Abstract
Cupressus sempervirens L., Juniperus communis L. and Cistus ladanifer L. are Mediterranean arboreal and shrub species that possess essential oils (EO) in their leaves and branches. This study aimed at characterizing the EOs obtained by steam distillation from the three species collected in different locations from Spain (Almazán, Andévalo, Barriomartín, Cerezal, Ermitas and Huéscar). For this purpose, volatiles composition was determined by GC-MS, and different bioactivities were evaluated. The highest content in terpenes was observed in C. sempervirens (Huéscar origin) followed by J. communis (Almazán origin), corresponding to 92% and 91.9% of total compounds, respectively. With exception of C. ladanifer from Cerezal that presented viridiflorol as the most abundant compound, all the three species presented in common the α-pinene as the major compound. The EOs from C. ladanifer showed high antibacterial potential, presenting MIC values from 0.3 to 1.25 mg/mL. Concerning other bioactivities, C. ladanifer EO revealed an oxidation inhibition of 83%, while J. communis showed cytotoxicity in the MCF-7 cell line, and C. sempervirens and C. ladanifer EOs exhibited the highest potential on NCI-H460 cell lines. Nevertheless, some EOs revealed toxicity against non-tumoral cells but generally presented a GI50 value higher than that of the tumor cell lines.
1. Introduction
For decades, the need to change from a fossil-based economy to bio-based systems has been discussed. It has been pushing the industry to transition towards a green, sustainable and circular economy, improving energy efficiency by using renewable raw materials [1,2]. In this sense, the use of lignocellulosic biomass, which represents 89.3% of the total biomass, is being explored as a potential substrate to obtain compounds of interest, such as bioactive molecules, through an integrated biorefinery approach.
Some crop and forest biomass resources can be an effective source of high-value compounds since this raw material is underused and naturally recycled into ecosystems. Thus, it is a low-cost feedstock that can assume a role of great importance for food, pharmaceutical and cosmetic industries due to beneficial effects, including antioxidant, antimicrobial, anti-inflammatory and anti-tumoral properties, that have been attributed to different compounds present in such agroforestry biomass [3,4].
The majority of the shrubs belonging to the Cupressaceae and Cistaceae families can be found all over the Mediterranean region and have the capacity of growing in open areas with deficient soils and harsh environments [5]. In addition, they are intensely aromatic plants due to the high content of essential oils (EOs) in their twigs and leaves [6]. In particular, the genus Cupressus and Juniperus (Cupressaceae) and Cistus (Cistaceae) are widespread include some species, such as Cupressus sempervirens L., Cistus ladanifer L. and Juniperus communis L., being their extracts or EOs used for many years in traditional medicine [7].
Moreover, bioactivity studies reported these species’ antimicrobial, anti-inflammatory, and antioxidant properties [8,9]. Several pharmacologically interesting compounds were already identified in their extracts, e.g., tannins, flavonoids, phenolic acids and terpenes [10,11]. Given their richness in bioactive molecules and bioactive power, these compounds, can effectively apply in different industries, such as food, cosmetics, and pharmaceuticals. [6,12].
The species C. sempervirens L. and J. communis L. are widely planted as ornamental shrubs in parks and gardens, while C. ladanifer L. is frequently found in wild areas. However, these species present a high potential to be grown in marginal lands, which are not used either for other agricultural or forestry purposes, allowing for improving soil fertility and organic carbon stocks while simultaneously generating biomass that can be used for bio-based value chains.
In the BBI-JU BeonNAT project, some of these species have been selected to create new dedicated plantations that can be used to produce valuable EOs. EOs are complex mixtures of hydrocarbons and oxygenated hydrocarbons from the isoprenoid pathways, mainly mono-, di-, and sesqui- terpenes [13]. So far, different studies have investigated the chemical composition of C. ladanifer, C. sempervirens and J. communis EOs by gas chromatography coupled to mass spectrometry (GC-MS) [14,15,16,17], with α-pinene being frequently reported as the major component in these three species [10].
However, they mainly refer to the essential oil obtained by laboratory hydrodistillation from the leaves of C. ladanifer and C. sempervirens, as also from J. communis berries with few or non-existing data regarding other plant parts. Hydro and steam distillations are some of the most traditional ways to isolate volatile compounds from medicinal and aromatic plants [18]. The extraction method is a recognized factor that may greatly impact the quality of EOs. Moreover, the chemical composition of essential oils can be affected by other factors, such as environmental conditions [19].
Therefore, as part of the BBI-JU BeonNAT project, the present study aimed at evaluating the chemical composition of the EOs extracted by steam distillation from the crown biomass (with branches diameter < 50 mm) of these three species and studying their bioactive properties, namely antioxidant, antibacterial, cytotoxic and anti-inflammatory, to access its potential as ingredients for bio-based products development in different industries. Moreover, each species was collected from two different locations in Spain to look for different chemotypes associated with different geographical locations and/or elevations.
2. Results and Discussion
2.1. Essential Oil Yields
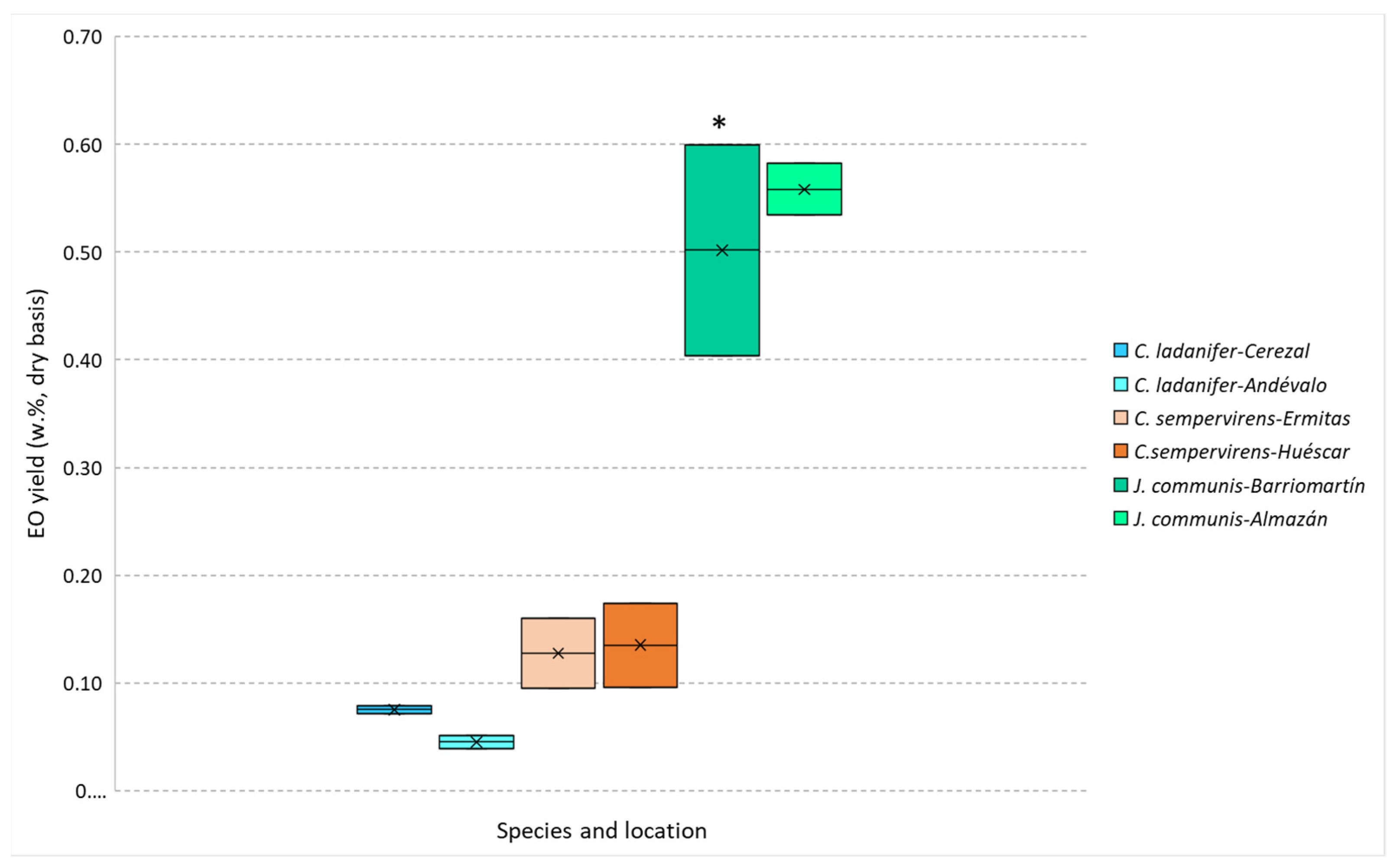
The extraction yield of the EOs obtained by steam distillation was higher for J. communis, followed by C. sempervirens and C. ladanifer, as can be observed in Figure 1. The obtained values are within the range reported in the literature for C. ladanifer (from 0.01 to 0.63%) [20,21,22,23] while being lower for C. sempervirens for which reported yields varied from 0.20 to 0.87% [24,25,26] and higher for J. communis (reported yields from 0.05 to 0.70%) [23,27,28,29,30]. These differences are most probably related to (i) the used samples (crown biomass that includes twigs, leaves and fruits instead of leaves or berries); (ii) the location where the plant samples were collected; (iii) the date when these samples were obtained, and (iv) the EO extraction methodology. These factors are important since the extraction yield of essential oils depends on some variables like the part of the plant material, seasonal variations, environmental and cultivation conditions, plant age, harvesting time, and type of distillation [17].
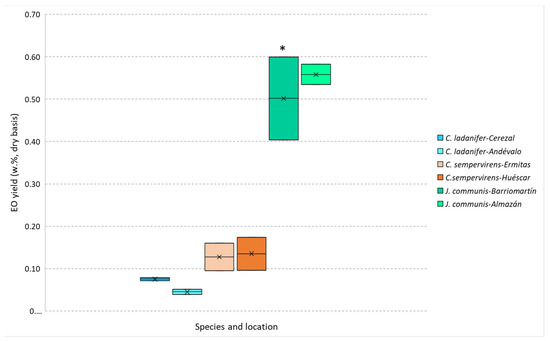
Figure 1.
J. communis, C. sempervirens and C. ladanifer EOs yields obtained by steam distillation. * Samples differ significantly (p < 0.05) between the different origins, obtained by Student’s t-test.
2.2. Chemical Composition
The composition of the EOs in terms of volatile compounds is presented in Table 1. The GC-MS analysis led to the identification of different components, representing between 84–92% of total oil constituents. The EOs of the three plant species showed four main chemical classes with a predominance of monoterpene hydrocarbons in all samples, except for C. ladanifer EO from Cerezal, for which oxygen-containing sesquiterpenes was the leading group. In both samples of J. communis, EO sesquiterpenes are also an important fraction, as they ranged from 19.3% to 26.2%. In general, the major compounds identified in C. ladanifer were common in both Cerezal and Andévalo samples but in different amounts: viridiflorol (24.13 ± 0.74 and 13.36 ± 1.41%), α-pinene (19.27 ± 0.26 and 42.50 ± 0.96%), ledol (6.94 ± 0.36 and 4.06 ± 0.17%), bornyl acetate (5.01 ± 0.02 and 4.16 ± 0.10%) and camphene (6.66 ± 0.01 and 2.15 ± 0.07%). While α-pinene is the predominant compound in the sample from Andévalo, in the one from Cerezal the main compound was viridiflorol. The obtained data are comparable and in line with the available reports described by other authors, despite some studies describing lower amounts of α-pinene than the ones herein reported. According to Verdeguer, et al. [31], the aerial parts of C. ladanifer collected in Spain presented high percentages of trans-pinocarveol (20%), followed by viridiflorol (13.59%), bornyl acetate (7.03%), terpinen-4-ol (6.37%), 2(10)-pinen-3-one (5.05%), α-pinene (4.70%) and camphene (1.17%). This report mentioned that other studies have previously found α-pinene (39%), viridiflorol (11.8%), ledol (3.3%) and bornyl acetate (3.1%) as major compounds in leaves and stems of C. ladanifer plants of Spanish origin collected in Corsica. Low amounts of α-pinene but higher in viridiflorol were reported in C. ladanifer fresh leaves and small branches from Morocco, which showed viridiflorol (19%), bornyl acetate (17%), camphene (12%), ledol (8%) and α-pinene (5%) as the main compounds [32]. Mediavilla et al., 2021 which used the same equipment and same steam distillation conditions for samples collected in Spain in two different periods, found α-pinene (39–52%), viridiflorol (6–10%), bornyl acetate (3%) and camphene (2–3%) as the main compounds in C. ladanifer samples. Zidane described a slightly different profile, Elmiz et al. (2013), who studied C. ladanifer fruits, stems, flowers and leaves and reported camphene (15.5%), borneol (11.1%), cyclohexanol-2,2,6-trimethyl (7.3%), terpineol-4 (6.3%) and α-pinene (4.2%) as the major compounds.

Table 1.
Chemical composition of essential oils extracted from tree and shrubs species.
Despite presenting some quantitative differences, the major compounds identified in the samples of J. communis were also similar in both locations. The major compounds in Barriomartín, Spain, were α-pinene (35.05 ± 0.02%), limonene (15.01 ± 0.26%), cis-thujopsene (8.04 ± 0.07%), sabinene (6.72 ± 0.40%), β-caryophyllene (3.51 ± 0.14%) and β-myrcene (3.24 ± 0.06%), while in Almazán, Spain, were α-pinene (23.96 ± 0.41%), limonene (21.30 ± 0.03%), cis-thujopsene (8.19 ± 0.14%), sabinene (7.86 ± 0.01%) and germacrene D (2.69 ± 0.03%). In general, the chemical composition was in good agreement with that of juniper berries essential oil defined in the European Pharmacopoeia (Ph. Eur. 10th), except for limonene (from 2–12%) [33]. Thus, these results suggest that juniper crown biomass can be a promising low-cost source of juniper EO due to the similar global composition with EO of juniper berries. Moreover, the obtained chemical profile is generally similar to that reported in previous studies regarding both juniper needles and berries. Chatzopoulou and Katsiotis [34] reported that the essential oil from the leaves of J. communis from northern Greece was dominated by α-pinene (41.3%) and sabinene (17.4%) while Cabral, et al. [35] and Caramiello, et al. [36] identified sabinene (30.5% and 41.4%, respectively) and α-pinene (29.6% and 13.4%, respectively) as major compounds in the needle oil from J. communis species grown in Turkey and the Northwestern Italian Alps, respectively. Mediavilla, Blázquez, Ruiz and Esteban [23] found α-pinene (16–21%), sabinene (18–34%), limonene (6–8%), β-myrcene (3–5%) and β-phellandrene (3–4%) as the major compounds in samples collected in Spain in two different periods. Höferl, Stoilova, Schmidt, Wanner, Jirovetz, Trifonova, Krastev and Krastanov [16] identified α-pinene (51.4%), myrcene (8.3%), sabinene (5.8%), limonene (5.1%) and β-pinene (5.0%) as the main compounds of J. communis berries while Koukos and Papadopoulou [37] detected α-pinene (27.22–62.08%), limonene (1.31–30.96%), myrcene (5.41–20.23%), sabinene (0.35–16.47%), citronellol (5.06–15.57%), β-caryophyllene (0.79–6.61%), borneol (0.86–4.51%) and β-pinene (1.89–3.47%). According to these authors, the minor compounds present in the fruit of J. communis were α-terpineol (0.50–2.64%), cedrol (0.56–1.84%), bornyl acetate (0.25–1.51%), eugenol (0.45–2.33%), geraniol (0.52–1.15%) and terpinolene (0.33–1.21%) [38]. These authors also found α-pinene (29.17%) and β-pinene (17.84%), sabinene (13.55%), limonene (5.52%), and myrcene (0.33%) as the main compounds present in the berries of this species. Therefore, from a qualitative point of view, the results obtained are in agreement with the available data on the literature.
Concerning the C. sempervirens species, the samples of the two evaluated locations in Spain presented similar qualitative and quantitative compositions. In both (Huéscar and Ermitas), the most abundant compound was α-pinene (52.32 ± 3.48 and 55.95 ± 0.46%, respectively) followed by 3-carene (16.18 ± 1.12 and 13.09 ± 2.70%) and cedrol (4.63 ± 0.25 and 2.88 ± 0.11%). Additionally, both locations presented amounts in the range of 2.5–5% for limonene (2.74 ± 0.23 and 2.66 ± 0.01%), germacrene D (3.13 ± 0.19 and 1.38 ± 0.01%) and terpinolene (3.58 ± 0.34 and 2.60 ± 0.02%), with samples from Huéscar presenting slightly higher amounts of these three compounds. The two locations also presented similar contents of other minor compounds, such as β-myrcene (2.63 ± 0.19 and 2.15 ± 0.07%), β-pinene (1.42 ± 0.13 and 1.25 ± 0.02%) and α-fenchene (0.64 ± 0.04 and 0.74 ± 0.03%). The compounds mentioned above are in good agreement with the ISO 20809 [33] concerning the Spanish type C. sempervirens, except for 3-carene from Ermitas location and cedrol from Huéscar location that fit the French type, and terpinolene that is not part of the chemical profile presented on ISO 20809 [33]. According to Selim, Adam, Hassan and Albalawi [24] α-pinene (48.6%), δ-3-carene (22.1%), limonene (4.6%) and α-terpinolene (4.5%) were the main components of the oil obtained from the aerials parts of C. sempervirens collected in Saudi Arabia. The same main compounds were found in the aerials parts of this species from Tunisia, being α-pinene (37.14%), δ-3-carene (19.67%), limonene (5.43%) and α-terpinolene (4.69%) as the most abundant compounds [25]. Mazari, et al. [39] found cedrol (8.3%) as the second most important constituent of the C. sempervirens oil. In another study, Rguez, Djébali, Ben Slimene, Abid, Hammemi, Chenenaoui, Bachkouel, Daami-Remadi, Ksouri and Hamrouni-Sellami [17] evaluated the importance of the phenological stages and found that the main constituents for all the stages were α-pinene, β-caryophyllene and germacrene D. Nevertheless, some differences between the phenological stages were noticed by the presence of specific minor compounds meaning that the vegetative stage was characterized by the appearance of γ-cadinene (2.75%) and γ-muurolene (2.95%), the flowering stage by camphene (0.33%), m-mentha-1,8 diene (0.32%) and α-farensene (0.21%) and the fructification stage was characterized by the presence of δ-3-carene.
2.3. Bioactive Evaluation
2.3.1. Antibacterial Activity
Table 2 presents the results of the antibacterial capacity against a panel of bacteria selected according to their importance in public health. None of the essential oils showed the potential to inhibit the growth of gram-negative bacteria K. pneumoniae and P. mirabilis. On the other hand, the E. coli strain was sensitive to all the tested EOs. At the same time, M. morganii was only sensitive to C. ladanifer EOs, being notably inhibited by the EO from Cerezal location (MIC = MBC = 0.6 mg/mL). Among the samples, C. ladanifer EO was the only one that presented inhibitory potential against the gram-negative bacteria P. aeruginosa, which is frequently associated with nosocomial infections.

Table 2.
Antimicrobial activity of the samples obtained by steam distillation (MIC and MBC values; mg/mL).
In general, gram-positive bacteria presented lower MIC values, being most susceptible compared with the gram-negative strains. E. faecalis was inhibited and killed by all the tested EOs, with MIC and MBC values ranging from 0.6 mg/mL to 2.5 mg/mL. The only sample that could not inhibit the growth of all the tested gram-positive bacteria was J. communis EO from Barriomartín location as it was not effective against L. monocytogenes.
In general, C. ladanifer exhibited the most potent antimicrobial potential, with samples from both locations showing the lowest MIC and MBC values for both gram-negative and gram-positive bacteria compared with other data described in the literature. Outstanding results were obtained against MRSA, a bacteria associated with nosocomial infections. According to Mohammed, Said, Fouzia, Kawtar, Zoubida, Abdelilah, Elhourri and Ghizlane [21], S. aureus shows high sensitivity (MIC = MBC = 6.25 mg/mL), and E. coli and P. aeruginosa respond very positively (MIC = MBC = 25 mg/mL for both strains) to the essential oil obtained from C. ladanifer stems and leaves. Curiously they found different compounds in major contents in the chemical characterization, suggesting that synergism between these compounds can occur. Identical results were reported by Benali, et al. [40] against S. aureus (MIC = MBC = 6.25 mg/mL), with much lower MIC and MBC values being observed for P. mirabilis (MIC = MBC = 0.19 mg/mL), which was the most sensitive strain when assessing the antimicrobial activity of C. ladanifer EO (aerial parts).
On the contrary, using a different method, the disk agar diffusion method, Tavares, Martins, Faleiro, Miguel, Duarte, Gameiro, Roseiro and Figueiredo [22] reported a weak antimicrobial activity for C. ladanifer essential oil against E. coli and S. aureus. Benayad, et al. [41] also studied the antimicrobial effect of C. ladanifer EO (full plant) and reported MIC values between 50–500 µg/mL, with the lower MIC being obtained against multiresistant S. aureus (MIC = 50 µg/mL). Although Benayad, Mennane, Charof, Hakiki and Mosaddak [41] obtained stronger activity with lower MIC values, they also verified that the EO was effective against both gram-positive and gram-negative bacteria, with better activity against the gram-positive, and no inhibition was observed for K. pneumoniae at the higher tested concentration. Although the studies reported by Benali, Bouyahya, Habbadi, Zengin, Khabbach, Achbani and Hammani [40] and Benayad, Mennane, Charof, Hakiki and Mosaddak [41] are in good agreement with the present ones, these authors did not analyze the chemical composition of the EO; thus it was not possible to corroborate the results with the chemical composition.
Regarding C. sempervirens EO, different studies reported its inability to inhibit the growth of P. aeruginosa, either using the agar dilution [42] or the broth microdilution [43] methodologies. Hammer, Carson and Riley [43] achieved identical results for P. aeruginosa; however, the oil obtained from the leaves and twigs was able to inhibit the growth of E. coli, S. aureus and E. faecalis at the maximal concentration tested (MICs > 2.0% v/v) which is in line with the herein obtained results. Similar results were obtained by [44], who reported that the EO from the aerial parts of C. sempervirens showed an antimicrobial activity more pronounced against gram-positive than gram-negative bacteria, with MIC and MBC values of 0.07 µg/mL and 0.31 µg/mL for S. aureus and E. coli respectively. One of the main compounds identified was δ-3-carene in both studies. Nevertheless, in this study the authors also reported that P. aeruginosa was sensitive to C. sempervirens EO (MIC = MBC= 0.31 µg/mL). Contrarily to previous studies and the results herein obtained, Mazari, Bendimerad, Bekhechi and Fernandez [39] reported that the EO from C. sempervirens (leaves) was ineffective against S. aureus, E. faecalis, E. coli and P. aeruginosa at the highest concentration tested (10 µL/mL). In this study, the common major compound (carene) was not identified, which probably justifies these results.
Regarding the results herein obtained for J. communis EO, they are in good agreement with previous studies that also reported the capacity of the EO obtained from different parts of the plant against S. aureus, E. faecalis and E. coli while not presenting antimicrobial activity at the highest tested concentration (2% v/v in the broth dilution and 5 mg/mL in the agar disc diffusion methods) for K. pneumoniae, P. mirabilis and P. aeruginosa [6,43]. Angioni, Barra, Russo, Coroneo, Dessí and Cabras [27] reported contradictory results, which showed that the antimicrobial activity of the J. communis EO from berries and leaves was generally nonsignificant against S. aureus and E. coli at the highest concentration tested (900 µg/mL). Falcão, et al. [45] evaluated the antimicrobial activity of two commercial samples of J. communis EO and one obtained by hydrodistillation of the berries and found that all were able to inhibit the growth of S. aureus, Bacillus cereus, Bacillus subtilis, E. coli, E. faecalis and K. pneumonia. In contrast, only one commercial EO presented activity against P. mirabilis, P. aeruginosa and Salmonella Typhimurium. The wider range of activity of this EO was related to its different chemical composition compared to the other samples, namely its higher content in oxygenated monoterpenes, such as terpinene-4-ol and 1,8-cineole, which have been associated with antimicrobial properties. The herein studied J. communis EO present very low content of terpinene-4-ol and 1,8-cineol was not detected, what can possibly explain the lower activity of these oils.
Comparing the antibacterial potential of the analyzed EOs with common antibiotics, none of them could compete with these commercial drugs. Nevertheless, commercial drugs are isolated compounds, while EOs are a mixture of different compounds. Nonetheless, given the growing resistance to antibiotics, new antibacterial agents are needed, and the exploitation of novel sources of antibacterials is a major research topic worldwide.
2.3.2. Antioxidant Activity
Analyzing the antioxidant values obtained from the cellular-based assays (Table 3), all the samples revealed the capacity to inhibit the oxidation process, highlighting the sample J. communis from Almazán that inhibited about 78% of oxidation, presenting a GI50 value of 324 ± 8 µg/mL, and C. ladanifer from Andévalo that inhibited about 83% of oxidation and exhibited a GI50 of 336 ± 8 µg/mL.

Table 3.
Antioxidant, cytotoxic and anti-inflammatory activities of C. ladanifer, C. sempervirens, and J. communis essential oils.
To the best of our knowledge, data are scarce in the literature regarding the antioxidant properties of the studied EO, with most studies available relying on the use of the DPPH method. Boukhris, Regane, Yangui, Sayadi and Bouaziz [44] measured the antioxidant activity of C. sempervirens EO by the radicals-scavenging effect on 2,2-diphenyl-1-picrylhydrazyl (DPPH) and reported an EC50 value of 7.70 ± 0.70 µg/mL, however, higher IC50 values (151 µg/mL and 290.09 µg/mL) have been reported for this species EO using the same methodology [25,26]. Additionally, using the DPPH assay, Höferl, Stoilova, Schmidt, Wanner, Jirovetz, Trifonova, Krastev and Krastanov [16] reported an IC50 of 944 µg/mL for Juniper berry oil. On the contrary, using this assay, no activity was found for C. ladanifer EO at the highest tested concentration (100 µg/mL) while a value of 0.1 ± 0.06 AAE/g was reported using the Ferric Reducing Antioxidant Power (FRAP) methodology.
2.3.3. Cytotoxic and Anti-Inflammatory Activity
The effects of the oils obtained by steam distillation on the growth of four human tumor cell lines (MCF-7, NCI-H460, CaCo2 and AGS) and two non-tumoral cell lines (Vero and PLP2), represented as the concentration that caused 50% of cell growth inhibition (GI50) are summarized in Table 3. The samples of C. ladanifer (both from Cerezal and Andévalo origins) exhibited higher potential in all the tested cell lines, presenting GI50 values that ranged from 14 to 78 μg/mL in the NCI-H460 and AGS cells, respectively. The sample J. communis, presented GI50 values of 30.88 ± 1.85 and 41.99 ± 3.60 μg/mL in MCF-7 cell line (from Almazán and Barriomartín, respectively), while C. sempervirens showed 20 ± 2 μg/mL in NCI-H460 cell-line (Ermitas origin). In general, all samples showed cytotoxicity in the non-tumor cell lines. However, in the majority of the cell lines, the value of GI50 was higher than that of the tumor cell lines, meaning that for particular cases, these EOs can be used without toxicity. Additionally, it can be stated that in vivo studies are needed to verify the toxicity of these oils for specific applications.
All the tested oils showed anti-inflammatory capacity, which is in agreement with Murbach Teles Andrade, Nunes Barbosa, da Silva Probst and Fernandes Júnior [42], who reported that various essential oils exert an anti-inflammatory action by increasing interleukin-10 production. For J. communis the plant from Barriomartín showed the best results, but lower than the other species. C. ladanifer from Andévalo presented the strongest activity (19 µg/mL), while C. sempervirens collected in Ermitas exhibited the highest activity from all the tested samples. Najar, et al. [46] found that C. ladanifer EO exhibited cytotoxic activity at 90 ppm for the MCF-7 cell line. However, no further activity was found for the tested cell lines with the essential oils from J. communis and C. sempervirens.
3. Materials and Methods
3.1. Plant Material Collection and Conditioning
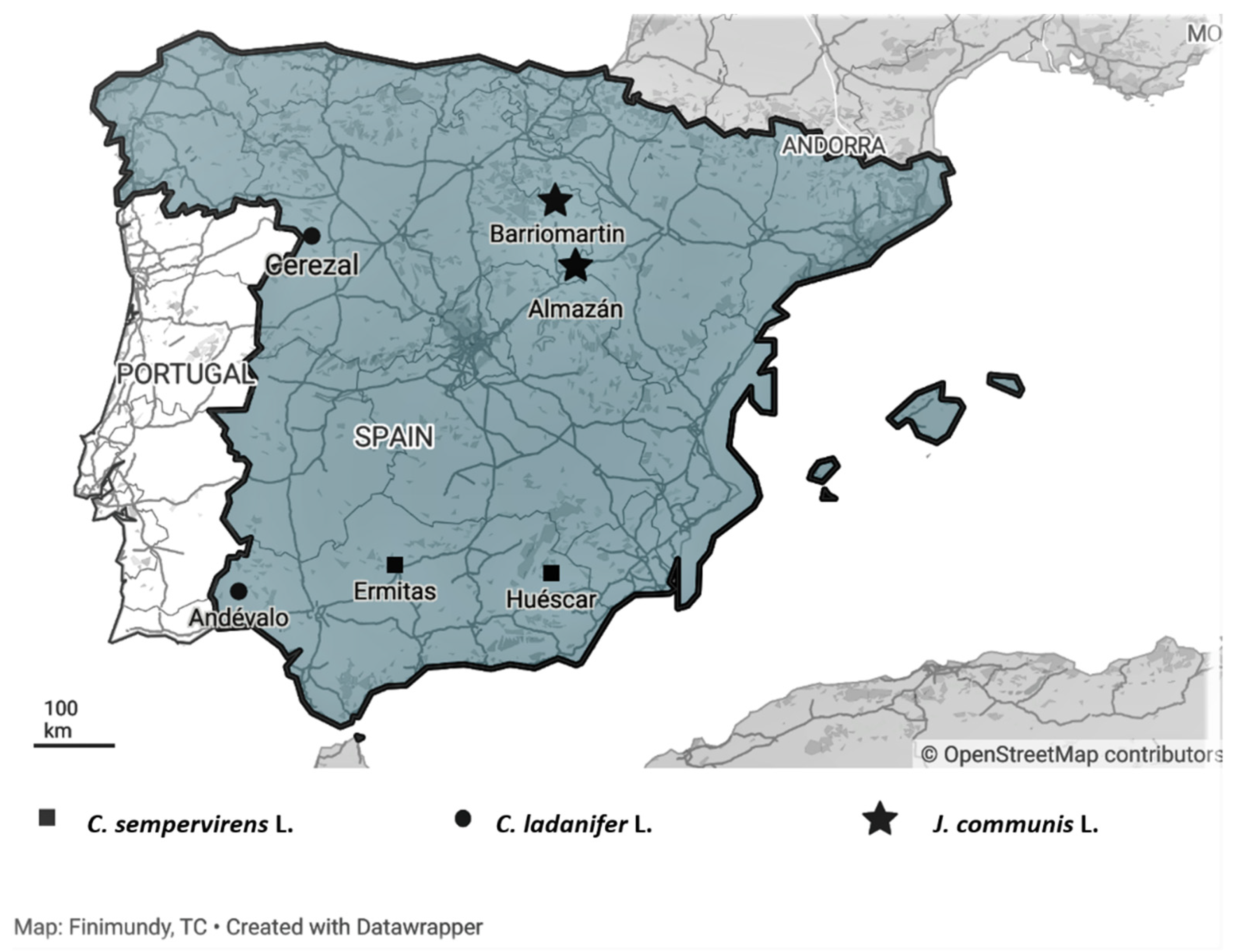
The plant material (crown biomass, with branches with a maximum stem diameter of 50 mm that included twigs, leaves and fruits) of each one of the species considered was collected in two different locations in Spain: C. ladanifer in Cerezal de Aliste and El Andévalo; C. sempervirens in Huéscar and Las Ermitas; and J. communis in Almazán and Barriomartín (Figure 2 and Table 4).
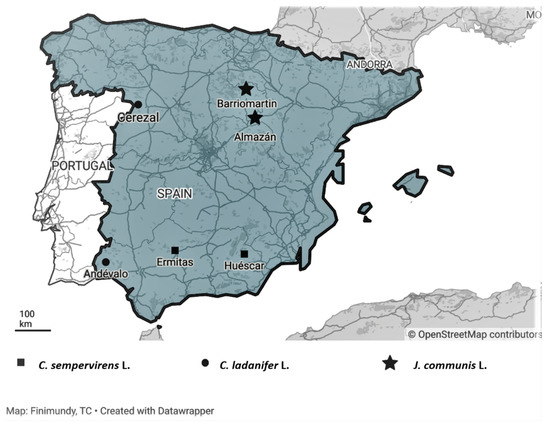
Figure 2.
Geographic locations of the collection sites of Spain shrubs populations. Maps generated by Datawapper Online tools.

Table 4.
Location and date of the plant material collection in Spain.
Samples were randomly taken from a minimum of 10 plants of a similar age, and the biomass of the different plants was mixed to obtain samples of 40 kg of green material from each species.
Previously to the steam distillation, fresh samples were air-dried in the shade at room temperature (10–15 °C) until moisture content was around 10–15%. Afterward, they were ground to a size of 20 mm using a shredder (90 kW, slow rotating single-shaft type, 70 rpm., SILMISA, Onil, Spain). Then, subsamples were taken to determine the moisture content following the standard ISO 18134-2:2017.
3.2. Essential Oils Extraction
The ground samples were distilled in a 50 L stainless steel still using steam produced in an electric boiler (ETE, Madrid, Spain). The steam conditions used for the extractions were 13 kg/h with a boiler pressure of 50 kPa. Batch extractions were performed, with two repetitions of 10 kg each per sample and an extraction duration of 2 h for each batch. Time was measured from the moment the first drop of distillate fell. The temperature inside the still was kept constant at 98 °C. The hydrolate and the essential oil were separated by density using a glass Florentine flask. The essential oil samples were then dried using anhydrous sodium sulfate and, after filtration, they were weighed and stored at 4 °C until further analysis. The oil yield for each sample was calculated as a percentage (w/w) on a biomass dry weight basis.
3.3. Gas Chromatography/Mass Spectrometry (GC-MS) Analyses
The EOs analysis was performed on a GC-MS Perkin Elmer system with a Clarus® 580 GC and a Clarus® SQ 8 S MS module, equipped with DB-5MS fused-silica column (30 m × 0.25 mm i.d., film thickness 0.25 μm; J&W Scientific, Inc., Folsom, CA, USA), according to Falcão, Bacém, Igrejas, Rodrigues, Vilas-Boas and Amaral [45]. The carrier gas was helium gas adjusted to a linear velocity of 30 cm/s. The oven temperature program was as follows: 40 °C for 4 min, raised at 3 °C/min to 175 °C, then at 15 °C/min to 300 °C and held for 10 min. The injector temperature was set at 260 °C, with a transfer line at 280 °C and an ion source at 220 °C. The ionization energy was 70 eV, and a scan range of 35–500 u with a scan time of 0.3 s was used. For each essential oil, 3 µL of sample diluted in HPLC grade n-hexane (1:100) was injected with a split ratio of 1:3. Identification of components was assigned by matching their mass spectra with NIST17 data and by determining the linear retention index (LRI) based on the retention times obtained for a mixture of n-alkanes (C8–C40, ref. 40147-U, Supelco) analyzed under identical conditions. When possible, comparisons were also performed with commercial standard compounds and published data. Quantification was performed using the relative peak area values obtained directly from the total ion current (TIC) values, and the results were expressed as the relative percentage of total volatiles.
3.4. Bioactive Evaluation
3.4.1. Antibacterial Activity
To evaluate the antibacterial activity of C. ladanifer, C. sempervirens and J. communis EO, five gram-negative (Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas aeruginosa and Morganella morganii) and three gram-positive bacteria (Enterococcus faecalis, Listeria monocytogenes and methicillin-resistant Staphylococcus aureus (MRSA)) were used. The bacterial strains were clinical isolates obtained from the Northeastern local health unit (Bragança, Portugal) and Hospital Center of Trás-os-Montes and Alto Douro (Vila Real, Portugal). These microorganisms were incubated at 37 °C in an appropriate fresh medium for 24 h before analysis to maintain the exponential growth phase. The antibacterial activity was evaluated through the broth microdilution method, based on the methodology described by Pires, et al. [47], determining the MIC (minimum inhibitory concentration) and the MBC (minimum bactericidal concentration), expressed in mg/mL. Briefly, the samples were serially diluted to obtain the concentration ranges of 2.5 mg/mL to 0.078 mg/mL. Different controls were prepared, namely two negative controls: MHB with Tween 80 and another one with the extract. Two positive controls with MHB with Tween 80 and each inoculum and one culture medium, antibiotics and bacteria. Ampicillin and Imipenem were used for all the tested Gram-negative bacteria and Listeria monocytogenes, while ampicillin and vancomycin were selected for Enterococcus faecalis and MRSA. After serial dilution in a 96 well microplate, each bacterial inoculum was pipetted to each well (corresponding to 1.5 × 108 Colony Forming Unit (CFU)/mL). The microplates were covered and incubated in a stirring board at 37 °C for 24 h. The MIC values were detected following the addition (50 μL) of 0.2 mg/mL p-iodonitrotetrazolium chloride (INT) and incubation at 37 °C for 30 min. MIC was defined as the lowest concentration that inhibits the visible bacterial growth determined by changing the coloration from yellow to pink if the microorganisms are viable. For the determination of MBC, 10 μL of liquid from each well showed no change in color was plated on solid medium, Blood agar (7% sheep blood) and incubated at 37 °C for 24 h. The lowest concentration that yielded no growth determines the MBC. MBC was defined as the lowest concentration required to kill bacteria.
3.4.2. Antioxidant Activity
The reducing power (RP) Kostić, et al. [48] and cellular antioxidant activity (CAA) [14,49,50] assays were performed to determine the antioxidant potential of the EOs. The reducing power was evaluated by determining the capacity of the extract to reduce Fe3+ to Fe2+ by measuring the absorbance at 690 nm. The results were expressed as EC50 values (μg/mL), corresponding to sample concentration with 0.5 of absorbance. For the CAA, the cells (RAW 264.7) were incubated with different EOs and AAPH (Azobis (2-methylpropionamidine) dihydrochloride), using DCFH-DA (2,7-Dichlorofluorescein diacetate) as a fluorescent marker [14,50]. DCF-DA is a compound that, once in the cell medium, is easily oxidized by peroxide radicals to a fluorescent compound, resulting in DCFH-DA. For the quantification of CAA, the efficacy of the antioxidant treatments was quantified by examining the percentage reduction in fluorescence, according to the formula:
where AUC sample and AUC control corresponds to the Area Under the Curve of the sample and control, respectively, cells were immediately placed on a microplate reader (FLX800 Biotek, Winooski, VT, USA), where real-time fluorescence was read initially and then every 5 min for 40 min. Fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 538 nm.
3.4.3. Cytotoxicity
To determine the cytotoxic potential of the different EOs, the Sulforhodamine B (SRB) assay [51] was performed on four different human tumor cells obtained from Leibniz-Institut DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen: NCI-H460 (lung carcinoma), MCF-7 (breast carcinoma), AGS (gastric carcinoma) and CaCo (colon carcinoma) and two normal cell lines: PLP2 (porcine liver cells) and VERO (monkey kidney cells). For hepatotoxicity evaluation, the porcine liver primary culture was prepared from a freshly harvested porcine liver. Ellipticine was used as the positive control, and the results were expressed in GI50 values (μg/mL), corresponding to the extract concentration that provides 50% of cell growth inhibition [52].
3.4.4. Anti-Inflammatory Activity
The anti-inflammatory activity was determined according to the method formerly reported by Mandim, et al. [53], in which the samples were tested for their capacity to inhibit the lipopolysaccharide (LPS)-induced NO (nitric oxide) production on a murine macrophage cell line (RAW 264.7). Dexamethasone was used as the positive control, and samples without LPS were used as a negative control. The results were expressed as IC50 values (µg/mL), corresponding to the extract concentration responsible for 50% of NO production inhibition.
3.4.5. Statistical Analysis
Experimental results were expressed as mean value ± standard deviation (SD). The obtained data were subjected to ANOVA post-hoc Tukey’s Honest Significant Difference (HSD) test, applied at p < 0.05, using the SPSS v.22.0 program. When less than three repetitions were available, the results were analyzed by the t-Student test as a form to determine the significant differences between two samples, with p = 0.05.
4. Conclusions
The obtained results suggest that the collection of these species in different geographical locations interfere with the essential oil’s yield and respective chemical composition, which can vary in terms of individual compounds’ contents, resulting in different chemotypes. Regardless, no relation was noticeable between the chemical composition and the location’s elevation. Compared with other parts of the plant, in literature, the crown biomass is qualitatively similar in terpenes profile, particularly when taking into consideration the composition specified by the International Organization for Standardization (ISO) and/or the European Pharmacopoeia for the branches and leaves of C. sempervirens and J. communis berries.
The evaluated species showed to be a viable and low-cost source of EOs that can be used for bio-based products development in different industries, such as the food, cosmetic and medicinal industries. All tested EOs showed the potential to inhibit the growth of gram-negative bacteria, especially E. coli, while C. ladanifer EO from Cerezal was the only one with the potential to inhibit the growth of M. morganii.
Nevertheless, according to the target application, the toxicity exhibited by some of the EOs in the tested tumor cell lines must be deeper analyzed by verifying this condition in specific toxicity models for each industry/product. Therefore, further studies are recommended to deepen the knowledge on these EOs and respective compounds towards different applications.
Author Contributions
Conceptualization, L.S.E. and L.B.; Formal analysis, V.X., T.C.F., J.S.A., J.V., T.C.S.P.P. and I.M.; Investigation, V.X., T.C.F., S.A.H., J.S.A., R.C.C., J.V., T.C.S.P.P., I.M., L.S.E. and L.B.; Methodology, V.X., T.C.F., J.S.A., R.C.C., J.V., T.C.S.P.P., I.M., L.S.E. and L.B.; Resources, L.B.; Supervision, S.A.H. and L.B.; Writing—original draft, V.X., T.C.F. and I.M.; Writing—review & editing, S.A.H., J.S.A., R.C.C., L.S.E., I.C.F.R.F. and L.B. All authors have read and agreed to the published version of the manuscript.
Funding
Foundation for Science and Technology (FCT, Portugal) for financial support through national funds FCT/MCTES to the CIMO (UIDB/00690/2020). L. Barros, R. Calhelha and S.A. Heleno (CEEC-IND/03040/2017) thank the national funding by FCT, P.I., through the institutional and individual scientific employment program-contract for their contracts. This project has received funding from the Bio Based Industries Joint Undertaking (JU) under grant agreement No 887917 BeonNAT. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and the Bio Based Industries Consortium.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Samples of the extracts are available from the authors.
References
- Serna-Loaiza, S.; Miltner, A.; Miltner, M.; Friedl, A. A Review on the Feed-Stocks for the Sustainable Production of Bioactive Compounds in Biorefineries. Sustainability 2019, 11, 6765. [Google Scholar] [CrossRef] [Green Version]
- Leipold, S.; Petit-Boix, A. The Circular Economy and the Bio-Based Sector—Perspectives of European and German Stakeholders. J. Clean. Prod. 2018, 201, 1125–1137. [Google Scholar] [CrossRef]
- De Corato, U.; De Bari, I.; Viola, E.; Pugliese, M. Assessing the Main Opportunities of Integrated Biorefining from Agro-Bioenergy Co/By-Products and Agroindustrial Residues into High-Value Added Products Associated to Some Emerging Markets: A Review. Renew. Sustain. Energy Rev. 2018, 88, 326–346. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and Sustainable Valorization of Bioactive Phenolic Compounds from Pinus By-Products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef]
- Nazzaro, F.; De Martino, L.; Fratianni, F.; De Feo, V. Essential Oils from Mediterranean Aromatic Plants. In The Mediterranean Diet, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 49; pp. 555–564. [Google Scholar]
- Raina, R.; Verma, P.K.; Peshin, R.; Kour, H. Potential of Juniperus communis L. as a Nutraceutical in Human and Veterinary Medicine. Heliyon 2019, 5, e02376. [Google Scholar] [CrossRef] [Green Version]
- Orav, A.; Koel, M.; Kailas, T.; Müürisepp, M. Comparative Analysis of the Composition of Essential Oils and Supercritical Carbon Dioxide Extracts from the Berries and Needles of Estonian Juniper (Juniperus communis L.). Proced. Chem. 2010, 2, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Gumral, N.; Kumbul, D.D.; Aylak, F.; Saygin, M.; Savik, E. Juniperus communis Linn Oil Decreases Oxidative Stress and Increases Antioxidant Enzymes in the Heart of Rats Administered a Diet Rich in Cholesterol. Toxicol. Ind. Health 2015, 31, 85–91. [Google Scholar] [CrossRef]
- Mediavilla, I.; Guillamón, E.; Ruiz, A.; Esteban, L.S. Essential Oils from Residual Foliage of Forest Tree and Shrub Species: Yield and Antioxidant Capacity. Molecules 2021, 26, 3257. [Google Scholar] [CrossRef] [PubMed]
- Ložienė, K.; Venskutonis, P.R. Juniper (Juniperus communis L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; Chapter 56; pp. 495–500. [Google Scholar]
- Pandey, A.K.; Kumar, P.; Saxena, M.J.; Maurya, P. Distribution of Aromatic Plants in the World and Their Properties. In Feed Additives; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 6; pp. 89–114. [Google Scholar]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Sousa, M.J.; Morais, J.S.; Ferreira, I.C.F.R. Aromatic Plants as a Source of Important Phytochemicals: Vitamins, Sugars and Fatty Acids in Cistus ladanifer, Cupressus lusitanica and Eucalyptus gunnii Leaves. Ind. Crops Prod. 2009, 30, 427–430. [Google Scholar] [CrossRef]
- Cox, R.E.; Yamamoto, S.; Otto, A.; Simoneit, B.R.T. Oxygenated Di- and Tricyclic Diterpenoids of Southern Hemisphere Conifers. Biochem. Syst. Ecol. 2007, 35, 342–362. [Google Scholar] [CrossRef]
- Gomes, P.B.; Mata, V.G.; Rodrigues, A.E. Characterization of the Portuguese-Grown Cistus ladanifer Essential Oil. J. Essent. Oil Res. 2005, 17, 160–165. [Google Scholar] [CrossRef]
- Upadhyay, N.; Singh, V.K.; Dwivedy, A.K.; Das, S.; Chaudhari, A.K.; Dubey, N.K. Cistus ladanifer L. Essential Oil as a Plant Based Preservative against Molds Infesting Oil Seeds, Aflatoxin B1 Secretion, Oxidative Deterioration and Methylglyoxal Biosynthesis. LWT 2018, 92, 395–403. [Google Scholar] [CrossRef]
- Höferl, M.; Stoilova, I.; Schmidt, E.; Wanner, J.; Jirovetz, L.; Trifonova, D.; Krastev, L.; Krastanov, A. Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism. Antioxidants 2014, 3, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Rguez, S.; Djébali, N.; Ben Slimene, I.; Abid, G.; Hammemi, M.; Chenenaoui, S.; Bachkouel, S.; Daami-Remadi, M.; Ksouri, R.; Hamrouni-Sellami, I. Cupressus sempervirens Essential Oils and Their Major Compounds Successfully Control Postharvest Grey Mould Disease of Tomato. Ind. Crops Prod. 2018, 123, 135–141. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Koidis, A. Methods for Extracting Essential Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; Chapter 4; pp. 31–38. [Google Scholar]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Zidane, H.; Elmiz, M.; Aouinti, F.; Tahani, A.; Wathelet, J.; Sindic, M.; Elbachiri, A. Chemical Composition and Antioxidant Activity of Essential Oil, Various Organic Extracts of Cistus ladanifer and Cistus libanotis Growing in Eastern Morocco. Afr. J. Biotechnol. 2013, 12, 5314–5320. [Google Scholar]
- Mohammed, B.; Said, C.; Fouzia, F.; Kawtar, F.; Zoubida, H.; Abdelilah, O.; Elhourri, M.; Ghizlane, E. Chemical Composition and Antimicrobial Activity of the Essential Oil of Cistus ladanifer var. maculatus Dun. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 925–930. [Google Scholar]
- Tavares, C.S.; Martins, A.; Faleiro, M.L.; Miguel, M.G.; Duarte, L.C.; Gameiro, J.A.; Roseiro, L.B.; Figueiredo, A.C. Bioproducts from Forest Biomass: Essential Oils and Hydrolates from Wastes of Cupressus lusitanica Mill. and Cistus ladanifer L. Ind. Crops Prod. 2020, 144, 112034. [Google Scholar] [CrossRef]
- Mediavilla, I.; Blázquez, M.A.; Ruiz, A.; Esteban, L.S. Influence of the Storage of Cistus ladanifer L. Bales from Mechanised Harvesting on the Essential Oil Yield and Qualitative Composition. Molecules 2021, 26, 2379. [Google Scholar] [CrossRef]
- Selim, S.A.; Adam, M.E.; Hassan, S.M.; Albalawi, A.R. Chemical Composition, Antimicrobial and Antibiofilm Activity of the Essential Oil and Methanol Extract of the Mediterranean Cypress (Cupressus sempervirens L.). BMC Complement. Altern. Med. 2014, 14, 179. [Google Scholar] [CrossRef]
- Ben Nouri, A.; Dhifi, W.; Bellili, S.; Ghazghazi, H.; Aouadhi, C.; Chérif, A.; Hammami, M.; Mnif, W. Chemical Composition, Antioxidant Potential, and Antibacterial Activity of Essential Oil Cones of Tunisian Cupressus sempervirens. J. Chem. 2015, 2015, 538929. [Google Scholar] [CrossRef] [Green Version]
- Sayed, A.F. Chemical Composition, Antioxidant, Anticancer Properties and Toxicity Evaluation of Leaf Essential Oil of Cupressus sempervirens. Not. Bot. Horti Agrobot. 2015, 43, 320–326. [Google Scholar]
- Angioni, A.; Barra, A.; Russo, M.T.; Coroneo, V.; Dessí, S.; Cabras, P. Chemical Composition of the Essential Oils of Juniperus from Ripe and Unripe Berries and Leaves and Their Antimicrobial Activity. J. Agric. Food Chem. 2003, 51, 3073–3078. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Yadav, L.B.S.; Ahmad, J.; Dubey, N.; Puri, S. Chemical Composition of Commercial Juniperus communis L. Leaf Oil. J. Essent. Oil Bear. Plant. 2007, 10, 310–313. [Google Scholar] [CrossRef]
- Bais, S.; Gill, N.S.; Rana, N.; Shandil, S. A Phytopharmacological Review on a Medicinal Plant: Juniperus communis. Int. Sch. Res. Not. 2014, 2014, 634723. [Google Scholar] [CrossRef] [Green Version]
- Yaglioglu, A.S.; Eser, F.; Yaglioglu, M.S.; Demirtas, I. The Antiproliferative and Antioxidant Activities of the Essential Oils of Juniperus Species from Turkey. Flavour Fragr. J. 2020, 35, 511–523. [Google Scholar] [CrossRef]
- Verdeguer, M.; Blázquez, M.A.; Boira, H. Chemical Composition and Herbicidal Activity of the Essential Oil from a Cistus ladanifer L. Population from Spain. Nat. Prod. Res. 2012, 26, 1602–1609. [Google Scholar] [CrossRef]
- Greche, H.; Mrabet, N.; Zrira, S.; Ismaïli-Alaoui, M.; Benjilali, B.; Boukir, A. The Volatiles of the Leaf Oil of Cistus ladanifer L. var. albiflorus and Labdanum Extracts of Moroccan Origin and Their Antimicrobial Activities. J. Essent. Oil Res. 2009, 21, 166–173. [Google Scholar] [CrossRef]
- ISO. Essential oil of cypress (Cupressus sempervirens L.). In ISO 20809; ISO Copyright Office: Geneva, Switzerland, 2017. [Google Scholar]
- Chatzopoulou, P.S.; Katsiotis, S.T. Chemical Investigation of the Leaf Oil of Juniperus communis L. J. Essent. Oil Res. 1993, 5, 603–607. [Google Scholar] [CrossRef]
- Cabral, C.; Francisco, V.; Cavaleiro, C.; Gonçalves, M.J.; Cruz, M.T.; Sales, F.; Batista, M.T.; Salgueiro, L. Essential Oil of Juniperus communis subsp. alpina (Suter) Čelak Needles: Chemical Composition, Antifungal Activity and Cytotoxicity. Phytother. Res. PTR 2012, 26, 1352–1357. [Google Scholar] [CrossRef]
- Caramiello, R.; Bocco, A.; Buffa, G.; Maffei, M. Chemotaxonomy of Juniperus communis, J. sibirica and J. intermedia. J. Essent. Oil Res. 1995, 7, 133–145. [Google Scholar] [CrossRef]
- Koukos, P.K.; Papadopoulou, K.I. Essential Oil of Juniperus communis L. Grown in Northern Greece: Variation of Fruit Oil Yield and Composition. J. Essent. Oil Res. 1997, 9, 35–39. [Google Scholar] [CrossRef]
- Pepeljnjak, S.; Kosalec, I.; Kalodera, Z.; Blazević, N. Antimicrobial Activity of Juniper Berry Essential Oil (Juniperus communis L., Cupressaceae). Acta Pharm. 2005, 55, 417–422. [Google Scholar]
- Mazari, K.; Bendimerad, N.; Bekhechi, C.; Fernandez, X. Chemical Composition and Antimicrobial Activity of Essential Oils Isolated from Algerian Juniperus phoenicea L. and Cupressus sempervirens L. J. Med. Plant. Res. 2010, 4, 959–964. [Google Scholar]
- Benali, T.; Bouyahya, A.; Habbadi, K.; Zengin, G.; Khabbach, A.; Achbani, E.H.; Hammani, K. Chemical Composition and Antibacterial Activity of the Essential Oil and Extracts of Cistus ladaniferus subsp. ladanifer and Mentha suaveolens against Phytopathogenic Bacteria and Their Ecofriendly Management of Phytopathogenic bacteria. Biocatal. Agric. Biotechnol. 2020, 28, 101696. [Google Scholar] [CrossRef]
- Benayad, N.; Mennane, Z.; Charof, R.; Hakiki, A.; Mosaddak, M. Antibacterial Activity of Essential Oil and Some Extracts of Cistus ladaniferus from Oulmes in Morocco. J. Mater. Environ. Sci. 2013, 4, 1066–1071. [Google Scholar]
- Murbach Teles Andrade, B.F.; Nunes Barbosa, L.; da Silva Probst, I.; Fernandes Júnior, A. Antimicrobial Activity of Essential Oils. J. Essent. Oil Res. 2014, 26, 34–40. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial Activity of Essential Oils and Other Plant Extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boukhris, M.; Regane, G.; Yangui, T.; Sayadi, S.; Bouaziz, M. Chemical Composition and Biological Potential of Essential Oil from Tunisian Cupressus sempervirens L. J. Arid Land Stud. 2012, 22, 329–332. [Google Scholar]
- Falcão, S.; Bacém, I.; Igrejas, G.; Rodrigues, P.J.; Vilas-Boas, M.; Amaral, J.S. Chemical Composition and Antimicrobial Activity of Hydrodistilled Oil from Juniper Berries. Ind. Crops Prod. 2018, 124, 878–884. [Google Scholar] [CrossRef] [Green Version]
- Najar, B.; Shortrede, J.E.; Pistelli, L.; Buhagiar, J. Chemical Composition and In Vitro Cytotoxic Screening of Sixteen Commercial Essential Oils on Five Cancer Cell Lines. Chem. Biodivers. 2020, 17, e1900478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, T.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.; Santos-Buelga, C.; Ferreira, I. Antioxidant and Antimicrobial Properties of Dried Portuguese Apple Variety (Malus domestica Borkh. cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostić, M.; Smiljković, M.; Petrović, J.; Glamočlija, J.; Barros, L.; Ferreira, I.; Ćirić, A.; Soković, M. Chemical, Nutritive Composition and a Wide Range of Bioactive Properties of Honey Mushroom Armillaria mellea (Vahl: Fr.) Kummer. Food Funct. 2017, 8, 3239–3249. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R.H. Cellular Antioxidant Activity of Common Fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Structure-Activity Relationships of Flavonoids in the Cellular Antioxidant Activity Assay. J. Agric. Food Chem. 2008, 56, 8404–8411. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and Chemical Characterization in Hydrophilic and Lipophilic Compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Petrova, K.T.; Potewar, T.M.; Correia-da-Silva, P.; Barros, M.T.; Calhelha, R.C.; Ćiric, A.; Soković, M.; Ferreira, I.C. Antimicrobial and Cytotoxic Activities of 1,2,3-Triazole-Sucrose Derivatives. Carbohydr. Res. 2015, 417, 66–71. [Google Scholar] [CrossRef]
- Mandim, F.; Barros, L.; Calhelha, R.C.; Abreu, R.M.V.; Pinela, J.; Alves, M.J.; Heleno, S.; Santos, P.F.; Ferreira, I.C.F.R. Calluna vulgaris (L.) Hull: Chemical Characterization, Evaluation of Its Bioactive Properties and Effect on the Vaginal Microbiota. Food Funct. 2019, 10, 78–89. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).