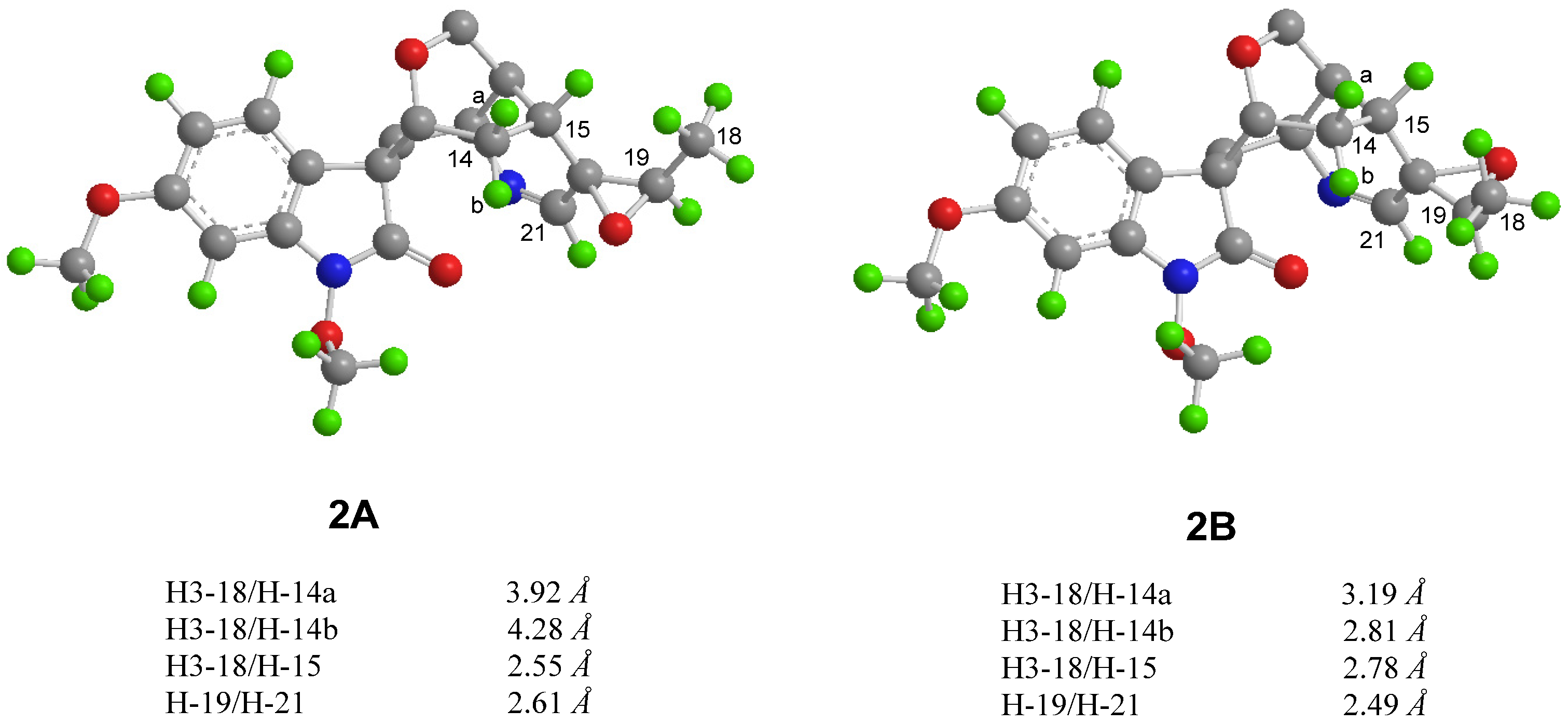
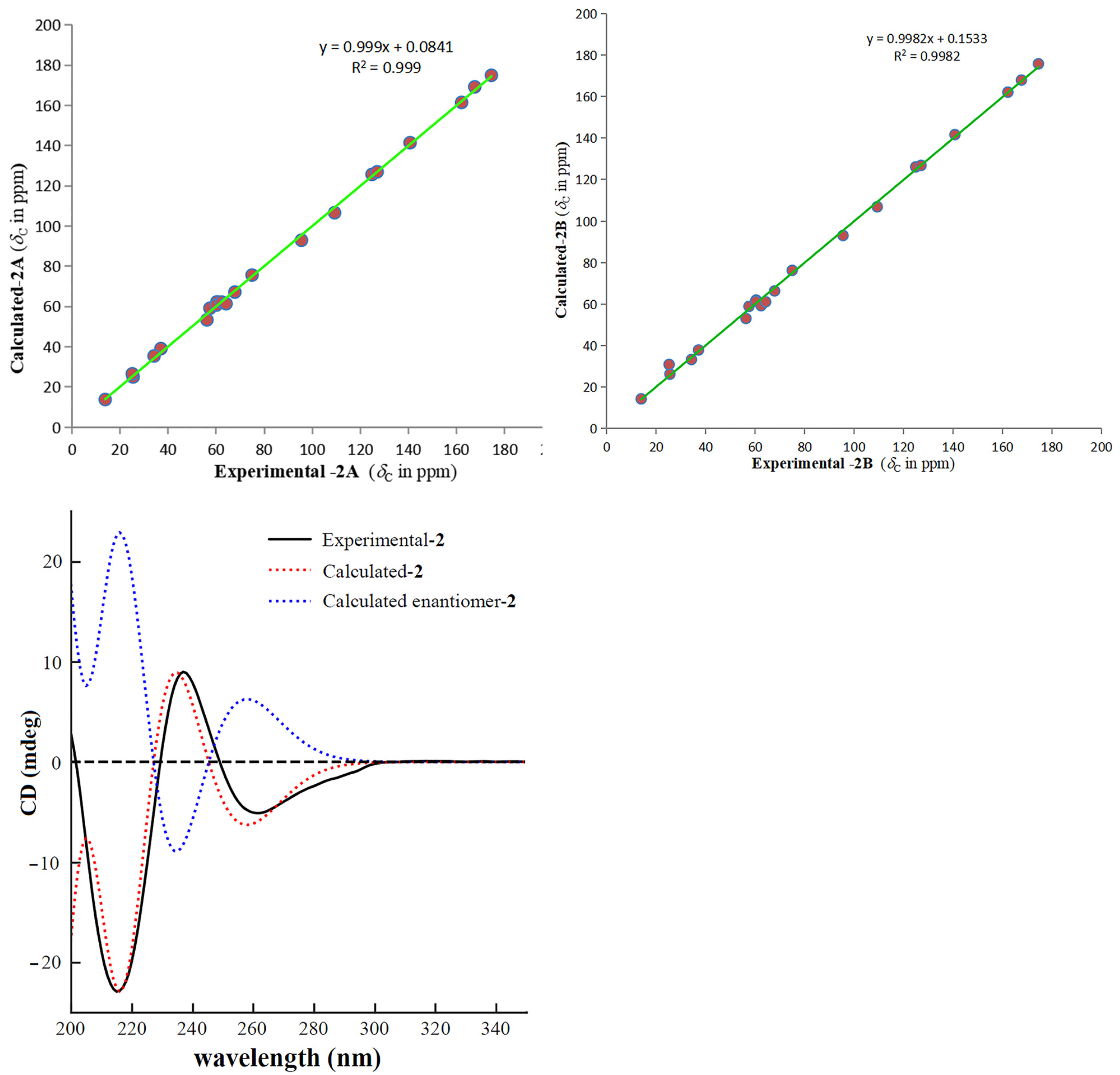
2.1. Structure Elucidation
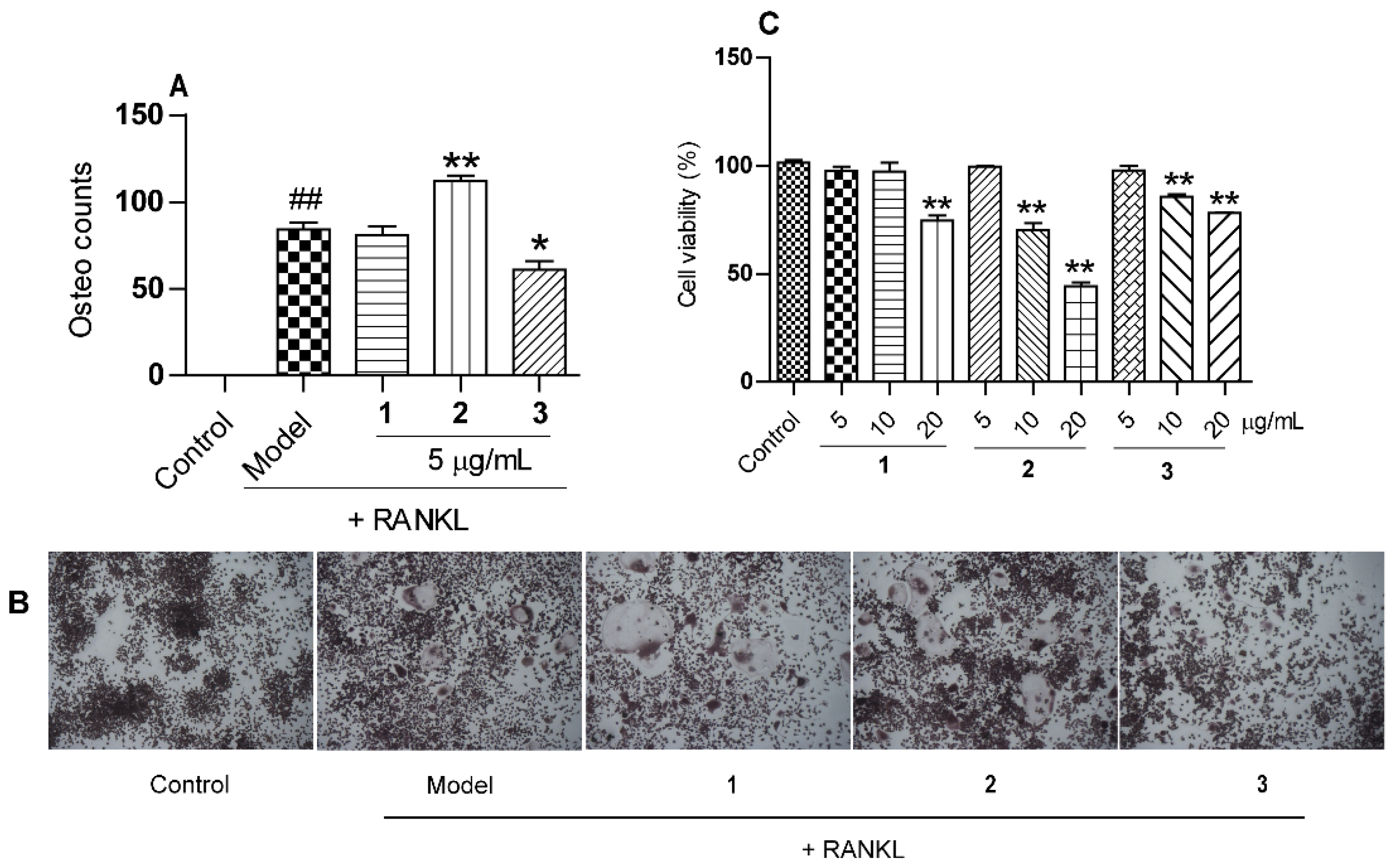
2.2. The Effects of Compounds 1–3 on Osteoclastogenesis Induced by RANKL
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Gelselegandine D (1)
3.3.2. Gelselegandine E (2)
3.4. ECD Calculation
3.5. 13C NMR Calculation
3.6. Cell Culture and Cell Viability Assay
3.7. Osteoclastogenesis Assay In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yang, M.; Ma, Y.; Wang, Z.; Khan, A.; Zhou, W.; Zhao, T.; Cao, J.; Cheng, G.; Cai, S. Phenolic constituents, antioxidant and cytoprotective activities of crude extract and fractions from cultivated artichoke inflorescence. Ind. Crops Prod. 2020, 143, 111433. [Google Scholar] [CrossRef]
- Li, F.R.; Liu, L.; Liu, Y.P.; Wang, J.T.; Yang, M.L.; Khan, A.; Qin, X.J.; Wang, Y.D.; Cheng, G.G. HRESIMS-guided isolation of aspidosperma-scandine type bisindole alkaloids from Melodinus cochinchinensis and their anti-inflammatory and cytotoxic activities. Phytochemistry 2021, 184, 112–673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, M.; Khan, A.; Cai, S.; Li, X.; Liu, J.; Kai, G.; Zhao, T.; Cheng, G.; Cao, J. Epigynumgenane-type pregnane glycosides from Epigynum cochinchinensis and their immunosuppressive activity. Phytochemistry 2019, 168, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Y.; He, S.; Khan, A.; Xue, Q.; Cui, Q.; Liu, L.; Liu, Y.; Cheng, G. Targeted isolation of terpenoid indole alkaloids from Melodinus cochinchinensis (Lour.) Merr. using molecular networking and their biological activities. Ind. Crops Prod. 2020, 157, 112922. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, X.; Zhang, L.Y.; Hu, X.Y.; Zhou, Y.Q.; Zhou, Y. Chemical constituents of Sophora tonkinensis. Chem. Nat. Compd. 2020, 56, 1140–1142. [Google Scholar] [CrossRef]
- Wei, X.; Dai, Z.; Yang, J.; Khan, A.; Yu, H.F.; Zhao, Y.L.; Wang, Y.F.; Liu, Y.P.; Yang, Z.F.; Huang, W.Y.; et al. Unprecedented sugar bridged bisindoles selective inhibiting glioma stem cells. Bioorganic Med. Chem. 2018, 26, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L.; Khosla, S.; Melton, L.J., 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Ti, Y.F.; Wang, R.; Zhao, J.N. Mechanism of osteoclast in bone resorption, China J. Orthop. Trauma 2014, 27, 529–532. [Google Scholar]
- De Villiers, T.J.; Gass, M.L.S.; Haines, C.J.; Hall, J.E.; Lobo, R.A.; Pierroz, D.D.; Rees, M. Global consensus statement on menopausalhormone therapy. Climacteric 2013, 16, 203–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adomaityte, J.; Farooq, M.; Qayyum, R. Effect of raloxifene therapy on venous thromboembolism in postmenopausal women: A Meta-analysis. Thromb. Haemost. 2008, 99, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Huang, X.T.; Zhang, L.Y.; Hu, X.Y.; Zhang, W.; Zhou, Y.Q.; Yu, H.F.; Ding, C.F.; Zhang, L.C.; Liu, X.; et al. New oxindole alkaloids with selective osteoclast inhibitory activity from Gelsemium elegans. Nat. Prod. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yang, J.; Ma, H.X.; Ding, C.F.; Yu, H.F.; Zhao, Y.L.; Liu, Y.P.; Khan, A.; Wang, Y.F.; Yang, Z.F.; et al. Antimicrobial indole alkaloids with adductive C9 aromatic unit from Gelsemium elegans. Tetrahedron Lett. 2018, 59, 2066–2070. [Google Scholar] [CrossRef]
- Xu, Y.K.; Yang, L.; Liao, S.G.; Cao, P.; Wu, B.; Hu, H.B.; Guo, J.; Zhang, P. Koumine, Humantenine, and Yohimbane Alkaloids from Gelsemium elegans. J. Nat. Prod. 2015, 78, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.L.; Su, Y.P.; Liu, M.; Xu, Y.; Yang, J.; Liao, K.J.; Yu, C.X. Medicinal plants of the genus Gelsemium (Gelsemiaceae, Gentianales)—A review of their phytochemistry, pharmacology, toxicology and traditional use. J. Ethnopharmacol. 2014, 152, 33–52. [Google Scholar] [CrossRef]
- Zhang, Z.; Di, Y.T.; Wang, Y.H.; Zhang, Z.; Mu, S.Z.; Fang, X.; Zhang, Y.; Tan, C.J.; Zhang, Q.; Yan, X.H.; et al. Gelegamines A–E: Five new oxindole alkaloids from Gelsemium elegans. Tetrahedron 2009, 65, 4551–4556. [Google Scholar] [CrossRef]
- Xu, H.C.; Hu, K.; Shi, X.H.; Tang, J.W.; Li, X.N.; Sun, H.D.; Puno, P.T. Synergistic use of NMR computation and quantitative interproton distance analysis in the structural determination of neokadcoccitane A, a rearranged triterpenoid featuring an aromatic ring D from Kadsura coccinea. Org. Chem. Front. 2019, 6, 1619–1626. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhao, Z.Z.; Cheng, G.G.; Zhao, K.; Han, K.Y.; Zhou, L.; Feng, T.; Li, Z.H.; Liu, J.K. Immunosuppressive Nor-isopimarane Diterpenes from Cultures of the Fungicolous Fungus Xylaria longipes HFG1018. J. Nat. Prod. 2020, 83, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.P.; Nishishita, K.; Sakai, E.; Yoshida, H.; Kato, Y.; Tsukuba, T.; Okamoto, K. Berberine inhibits RANKL-induced osteoclast formation and survival through suppressing the NF-kappaB and Akt pathways. Eur. J. Pharmacol. 2008, 580, 70–79. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Vandermeersch, T.; Flynn, C.J.; Maguire, A.R.; Hutchison, G.R. Confab-Systematic generation of diverse low-energy conformers. J. Cheminform. 2011, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational prediction of 1H and 13C chemical shifts: A useful tool for natural product, mechanistic, and synthetic organic chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef] [PubMed]

 ), 1H-1H COSY (
), 1H-1H COSY (  ), and ROESY (
), and ROESY (  ) correlations of compounds 1 and 2.
) correlations of compounds 1 and 2.

| NO. | 1 a | 2 a | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 2 | 174.4 | 174.5 | ||
| 3 | 3.57 (d, J = 8.2, 1H), | 74.8 | 3.63 (d, J = 7.8, 1H) | 74.9 |
| 5 | 4.32 (tt, J = 7.3, 2.1, 1H) | 60.7 | 4.26 (tt, J = 7.2, 2.4, 1H) | 60.4 |
| 6 | 2.66 (dd, J = 15.8, 7.2, 1H), 2.21 (d, J = 15.8, 1H) | 37.1 | 2.61 (dd, J = 15.7, 7.2, 1H), 2.14 (dd, J = 15.7, 2.2, 1H) | 37.1 |
| 7 | 57.4 | 57.4 | ||
| 8 | 125.1 | 124.8 | ||
| 9 | 7.39 (d, J = 8.3, 1H) | 127.0 | 7.40 (d, J = 8.3, 1H) | 127.0 |
| 10 | 6.66 (dd, J = 8.3, 2.4, 1H) | 109.3 | 6.66 (dd, J = 8.3, 2.4, 1H) | 109.3 |
| 11 | 162.1 | 162.1 | ||
| 12 | 6.58 (d, J = 2.4, 1H) | 95.4 | 6.58 (d, J = 2.4, 1H) | 95.5 |
| 13 | 140.6 | 140.6 | ||
| 14 | 2.55 (m, 1H), 2.05 (ddd, J = 15.3, 11.2, 8.3, 1H) | 25.0 | 2.57 (dd, J = 15.2, 5.4, 1H), 2.07 (ddd, J = 15.2, 11.5, 7.9, 1H) | 25.5 |
| 15 | 1.91 (m, 1H) | 31.3 | 2.18 (m, 1H) | 25.1 |
| 16 | 2.56 (m, 1H) | 34.2 | 2.44 (m, 1H) | 34.2 |
| 17 | 4.41 (d, J = 10.5, 1H), 4.03 (dd, J = 10.5, 4.0, 1H) | 67.9 | 4.40 (d, J = 10.5, 1H), 4.06 (dd, J = 10.5, 3.9, 1H) | 67.8 |
| 18 | 1.45 (d, J = 5.6, 3H) | 14.7 | 1.40 (d, J = 5.4, 3H) | 13.9 |
| 19 | 3.24 (q, J = 5.6, 1H) | 64.6 | 3.33 (m, 1H) | 60.1 |
| 20 | 63.4 | 62.3 | ||
| 21 | 7.61 (s, 1H) | 165.2 | 7.25 (s, 1H) | 167.6 |
| NOMe | 3.93 (s, 3H) | 64.2 | 3.93 (s, 3H) | 64.2 |
| ArOMe | 3.82 (s, 3H) | 56.2 | 3.82 (s, 3H) | 56.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Guo, R.; Wang, X.; Liang, J.-J.; Yu, H.-F.; Ding, C.-F.; Feng, T.-T.; Zhang, L.-Y.; Liu, X.; Hu, X.-Y.; et al. New Monoterpenoid Indoles with Osteoclast Activities from Gelsemium elegans. Molecules 2021, 26, 7457. https://doi.org/10.3390/molecules26247457
Wei X, Guo R, Wang X, Liang J-J, Yu H-F, Ding C-F, Feng T-T, Zhang L-Y, Liu X, Hu X-Y, et al. New Monoterpenoid Indoles with Osteoclast Activities from Gelsemium elegans. Molecules. 2021; 26(24):7457. https://doi.org/10.3390/molecules26247457
Chicago/Turabian StyleWei, Xin, Rui Guo, Xiao Wang, Jia-Jun Liang, Hao-Fei Yu, Cai-Feng Ding, Ting-Ting Feng, Li-Yan Zhang, Xia Liu, Xin-Yue Hu, and et al. 2021. "New Monoterpenoid Indoles with Osteoclast Activities from Gelsemium elegans" Molecules 26, no. 24: 7457. https://doi.org/10.3390/molecules26247457







