Phosphonodithioester–Amine Coupling as a Key Reaction Step for the Design of Cationic Amphiphiles Used for Gene Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization
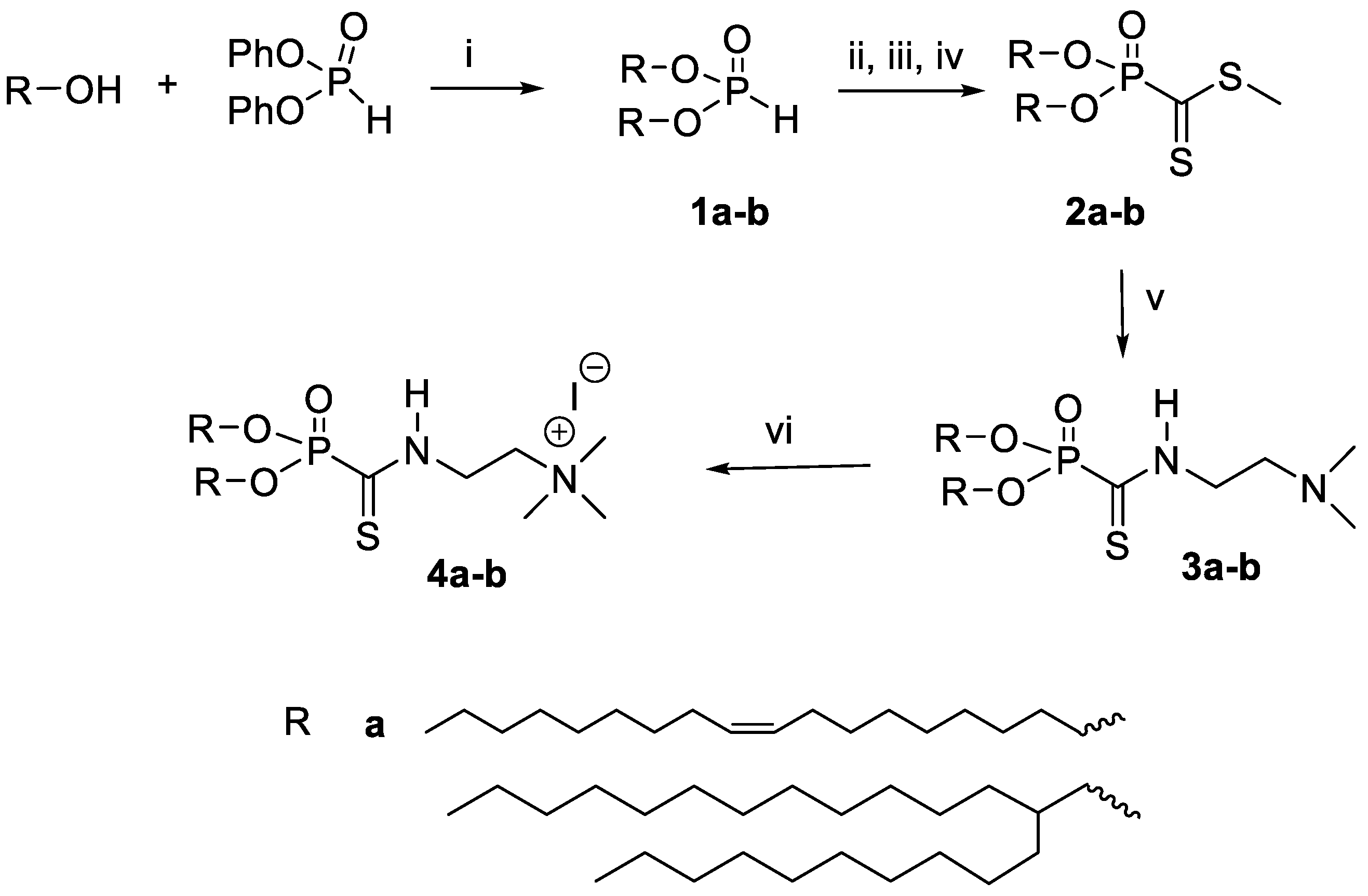
2.2.1. Dioleylphosphite 1a
2.2.2. Bis(2-decyltetradecyl)phosphite 1b
2.2.3. Methyl Dioleylphosphonodithioformate 2a
2.2.4. Methyl Bis-(2-decanyltetradecyl)phosphonodithioformate 2b
2.2.5. Dioleyl ((2-(dimethylamino)ethyl)carbamothioyl)phosphonate 3a
2.2.6. Bis(2-decanyltetradecyl) ((2-(dimethylamino)ethyl)carbamothioyl)phosphonate 3b
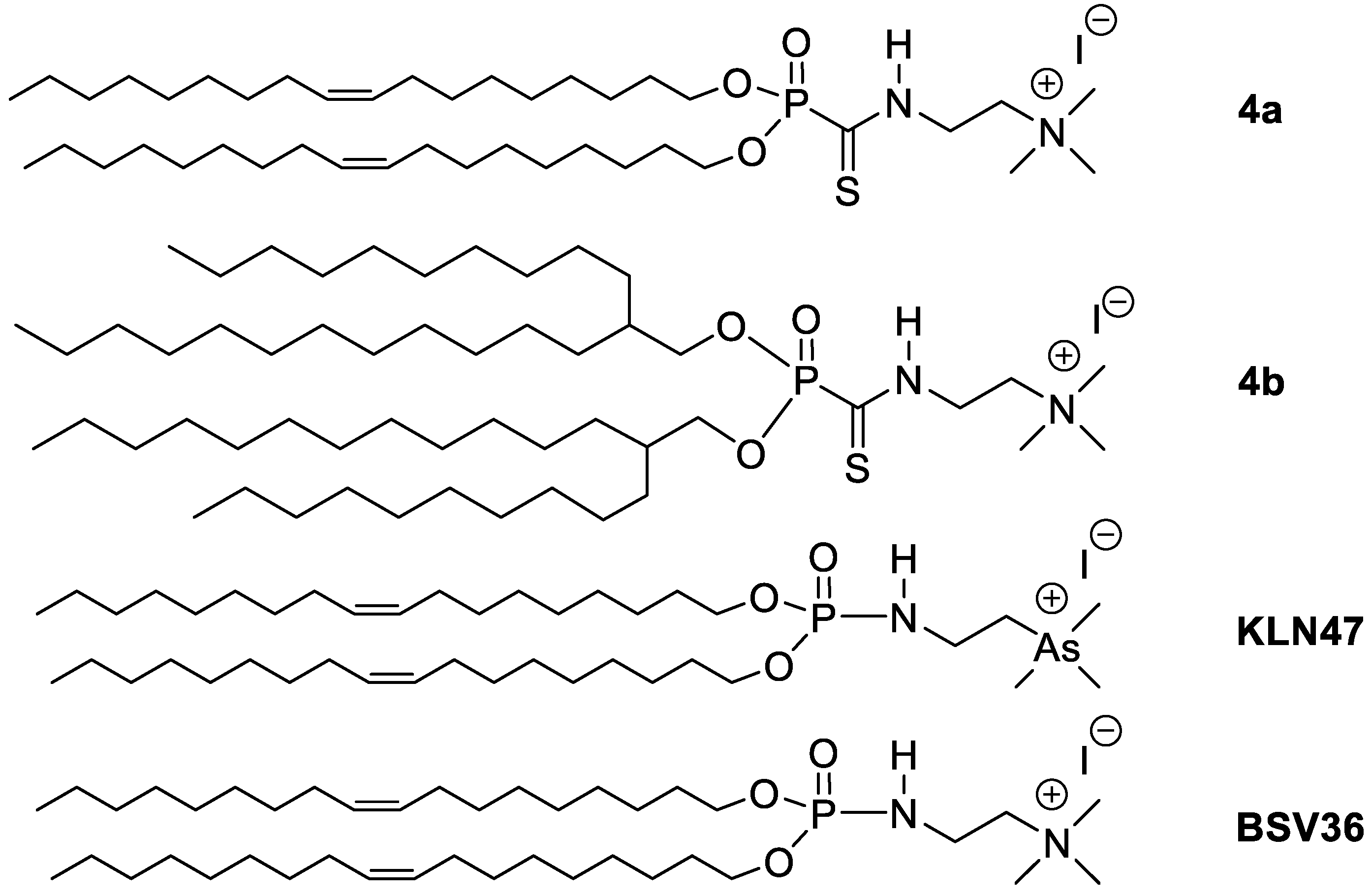
2.2.7. 2-((Dioleyloxyphosphoryl)methanethioamido)-N,N,N-trimethylethan-1-aminium iodide 4a
2.2.8. 2-((Bis(2-decanyltetradecyloxy)phosphoryl)methanethioamido)-N,N,N-trimethylethan-1-ammonium iodide 4b
2.3. Preparation of Liposomes
2.4. Size and Zeta Measurements
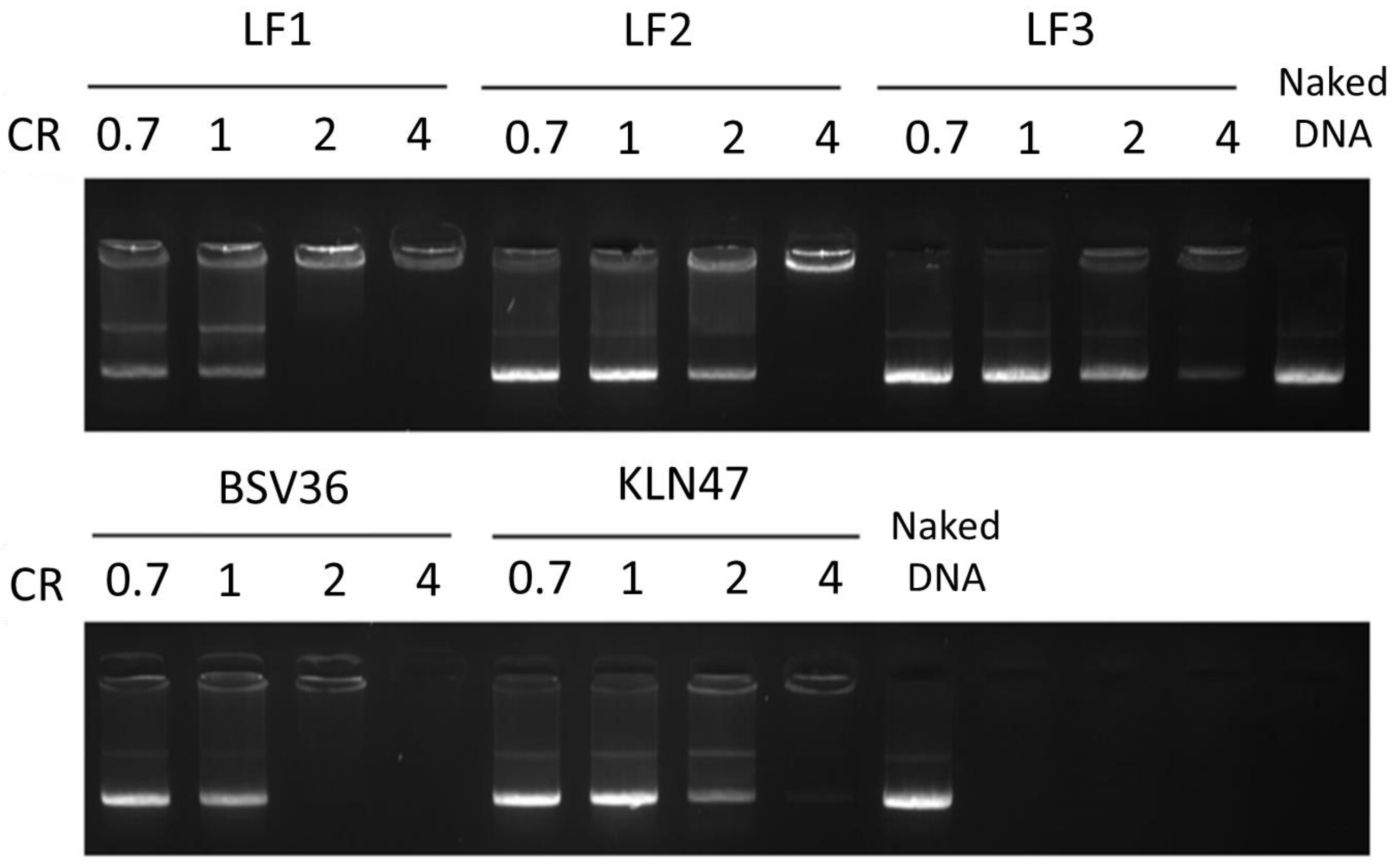
2.5. DNA Complexation
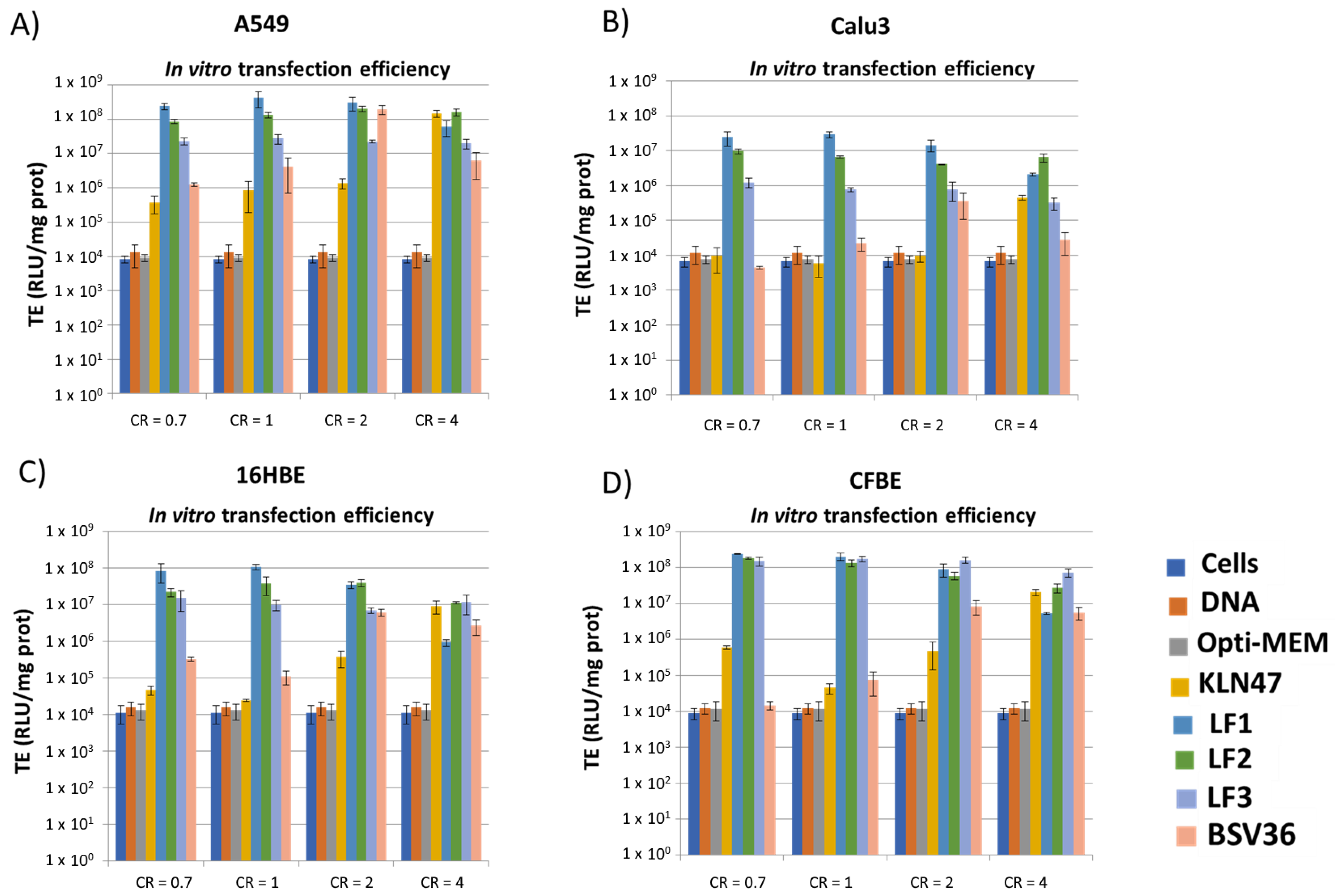
2.6. In Vitro Transfection Efficacies
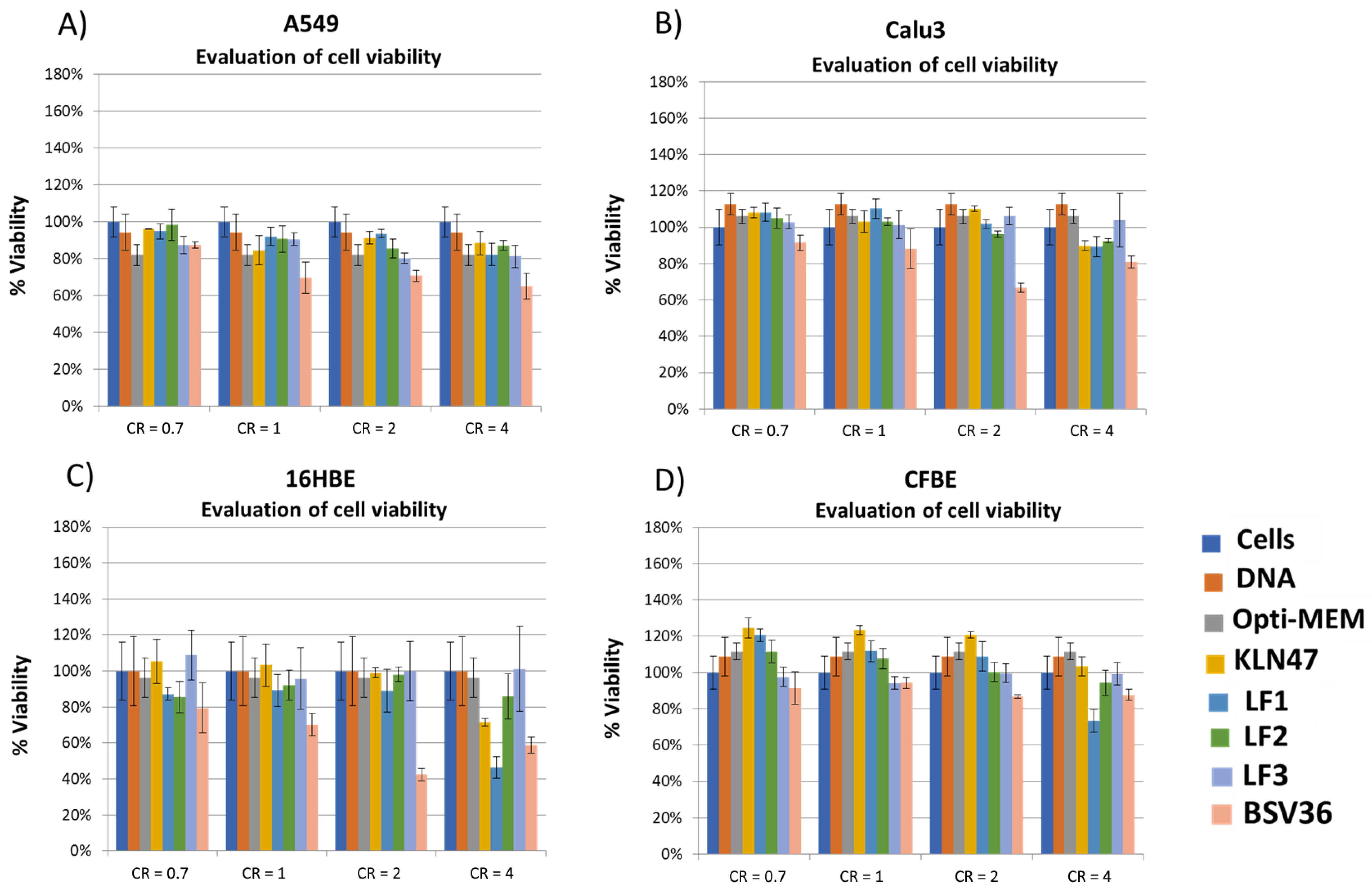
2.7. Cell Viability
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rädler, J.O.; Koltover, I.; Salditt, T.; Safinya, C.R. Structure of DNA–Cationic Liposome Complexes: DNA Intercalation in Multilamellar Membranes in Distinct Interhelical Packing Regimes. Science 1997, 275, 810–814. [Google Scholar] [CrossRef] [Green Version]
- Pozharski, E.; MacDonald, R.C. Lipoplex Thermodynamics: Determination of DNA-Cationic Lipoid Interaction Energies. Biophys. J. 2003, 85, 3969–3978. [Google Scholar] [CrossRef] [Green Version]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef] [Green Version]
- Mintzer, M.A.; Simanek, E.E. Nonviral Vectors for Gene Delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef]
- Zhi, D.; Bai, Y.; Yang, J.; Cui, S.; Zhao, Y.; Chen, H.; Zhang, S. A review on cationic lipids with different linkers for gene delivery. Adv. Colloid Interface Sci. 2018, 253, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.F.; Zhang, S.B.; Wang, B.; Zhao, Y.N.; Yang, B.L.; Yu, S.J. Transfection efficiency of cationic lipids with different hydrophobic domains in gene delivery. Bioconjugate Chem. 2010, 21, 563–577. [Google Scholar] [CrossRef]
- Bajaj, A.; Mishra, S.K.; Kondaiah, P.; Bhattachary, S. Effect of the headgroup variation on the gene transfer properties of cholesterol based cationic lipids possessing ether linkage. Biochim. Biophys. Acta 2008, 1778, 1222–1236. [Google Scholar] [CrossRef] [Green Version]
- Billiet, L.; Gonçalves, C.; Gomez, J.P.; Lodewick, J.; Berchel, M.; Jaffrès, P.A.; Montier, T.; Lehn, P.; Bertrand, E.; El-Ghoul, Y.; et al. Gene transfer by chemical vectors: Comparative study of the endocytosis routes of polyplexes, lipoplexes and lipopolyplexes in a myoblast cell line. Biomaterials 2012, 33, 2980–2990. [Google Scholar] [CrossRef]
- Singh, R.S.; Gonçalves, C.; Sandrin, P.; Pichon, C.; Midoux, P.; Chaudhuri, A. On the Gene Delivery Efficacies of pH-Sensitive Cationic Lipids via Endosomal Protonation: A Chemical Biology Investigation. Chem. Biol. 2004, 11, 713–723. [Google Scholar] [CrossRef]
- Mével, M.; Neveu, C.; Gonçalves, C.; Yaouanc, J.J.; Pichon, C.; Jaffrès, P.A.; Midoux, P. Novel neutral imidazole-lipophosphoramides for transfection assays. Chem. Commun. 2008, 3124–3126. [Google Scholar] [CrossRef] [Green Version]
- Fraix, A.; Le Gall, T.; Berchel, M.; Denis, C.; Lehn, P.; Montier, T.; Jaffrès, P.A. Cationic lipophosphoramidates with two disulfide motifs: Synthesis, behaviour in reductive media and gene transfection activity. Org. Biomol. Chem. 2013, 11, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Puchkov, P.A.; Shmendel, E.V.; Luneva, A.S.; Zenkova, M.A.; Maslov, M.A. Position of Disulfide Bond Determines the Properties of Novel Stimuli-Responsive Cationic Lipids. Chem. Sel. 2020, 5, 4509–4514. [Google Scholar] [CrossRef]
- Reinhard, S.; Wang, Y.; Dengler, S.; Wagner, E. Precise Enzymatic Cleavage Sites for Improved Bioactivity of siRNA Lipo-Polyplexes. Bioconjugate Chem. 2018, 29, 3649–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buck, J.; Grossen, P.; Cullis, P.R.; Huwyler, J.; Witzigmann, D. Lipid-Based DNA Therapeutics: Hallmarks of Non-Viral Gene Delivery. ACS Nano 2019, 13, 3754–3782. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new aera in vaccinology. Nature Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Delalande, A.; Gosselin, M.P.; Suwalski, A.; Guilmain, W.; Leduc, C.; Berchel, M.; Jaffrès, P.A.; Baril, P.; Midoux, P.; Pichon, C. Enhanced Achilles tendon healing by fibromodulin gene transfer. Nanomed. NBM 2015, 11, 1735–1744. [Google Scholar] [CrossRef]
- Lindberg, M.F.; Le Gall, T.; Carmoy, N.; Berchel, M.; Hyde, S.C.; Gill, D.R.; Jaffrès, P.A.; Lehn, P.; Montier, T. Efficient in vivo transfection and safety profile of a CpG-free and codon optimized luciferaseplasmid using a cationic lipophosphoramidate in a multiple intravenous administration procedure. Biomaterials 2015, 59, 1–11. [Google Scholar] [CrossRef]
- Gaspar, D.P.; Vital, J.; Leiva, M.C.; Goncalves, L.M.D.; Taboada, P.; Remunan-Lopez, C.; Vitor, J.; Almeida, A.J. Transfection of pulmonary cells by stable pDNA-polycationic hybrid nanostructured particles. Nanomedicine 2019, 14, 407–429. [Google Scholar] [CrossRef] [PubMed]
- Künnapuu, K.; Veiman, K.L.; Porosk, L.; Rammul, E.; Kiisholts, K.; Langel, U.; Kurrikoff, K. Tumor gene therapy by systemic delivery of plasmid DNA with cell-penetrating peptides. FASEB BioAdv. 2019, 1, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, P.Y.; Cheng, W.; Hedrick, J.L.; Yang, Y.Y. Co-deliveryofdrugsandplasmidDNAforcancertherapy. Adv. Drug Deliv. Rev. 2016, 98, 41–63. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Bouraoui, A.; Ghanem, R.; Berchel, M.; Deschamps, L.; Vié, V.; Paboeuf, G.; Le Gall, T.; Montier, T.; Jaffrès, P.A. Cationic amphiphiles producing hexagonal aggregates: Physico-chemical characterization and application to gene delivery. Org. Biomol. Chem. 2020, 18, 337–345. [Google Scholar] [CrossRef]
- Afonso, D.; Le Gall, T.; Couthon-Gourvès, H.; Grélard, A.; Prakash, S.; Berchel, M.; Kervarec, N.; Dufourc, E.J.; Montier, T.; Jaffrès, P.A. Thiol-ene click reaction applied to cationic amphiphiles: Impacts on supramolecular packing and gene transfection properties. Soft Matter 2016, 12, 4516–4520. [Google Scholar] [CrossRef] [PubMed]
- Fraix, A.; Montier, T.; Carmoy, N.; Loizeau, D.; Burel-Deschamps, L.; Le Gall, T.; Giamarchi, P.; Couthon-Gourvès, H.; Haelters, J.P.; Lehn, P.; et al. Cationic Lipothiophosphoramidates for in vitro gene delivery: Synthesis, physico-chemical characterizations and transfection assays- comparison with lipophosphoramidates. Org. Biomol. Chem. 2011, 9, 2422–2432. [Google Scholar] [CrossRef]
- Van der Jeught, K.; De Koker, S.; Bialkowski, L.; Heirman, C.; Tjok Joe, P.; Bevers, S.; Broos, K.; Deswarte, K.; Malard, V.; Hammad, H.; et al. Systemic delivery of modified mRNA through lipopolyplexes combines strong antitumor T cell immunity with improved inflammatory safety. ACS Nano 2018, 12, 9815–9829. [Google Scholar] [CrossRef]
- Dey, S.; Gupta, A.; Saha, A.; Pal, S.; Kumar, S.; Manna, D. Sunlight-Mediated Thiol−Ene/Yne Click Reaction: Synthesis and DNA Transfection Efficiency of New Cationic Lipids. ACS Omega 2020, 5, 735–750. [Google Scholar] [CrossRef]
- Naik, S.S.; Chan, J.W.; Comer, C.; Hoyle, C.E.; Savin, D.A. Thiol–yne ‘click’ chemistry as a route to functional lipid mimetics. Polym. Chem. 2011, 2, 303–305. [Google Scholar] [CrossRef]
- Saha, A.; Panda, S.; Pradhan, N.; Kalita, K.; Trivedi, V.; Manna, D. Azidophosphonate Chemistry as a Route for a Novel Class of Vesicle-Forming Phosphonolipids. Chem. Eur. J. 2018, 24, 1121–1127. [Google Scholar] [CrossRef]
- Bulpin, A.; Le Roy-Gourvennec, S.; Masson, S. The amination of phosphonodithioformates; a preparation of new functionalised phosphonates. Phosphorus Sulfur Sil. 1994, 89, 119–132. [Google Scholar] [CrossRef]
- Lebouc, F.; Dez, I.; Gulea, M.; Madec, P.J.; Jaffrès, P.A. Synthesis of phosphorus-containing chitosan derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 872–889. [Google Scholar] [CrossRef]
- Khalil, M.; Jeanne Dit Fouque, D.; Berchel, M.; Fraix, A.; Dupont, A.; Sortino, S.; Memboeuf, A.; Jaffrès, P.A. Phosphonodithioester-Amine Coupling in water: A fast reaction to modify the surface of liposomes. Org. Biomol. Chem. 2021, 19, 6392–6396. [Google Scholar] [CrossRef]
- Pfund, E.; Lequeux, T.; Masson, S.; Vazeux, M.; Cordi, A.; Pierre, A.; Serred, V.; Herve, G. Efficient synthesis of fluorothiosparfosic acid analogues with potential antitumoral activity. Bioorg. Med. Chem. 2005, 13, 4921–4928. [Google Scholar] [CrossRef] [PubMed]
- Picquet, E.; Le Ny, K.; Delépine, P.; Montier, T.; Yaouanc, J.J.; Cartier, D.; des Abbayes, H.; Férec, C.; Clément, J.C. Cationic Lipophosphoramidates and Lipophosphoguanidines Are Very Efficient for in Vivo DNA Delivery. Bioconjugate Chem. 2005, 16, 1051–1053. [Google Scholar] [CrossRef]
- Le Corre, S.S.; Berchel, M.; Belmadi, N.; Denis, C.; Haelters, J.P.; Le Gall, T.; Lehn, P.; Montier, T.; Jaffrès, P.A. Cationic lipophosphoramidates with two different lipid chains: Synthesis and evaluation as gene carriers. Org. Biomol. Chem. 2014, 12, 1463–1474. [Google Scholar] [CrossRef]
- Bouraoui, A.; Berchel, M.; Ghanem, R.; Vié, V.; Paboeuf, G.; Deschamps, L.; Lozach, O.; Le Gall, T.; Montier, T.; Jaffrès, P.A. Thiol-ene click reaction to construct the lipid chains of cationic amphiphiles: Physico-chemical behaviour and application for gene delivery. Org. Biomol. Chem. 2019, 17, 3609–3616. [Google Scholar] [CrossRef]
- Le Corre, S.S.; Berchel, M.; Le Gall, T.; Haelters, J.P.; Lehn, P.; Montier, T.; Jaffrès, P.A. Cationic trialkylphosphates: Synthesis and transfection efficacies compared to phosphoramidate analogues. Eur. J. Org. Chem. 2014, 8041–8048. [Google Scholar] [CrossRef]
- Delbeke, E.I.P.; Lozach, O.; Le Gall, T.; Berchel, M.; Montier, T.; Jaffrès, P.A.; Van Geem, K.M.; Stevens, C.V. Evaluation of the transfection efficacies of quaternary ammonium salts prepared from sophorolipids. Org. Biomol. Chem. 2016, 14, 3744–3751. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, T.; Loizeau, D.; Picquet, E.; Carmoy, N.; Yaouanc, J.J.; Burel-Deschamps, L.; Delépine, P.; Giamarchi, P.; Jaffès, P.A.; Lehn, P.; et al. A Novel Cationic Lipophosphoramide with Diunsaturated Lipid Chains: Synthesis, Physicochemical Properties, and Transfection Activities. J. Med. Chem. 2010, 53, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Grisley, D.W. The reaction of sodium dialkyl phosphonates with carbonyl sulfide and with carbon disulfide. J. Org. Chem. 1961, 26, 2544–2546. [Google Scholar] [CrossRef]
- Heuzé, B.; Lemarié, M.; Vazeux, M.; Gulea, M.; Masson, S.; Sene, A.; Jaffrès, P.A.; Alberti, A.; Macciantelli, D. Synthesis of methylene diphosphonates from carbon disulfide and phosphites via desulfurization: A mechanistic study. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 820–829. [Google Scholar] [CrossRef]
- Hyde, S.C.; Pringle, I.A.; Abdullah, S.; Lawton, A.E.; Davies, L.A.; Varathalingam, A.; Nunez-Alonso, G.; Green, A.M.; Bazzani, R.P.; Sumner-Jones, S.G.; et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat. Biotechnol. 2008, 26, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Laurent, V.; Fraix, A.; Montier, T.; Cammas-Marion, S.; Ribault, C.; Benvegnu, T.; Jaffrès, P.A.; Loyer, P. Highly efficient gene transfer into hepatocyte-like HepaRG cells: New means for drug metabolism and toxicity studies. Biotechnol. J. 2010, 5, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Arora, S.; Singh, J.; Layek, B. A review of the tortuous path of nonviral gene delivery and recent progress. Inter. J. Biol. Macromol. 2021, 183, 2055–2073. [Google Scholar] [CrossRef]
| Formulations | Composition | Ratio | Concentration | Method |
|---|---|---|---|---|
| BSV36 | BSV36 | 1 | 1.5 mM | Lipid film hydration |
| KLN47 | KLN47 | 1 | 1.5 mM | Lipid film hydration |
| LF1 | 4a: DOPE | 1: 1 | 1.5 mM in 4a | Lipid film hydration |
| LF2 | 4a: Chol: DOPE | 1: 1: 1 | 1.5 mM in 4a | Lipid film hydration |
| LF3 | 4b: DOPE | 1: 1 | 1.5 mM in 4b | Ethanol injection |
| Formulations | Size (nm) | PDI | Potential Zeta (mV) |
|---|---|---|---|
| BSV36 | 169 ± 9 | 0.24 | 58 ± 0.06 |
| KLN47 | 126 ± 7 | 0.33 | 28 ± 1.16 |
| LF1 | 227 ± 41 | 0.46 | 61 ± 1.7 |
| LF2 | 194 ± 16 | 0.44 | 79 ± 0.6 |
| LF3 | 229 ± 15 | 0.33 | 57 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, M.; Hocquigny, A.; Berchel, M.; Montier, T.; Jaffrès, P.-A. Phosphonodithioester–Amine Coupling as a Key Reaction Step for the Design of Cationic Amphiphiles Used for Gene Delivery. Molecules 2021, 26, 7507. https://doi.org/10.3390/molecules26247507
Khalil M, Hocquigny A, Berchel M, Montier T, Jaffrès P-A. Phosphonodithioester–Amine Coupling as a Key Reaction Step for the Design of Cationic Amphiphiles Used for Gene Delivery. Molecules. 2021; 26(24):7507. https://doi.org/10.3390/molecules26247507
Chicago/Turabian StyleKhalil, Montassar, Alexis Hocquigny, Mathieu Berchel, Tristan Montier, and Paul-Alain Jaffrès. 2021. "Phosphonodithioester–Amine Coupling as a Key Reaction Step for the Design of Cationic Amphiphiles Used for Gene Delivery" Molecules 26, no. 24: 7507. https://doi.org/10.3390/molecules26247507
APA StyleKhalil, M., Hocquigny, A., Berchel, M., Montier, T., & Jaffrès, P.-A. (2021). Phosphonodithioester–Amine Coupling as a Key Reaction Step for the Design of Cationic Amphiphiles Used for Gene Delivery. Molecules, 26(24), 7507. https://doi.org/10.3390/molecules26247507








