In Vitro Phytochemical Screening, Cytotoxicity Studies of Curcuma longa Extracts with Isolation and Characterisation of Their Isolated Compounds
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Material
2.2. Preparation of Plant Extracts
2.3. Screening Test for Phytochemicals
3. Isolation of Curcuminoids
3.1. HPLC Analysis of Curcuminoids
3.2. Isolation of Bisdemethoxycurcumin
3.3. Melting Point Determination
3.4. HPLC Analysis of Bisdemethoxycurcumin
3.5. IR (Infrared) Spectroscopy
3.6. NMR (Nuclear Magnetic Resonance)
3.7. Mass Spectrometry
4. Cancer Cell Lines
5. Cytotoxicity Assay
6. Preparation of Test Solutions
Test Sample Preparation
7. Statistical Analysis
8. Results and Discussion
8.1. Percentage Yield of Extraction
8.2. Phytochemical Screening
8.3. Isolation of Curcuminoids
8.4. HPLC Analysis of Curcuminoids
8.5. Isolation of Bisdemethoxycurcumin
8.6. Melting Point
8.7. Characterisation
8.7.1. HPLC Analysis of Bisdemethoxycurcumin
8.7.2. FT-IR Spectra
8.7.3. Nuclear Magnetic Resonance

8.7.4. Mass Spectrum
8.8. Cytotoxicity
9. Conclusions
10. Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- He, L.; Gu, J.; Lim, L.Y.; Yuan, Z.; Mo, J. Nanomedicine-Mediated Therapies to Target Breast Cancer Stem Cells. Front. Pharmacol. 2016, 7, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Huang, G.; Chen, Z.; Zhang, Y. Nanomaterials in Targeting Cancer Stem Cells for Cancer Therapy. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Tirumala, M.G.; Anchi, P.; Raja, S.; Rachamalla, M.; Godugu, C. Novel Methods and Approaches for Safety Evaluation of Nanoparticle Formulations: A Focus towards in Vitro Models and Adverse Outcome Pathways (AOP). Front. Pharmacol. 2021, 12, 612659. [Google Scholar] [CrossRef]
- Zhang, L.-Q.; Lv, R.-W.; Qu, X.-D.; Chen, X.-J.; Lu, H.-S.; Wang, W. Aloesin Suppresses Cell Growth and Metastasis in Ovarian Cancer SKOV3 Cells through the Inhibition of the MAPK Signaling Pathway. Anal. Cell. Pathol. 2017, 2017, 8158254. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA A Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Rachamalla, M.; Nandekar, P.; Khatik, G.L.; Sangamwar, A.T.; Tikoo, K.; Nair, V.A. Design and Synthesis of Optically Pure 3-Aryl-6-Methyl-2-Thioxotetrahydropyrimidin-4(1 H )-Ones as Anti-Prostate Cancer Agents. RSC Adv. 2014, 4, 37868–37877. [Google Scholar] [CrossRef]
- GLOBOCAN 2020: New Global Cancer Data|UICC. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 27 September 2021).
- Jain, S.; Rachamalla, M.; Kulkarni, A.; Kaur, J.; Tikoo, K. Pulmonary Fibrotic Response to Inhalation of ZnO Nanoparticles and Toluene Co-Exposure through Directed Flow Nose Only Exposure Chamber. Inhal. Toxicol. 2013, 25, 703–713. [Google Scholar] [CrossRef]
- Chauthe, S.K.; Mahajan, S.; Rachamalla, M.; Tikoo, K.; Singh, I.P. Synthesis and Evaluation of Linear Furanocoumarins as Potential Anti-Breast and Anti-Prostate Cancer Agents. Med. Chem. Res. 2014, 24, 2476–2484. [Google Scholar] [CrossRef]
- Mukherjee, S.; Patra, C.R. Therapeutic Application of Anti-Angiogenic Nanomaterials in Cancers. Nanoscale 2016, 8, 12444–12470. [Google Scholar] [CrossRef]
- Patra, C.R.; Mukherjee, S.; Kotcherlakota, R. Biosynthesized Silver Nanoparticles: A Step Forward for Cancer Theranostics? Nanomedicine 2014, 9, 1445–1448. [Google Scholar] [CrossRef]
- Van Iersel, M.L.; Verhagen, H.; van Bladeren, P.J. The Role of Biotransformation in Dietary (Anti)Carcinogenesis. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 1999, 443, 259–270. [Google Scholar] [CrossRef]
- Xiao, D.; Srivastava, S.K.; Lew, K.L.; Zeng, Y.; Hershberger, P.; Johnson, C.S.; Trump, D.L.; Singh, S.V. Allyl Isothiocyanate, a Constituent of Cruciferous Vegetables, Inhibits Proliferation of Human Prostate Cancer Cells by Causing G2/M Arrest and Inducing Apoptosis. Carcinogenesis 2003, 24, 891–897. [Google Scholar] [CrossRef]
- Mehrotra, S.; Agnihotri, G.; Singh, S.; Jamal, F. Immunomodulatory Potential of Curcuma longa: A Review. South Asian J. Exp. Biol. 2013, 3, 299–307. [Google Scholar] [CrossRef]
- Ijaz, S.; Akhtar, N.; Khan, M.S.; Hameed, A.; Irfan, M.; Arshad, M.A.; Ali, S.; Asrar, M. Plant Derived Anticancer Agents: A Green Approach towards Skin Cancers. Biomed. Pharmacother. 2018, 103, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, M.; Poi, R.; Banerjee, H.; Bandyopadhyay, A. High-Performance Thin Layer Chromatographic Method for Quantitative Determination of Curcuminoids in Curcuma longa Germplasm. Food Chem. 2009, 113, 640–644. [Google Scholar] [CrossRef]
- Kalpravidh, R.W.; Siritanaratkul, N.; Insain, P.; Charoensakdi, R.; Panichkul, N.; Hatairaktham, S.; Srichairatanakool, S.; Phisalaphong, C.; Rachmilewitz, E.; Fucharoen, S. Improvement in Oxidative Stress and Antioxidant Parameters in Beta-Thalassemia/Hb E Patients Treated with Curcuminoids. Clin. Biochem. 2010, 43, 424–429. [Google Scholar] [CrossRef]
- AlBasher, G.; Abdel-Daim, M.M.; Almeer, R.; Ibrahim, K.A.; Hamza, R.Z.; Bungau, S.; Aleya, L. Synergistic Antioxidant Effects of Resveratrol and Curcumin against Fipronil-Triggered Oxidative Damage in Male Albino Rats. Environ. Sci. Pollut. Res. 2020, 27, 6505–6514. [Google Scholar] [CrossRef] [PubMed]
- Changtam, C.; de Koning, H.P.; Ibrahim, H.; Sajid, M.S.; Gould, M.K.; Suksamrarn, A. Curcuminoid Analogs with Potent Activity against Trypanosoma and Leishmania Species. Eur. J. Med. Chem. 2010, 45, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.A.; El-Sayed, S.A.E.-S.; Igarashi, I. Effects of Methanolic Extract from Turmeric (Curcuma longa) against the In Vitro Multiplication of Several Babesia Species and Theileria Equi. Parasitologia 2021, 1, 20. [Google Scholar] [CrossRef]
- Lim, H.S.; Park, S.H.; Ghafoor, K.; Hwang, S.Y.; Park, J. Quality and Antioxidant Properties of Bread Containing Turmeric (Curcuma longa, L.) Cultivated in South Korea. Food Chem. 2011, 124, 1577–1582. [Google Scholar] [CrossRef]
- Péret-Almeida, L.; Cherubino, A.P.F.; Alves, R.J.; Dufossé, L.; Glória, M.B.A. Separation and Determination of the Physico-Chemical Characteristics of Curcumin, Demethoxycurcumin and Bisdemethoxycurcumin. Food Res. Int. 2005, 38, 1039–1044. [Google Scholar] [CrossRef]
- Aditya, N.P.; Chimote, G.; Gunalan, K.; Banerjee, R.; Patankar, S.; Madhusudhan, B. Curcuminoids-Loaded Liposomes in Combination with Arteether Protects against Plasmodium Berghei Infection in Mice. Exp. Parasitol. 2012, 131, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; El-Khatib, R.; Rainsford, K.D.; Whitehouse, M.W. Synthesis and Anti-Inflammatory Properties of Some Aromatic and Heterocyclic Aromatic Curcuminoids. Bioorganic Chem. 2012, 40, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Behl, T.; Sachdeva, M.; Bungao, S.; Aleya, L.; Setia, D. Focus on Multi-Targeted Role of Curcumin: A Boon in Therapeutic Paradigm. Environ. Sci. Pollut. Res. 2021, 28, 18893–18907. [Google Scholar] [CrossRef]
- Yue, G.G.; Chan, B.C.; Hon, P.M.; Kennelly, E.J.; Yeung, S.K.; Cassileth, B.R.; Fung, K.P.; Leung, P.C.; Lau, C.B. Immunostimulatory Activities of Polysaccharide Extract Isolated from Curcuma longa. Int. J. Biol. Macromol. 2010, 47, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Tapal, A.; Tiku, P.K. Complexation of Curcumin with Soy Protein Isolate and Its Implications on Solubility and Stability of Curcumin. Food Chem. 2012, 130, 960–965. [Google Scholar] [CrossRef]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Hosseini Nouzari, S.M.; Jalalian, H.R.; Sahebkar, A. Antioxidant Effects of Bioavailability-Enhanced Curcuminoids in Patients with Solid Tumors: A Randomized Double-Blind Placebo-Controlled Trial. J. Funct. Foods 2014, 6, 615–622. [Google Scholar] [CrossRef]
- Zhan, P.Y.; Zeng, X.H.; Zhang, H.M.; Li, H.H. High-Efficient Column Chromatographic Extraction of Curcumin from Curcuma longa. Food Chem. 2011, 129, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Pinsornsak, P.; Niempoog, S. The Efficacy of Curcuma longa, L. Extract as an Adjuvant Therapy in Primary Knee Osteoarthritis: A Randomized Control Trial. J. Med. Assoc. Thai 2012, 95 (Suppl. 1), S51–S58. [Google Scholar]
- Mishra, S.; Palanivelu, K. The Effect of Curcumin (Turmeric) on Alzheimer’s Disease: An Overview. Ann. Indian Acad. Neurol. 2008, 11, 13. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Cole, G.M.; Masterman, D.L.; Cummings, J.L. A Potential Role of the Curry Spice Curcumin in Alzheimer’s Disease. Curr. Alzheimer Res. 2005, 2, 131. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Fu, M.; Gao, S.-H.; Liu, J.-L. Curcumin and Diabetes: A Systematic Review. Evid. Based Complementary Altern. Med. 2013, 2013, 16. [Google Scholar] [CrossRef]
- Labban, L. Medicinal and Pharmacological Properties of Turmeric (Curcuma longa): A Review. Int. J. Pharm. Biomed. Sci. 2014, 5, 17–23. [Google Scholar]
- Lee, J.; Jung, Y.; Shin, J.-H.; Kim, H.K.; Moon, B.C.; Ryu, D.H.; Hwang, G.-S. Secondary Metabolite Profiling of Curcuma Species Grown at Different Locations Using GC/TOF and UPLC/Q-TOF MS. Molecules 2014, 19, 9535. [Google Scholar] [CrossRef] [Green Version]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, V.K.; Joshi, A.; Dhiman, K.S. The Ayurvedic Pharmacopoeia of India, Development and Perspectives. J. Ethnopharmacol. 2017, 197, 32–38. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.; Pandya, D.; Mankad, A. Comparative Study of Phytochemical Screening and Antibacterial Activity of Curcuma longa (L.) and Curcuma Aromatica (Salib.). J. Med. Plants 2018, 6, 145–148. [Google Scholar]
- Revathy, S.; Elumalai, S.; Benny, M.; Antony, B. Isolation, Purification and Identification of Curcuminoids from Turmeric (Curcuma longa, L.) by Column Chromatography. J. Exp. Sci. 2011, 2, 21–25. [Google Scholar]
- Sobha, K.; Poornima, A.; Harini, P.; Veeraiah, K. A Study on Biochemical Changes in the Fresh Water Fish, Catla Catla (Hamilton) Exposed to the Heavy Metal Toxicant Cadmium Chloride. Kathmandu Univ. J. Sci. Eng. Technol. 1970, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sabir, S.M.; Zeb, A.; Mahmood, M.; Abbas, S.R.; Ahmad, Z.; Iqbal, N. Phytochemical Analysis and Biological Activities of Ethanolic Extract of Curcuma longa Rhizome. Braz. J. Biol. 2020, 81, 737–740. [Google Scholar] [CrossRef]
- Irshad, S.; Ashfaq, A.; Muazzam, A.; Yasmeen, A. Antimicrobial and Anti-Prostate Cancer Activity of Turmeric (Curcuma longa, L.) and Black Pepper (Piper nigrum, L.) Used in Typical Pakistani Cuisine. Pak. J. Zool. 2017, 49, 1665–1669. [Google Scholar] [CrossRef]
- Unnikrishnan, M.C.; Kuttan, R. Tumour Reducing and Anticarcinogenic Activity of Selected Spices. Cancer Lett. 1990, 51, 85–89. [Google Scholar] [CrossRef]
- Teiten, M.H.; Gaascht, F.; Eifes, S.; Dicato, M.; Diederich, M. Chemopreventive Potential of Curcumin in Prostate Cancer. Genes Nutr. 2010, 5, 61–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, C.M.; Su, Y.H.; Lin, H.Y.; Lin, J.N.; Liu, L.C.; Ho, C.T.; Way, T. der Demethoxycurcumin Modulates Prostate Cancer Cell Proliferation via AMPK-Induced down-Regulation of HSP70 and EGFR. J. Agric. Food Chem. 2012, 60, 8427–8434. [Google Scholar] [CrossRef]
- Belluti, S.; Orteca, G.; Semeghini, V.; Rigillo, G.; Parenti, F.; Ferrari, E.; Imbriano, C. Potent Anti-Cancer Properties of Phthalimide-Based Curcumin Derivatives on Prostate Tumor Cells. Int. J. Mol. Sci. 2018, 20, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dützmann, S.; Schiborr, C.; Kocher, A.; Pilatus, U.; Hattingen, E.; Weissenberger, J.; Geßler, F.; Quick-Weller, J.; Franz, K.; Seifert, V.; et al. Intratumoral Concentrations and Effects of Orally Administered Micellar Curcuminoids in Glioblastoma Patients. Nutr. Cancer 2016, 68, 943–948. [Google Scholar] [CrossRef]








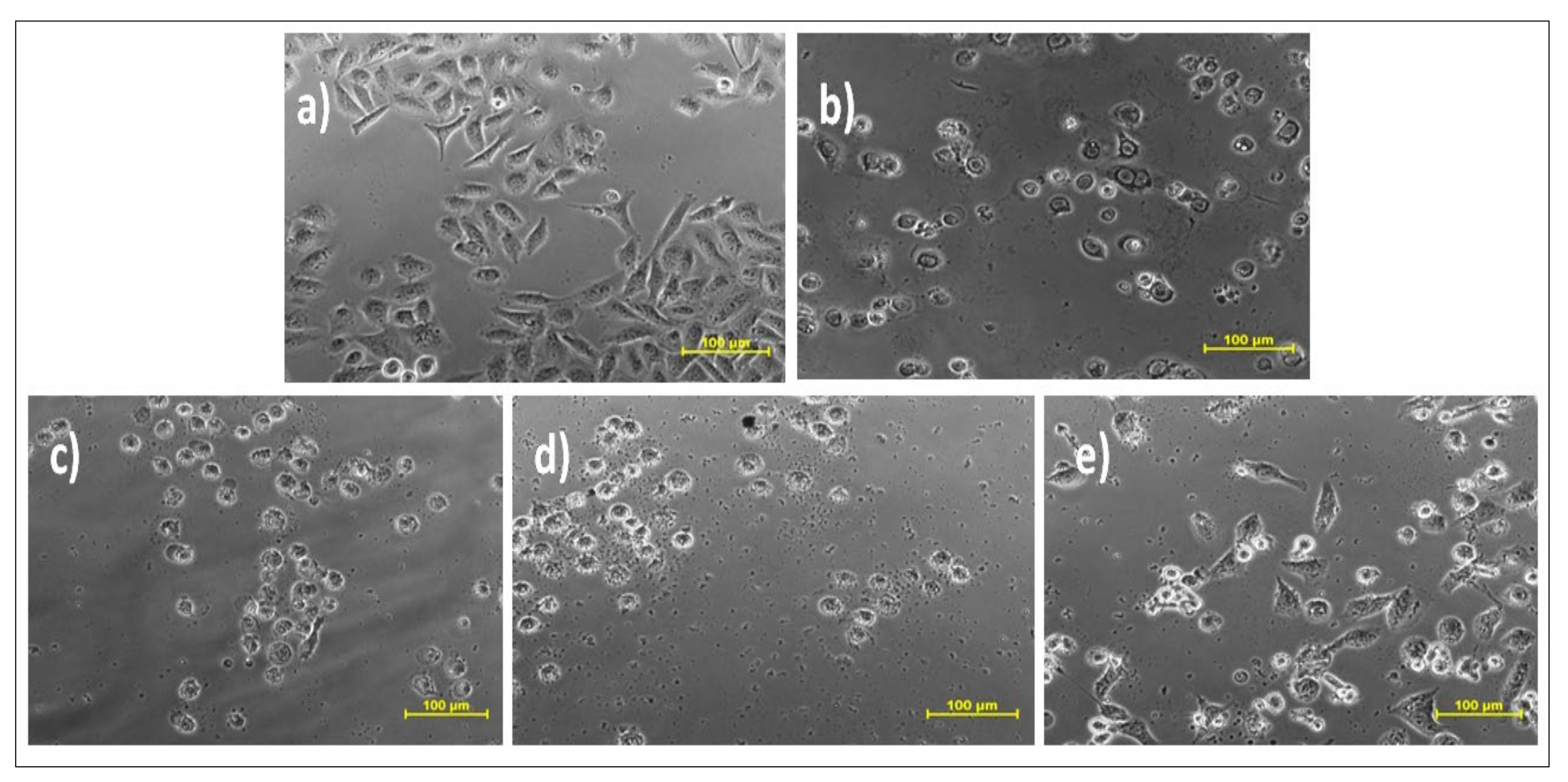

| Sample Number | Solvents | Extractive Value | % Yield (w/w) a |
|---|---|---|---|
| 1 | Chloroform | 249 mg | 2.49% |
| 2 | Ethanol | 1104 mg | 11.04% |
| 3 | Hydro-alcoholic (60:40) | 348 mg | 3.48% |
| Test | Ethanolic Extract (EC1) a | Chloroform Extract (CC1) a,b | Hydroalcoholic Extract (CMW) a,b |
|---|---|---|---|
| Alkaloids | + | + | + |
| Carbohydrates | + | + | + |
| Glycosides | + | + | + |
| Saponins | + | + | + |
| Steroids | + | + | + |
| Proteins | + | − | + |
| Terpenoids | + | − | + |
| Flavonoids | + | - | + |
| Anthraquinones | + | + | + |
| Phlobotannins | + | + | − |
| Tannins | + | − | + |
| Sample Number | Solvents | Quantity Taken | Extractive Value | % Yield (w/w) |
|---|---|---|---|---|
| 1 | Acetone | 20 gm | 3.2 gm | 16.0% |
| 2 | Acetone (for the other 2 replicas) | 20 × 2= 40 gm | 3.2 × 2 = 6.4 gm | 16.0% |
| Total yield = 3.2 + 6.4 = 9.6 gm for 60 gm of rhizomes | ||||
| Cell Type | Cell Line | IC50 (μg/mL) | |||||
|---|---|---|---|---|---|---|---|
| EC1 b | CMW b | CC1 b | C3 b | BD b | Dox a,b | ||
| Prostate Cancer | DU-145 | 19.88 ± 0.5 | 53.98 ± 0.27 | 1365.47 ± 0.36 | 17.82 ± 0.6 | 93.28 ± 0.5 | 19.5 ± 0.5 |
| Oral Cancer | SCC-29B | 11.27 ± 0.37 | 32.22 ± 0.51 | 426.896 ± 0.5 | 16.79 ± 0.56 | 106.91 ± 0.45 | 17.53 ± 0.5 |
| Normal Kidney cell line (healthy cells) | Vero cells | 525 ± 0.5 | 16.80 ± 0.66 | −107.915 ± 0.8 | 23.9 ± 0.45 | 202.1 ± 0.5 | 53.39 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grover, M.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Virmani, T.; Rachamalla, M.; Farasani, A.; Chigurupati, S.; Alsubayiel, A.M.; et al. In Vitro Phytochemical Screening, Cytotoxicity Studies of Curcuma longa Extracts with Isolation and Characterisation of Their Isolated Compounds. Molecules 2021, 26, 7509. https://doi.org/10.3390/molecules26247509
Grover M, Behl T, Sehgal A, Singh S, Sharma N, Virmani T, Rachamalla M, Farasani A, Chigurupati S, Alsubayiel AM, et al. In Vitro Phytochemical Screening, Cytotoxicity Studies of Curcuma longa Extracts with Isolation and Characterisation of Their Isolated Compounds. Molecules. 2021; 26(24):7509. https://doi.org/10.3390/molecules26247509
Chicago/Turabian StyleGrover, Madhuri, Tapan Behl, Aayush Sehgal, Sukhbir Singh, Neelam Sharma, Tarun Virmani, Mahesh Rachamalla, Abdullah Farasani, Sridevi Chigurupati, Amal M. Alsubayiel, and et al. 2021. "In Vitro Phytochemical Screening, Cytotoxicity Studies of Curcuma longa Extracts with Isolation and Characterisation of Their Isolated Compounds" Molecules 26, no. 24: 7509. https://doi.org/10.3390/molecules26247509
APA StyleGrover, M., Behl, T., Sehgal, A., Singh, S., Sharma, N., Virmani, T., Rachamalla, M., Farasani, A., Chigurupati, S., Alsubayiel, A. M., Felemban, S. G., Sanduja, M., & Bungau, S. (2021). In Vitro Phytochemical Screening, Cytotoxicity Studies of Curcuma longa Extracts with Isolation and Characterisation of Their Isolated Compounds. Molecules, 26(24), 7509. https://doi.org/10.3390/molecules26247509








