1. Introduction
Chemical elements are among the most widespread of the various pollutants originating from anthropogenic activities, particularly from mining, metallurgy and smelting waste sites. They are one of the most persistent pollutants in the environment, since they do not decompose, nor do they biodegrade into simpler and less harmful substances. Their concentrations in water are quite variable, with the highest concentrations found in the suspended matter (insoluble substances) and the lowest in the liquid phase [
1,
2,
3]. The distribution of the elements is primarily on the surfaces of sediments, suspended particles, and other solids, decreasing in the order: suspended matter > sediment > water [
4,
5]. Chemical processes occurring in natural waters, such as oxidation, can cause them to precipitate from the solution [
6].
As an integral part of natural water sources, sediments play an important role in the biogeochemical cycle of the elements, as they are the site of deposition and chemical transformation of many compounds entering the water bodies [
7]. The elements enter surface waters from many sources, in the form of atmospheric deposits, or are leached from rocks and soil. They are not biodegradable, but bind to proteins, thus being stored in the bodies of water organisms or excreted in their feces [
8], which under certain conditions leads to secondary contamination of the water bodies. In natural waters, chemical elements are found in many forms: as free ions (the most toxic forms for living organisms); in the form of various complexes; as precipitated compounds suspended in the aqueous phase; and adsorbed on the surface of other suspended or colloidal particles [
9].
The history of anthropogenic water pollution can be determined by analysis of sediments [
10,
11,
12]. Reservoir sediments provide fine-scale information on the historical record of metal pollution in a watershed [
13,
14,
15]. They have recorded the elemental deposition and thus allow establishing a connection between the temporal evolution of the pollution and historical changes in smelting and waste treatment processes [
13]. The resulting compositional datasets are usually tested by principal component analyses, self-organizing maps, and cluster analyses, with their pollution load index (PLI), index of geoaccumulation (
Igeo) and enrichment factors (EFs) being calculated [
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26].
Reservoir sediments accumulate at a high rate (usually > 2 cm/year [
27]), in contrast to river sediments (usually < 0.3 cm/year [
26]), which is why they can reveal the accumulation of the elements over time. Due to these sedimentation rates, reservoir sediments are considered to be slightly affected by early diagenesis processes and provide preserved historical elemental inputs. Reservoir sediments are also of great concern, since they can turn from a sink to a source of chemical elements for fluvial systems by diffusion at the water/sediment interface, bioturbation or resuspension due to dredging or flooding. Thus, it is important to determine the intensity of pollution by inventorying the elemental concentrations and their spatial distribution in sediments [
28].
Pchelina Reservoir was built in 1975 and serves not only as a secondary precipitator of the Struma River but also as a source for irrigation and industrial water supply of Pernik Municipality, Bulgaria. The total volume of the reservoir is 54.8 million m
3 (of which the useful volume is 19.3 million m
3), and its depth reaches 19 m [
29]. The main sources of water pollution at Pchelina Reservoir are defined as point and diffuse. Point sources are the sewerage systems from the settlements, discharged without purification in treatment plants, the wastewater treatment plants, the industrial sites, the tailings, and the mines. Diffuse sources of pollution are unregulated landfills for solid waste, settlements without sewerage, landfills, and agricultural activities such as animal husbandry. The main pollutant of the river Struma is the town of Pernik, the heavy industry of which has traditionally been dominated by mining activities and metallurgy. The industrial profile of the town changed in the 1990s, but the wastewaters discharged into the river are still collected in the Pchelina Reservoir. The only available studies of the sediments of Pchelina Reservoir were made by Meuser and co-authors [
30,
31].
This study aims to propose a methodology for assessing the temporal dynamics of anthropogenic impacts on sediments of Pchelina Reservoir. The proposed methodology includes: (i) analysis of chemical elements and 137Cs radionuclides of three sediment cores taken from three selected sampling points in Pchelina Reservoir, (ii) multivariate and time trend statistical analysis of the sediment cores data, and (iii) calculation of enrichment factors and geochemical indexes of surface sediments followed by ecotoxicity assessment using Phytotoxkit F ™ bioassay.
2. Results
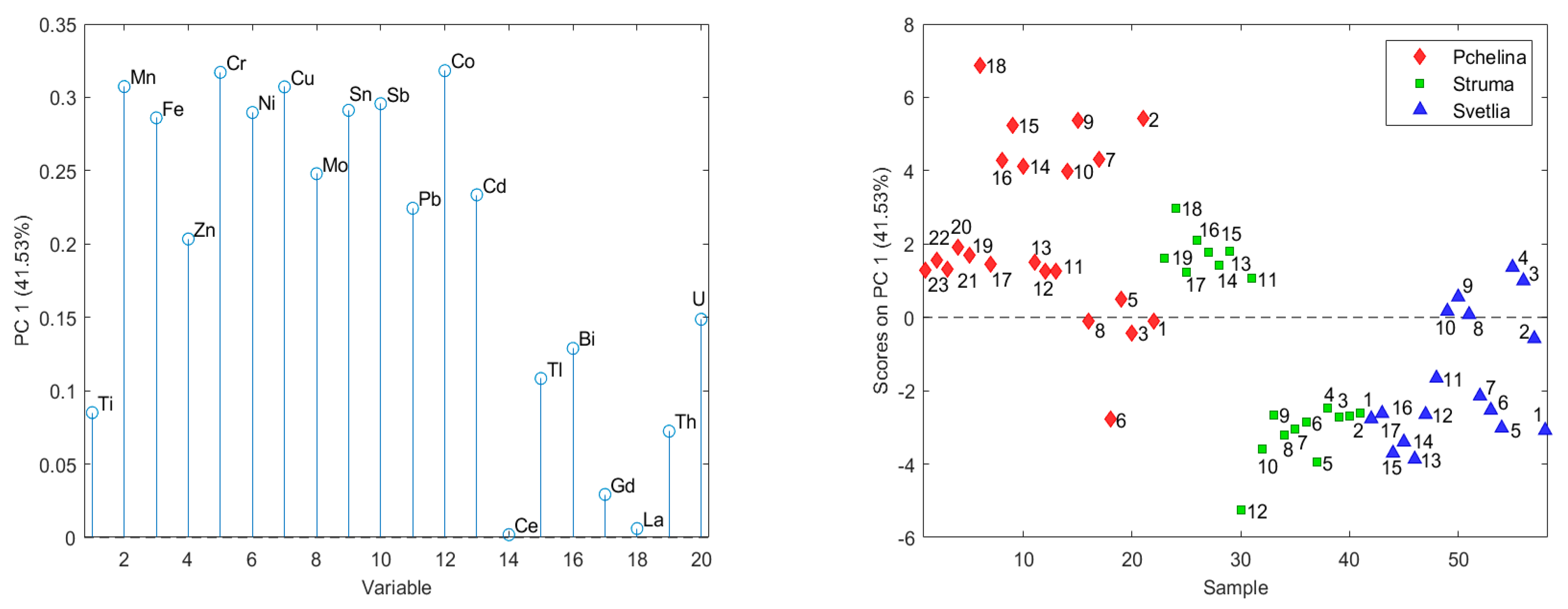
To reveal the relationships between the analyzed chemical elements and/or layers in the sediment cores, a principal component analysis (PCA) was applied. The input data set used for PCA consists of 58 objects (layers in the sedimentary cores) and 20 variables (analyzed chemical elements). PCA of the data from the three sedimentary cores revealed that the first three main components describe almost 80% of the variation of the data. The number of latent variables is determined based on their eigenvalues and the internal model validation error. In the formation of the first principal component, explaining 41.53% of data variance, the following elements have a significant impact: Mn, Fe, Cr, Ni, Cu, Mo, Sn, Sb and Co (
Figure 1). This component separates the elements into two groups according to their time trends in Struma sediment core: (i) Mn, Fe, Zn, Cr, Ni, Cu, Mo, Sn, Sb, Pb, Co, Cd and U have increased concentrations with time; and (ii) Ti, Ce, Tl, Bi, Gd, La and Th have decreased or non-significant time trends.
The second principal component (22.16%) is formed by Ce, Gd, La, Th, and Ti, which significantly decrease over time in the sedimentary cores at Struma and Svetlia rivers (
Figure 2). These decreasing trends do not lead to a significant change in the contents of the elements in the Pchelina Reservoir, which are comparable to the contents in the sedimentary cores near the Struma and Svetlia at the beginning of the period.
The third principal component (15.44%) is formed by the elements Zn, Pb, Cd, Bi and U
nat. The factor scores of the layers of the Pchelina core show a particularly pronounced positive trend, which leads to the formation of two groups of layers. The first group, covering the beginning of the studied period (1–11), has contents comparable to the sedimentary core of the Svetlia (anthropogenically undisturbed river), while the contents of the elements in the second group (12–23) are the highest for all the three studied sediment cores (
Figure 3). The layers in the Struma sediment core have medium factor scores between both groups of Pchelina sediment core.
137Cs has been widely applied as an environmental tracer in the study of sediment recent deposition history (usually within the last 50 years) [
32,
33]. Each of the 2 cm sediment core fractions was analyzed for its
137Cs content. Only in one of these fractions for each sediment core, radioactivity (γ-activity) was found and the content of
137Cs measured was between 32.8 Bq/kg to 55.3 Bq/kg specific activities. Based on these findings, a conclusion was drawn that, on average, 15 cm of sediment was deposited in the 34 years since the Chernobyl incident (1986). This means that the average sedimentation rate at the sampling points in Pchelina Reservoir is 0.44 cm/year. Such results differ from the literature values—usually > 2 cm/year [
27,
34]—but are closer to the reported values for the rates of river sediment—usually < 0.3 cm/year) [
30].
The element depth profiles of sediments from Pchelina Reservoir at the different sampling points (Pchelina, Struma and Svetlia) are shown in
Figure 4. Red points indicate the sample in which the highest radioactivity (γ-activity) has been registered, which corresponds to the Chernobyl pollution of 1986. A similar approach was used by Audry et al. [
13] for the sediment core dating of the Lot River reservoirs.
To determine whether significant time trends of elements were observed, the Mann–Kendell test was performed. The results are presented in
Table 1, with significant trends (
p < 0.05) marked with “+” for increasing and “−” for decreasing trends. The significant time tendencies in the sediment cores influenced by the flowing rivers reveal that Struma River (sampling point 1) is the main source of Pchelina Reservoir pollution.
According to the sedimentation rate calculations, it is assumed that any concentration of each element in a layer more than 6 cm below the sample, which corresponds to the Chernobyl pollution (marked in red in
Figure 4), is a background concentration. The average result for Fe (used as a conservative element) of all such samples was calculated for each sampling point and used to obtain the enrichment factor for the sample corresponding to 2020 (top layers,
Table 2).
Significant enrichment is observed only in the reference point (Pchelina), in terms of Zn and Cd (7.6 and 7.5, respectively). Regarding Cd, the enrichment in Svetlia is moderate (3.2). The enrichment factor is 1.8 in the Struma, which shows significant pollution with this metal in the entire Reservoir. Except for these elements, moderate enrichment is observed for Cu, Pb, Ce and Th only at Pchelina, as well as for Tl and Unat in Svetlia and Pchelina.
The same approach for determination of the reference concentration (average of all results for the layers more than 6 cm below the sample, which corresponds to the Chernobyl pollution—marked in red in
Figure 4) for each of the studied elements was used to determine the geoaccumulation index. The results are presented in
Table 3.
It is evident from
Table 3 that for most elements, the geoaccumulation index corresponds to an uncontaminated sample. This result is especially important for the sediments at the inflow of the Svetlia River (sampling point 2), where only for Cd, Tl and U
nat the index corresponds to an unpolluted to moderately polluted sample. The geoaccumulation index in the sediments at the inflow of the Struma River (sampling point 1) corresponds to an unpolluted to moderately contaminated sample for Fe, Cr, Mo and Sn and to a moderately polluted sample for Sb and Cd. The geoaccumulation index for the sediments of Pchelina Reservoir from the village of Radibosh (sampling point 3), which corresponds to an unpolluted to moderately polluted sample in terms of Pb, Tl and U
nat (similar to the sediments in Svetlia) and a moderately polluted sample in terms of Zn and Cd (analogous to the sediments in the Struma). The moderate contamination of the sediments of Pchelina Reservoir in all sampling points with Cd is impressive. Similar results were obtained for sediments of Wadi Al-Arab Dam, Jordan [
35], where sediments were found uncontaminated with Mn, Fe, and Cu, moderately contaminated with Zn, and strongly to extremely contaminated with Cd. Ye et al. [
36] compared deposits of “heavy metals”, accumulated in the water-level-fluctuation zone before and after the submergence period and found that Cd is the main pollutant of the sediment.
The results of the conducted biotest Phytotoxkit F ™ are presented in
Table 4 and reveal the low ecotoxicity of the surface sediments from Pchelina Reservoir.
The indicator related to seed germination (SG) of Sinapis alba shows the lack of ecotoxicological effect. This means that the number of germinated seeds in the analyzed sediments is equal to their number in the control sample, in this case, all 10 seeds have germinated. The ecotoxicological effect, reflecting the growth of the roots of Sinapis alba, is relatively weak, as it is highest in the sample from Struma River, and even shows a negative value in Pchelina (hormesis). This means that the roots of the test species used are longer than the control sample.
3. Discussion
The background concentrations strongly depend on geological characteristics such as mineral composition, grain size distribution and organic matter content. Thus, establishing geochemical background concentrations of chemical elements is a very important step in environmental pollution assessment [
37].
137Cs as an indicator of sedimentation processes is consistent as it binds almost irreversibly to clay and silt particles and because of its relatively long half-life (
t1/2 = 30.2 years). Moreover, the Chernobyl nuclear accident of 1986 has been recorded by the European sediments. Thus,
137Cs activity depth profiles are often used for sediment core dating [
38].
Based on the measurement of γ-activity and the calculated sedimentation rate in the Pchelina Reservoir of an average of 0.44 cm/year, it can be assumed that three of the points under 1986 (marked in red) are samples of sediment (approximately 6 cm), and the rest are from the natural soil cover, flooded during the construction of the Reservoir (1975). The remaining samples should contain levels of the elements that correspond to the background concentrations of these elements before they are anthropogenically affected. This was the basis for the analysis of the elements’ core depth profiles in the present study. It is noteworthy that the concentrations of most of the studied elements increase, except for Bi, La, Ti, Tl, where the concentrations decrease over time, while Ce, Gd, Th practically do not change.
The PCA results divided the analyzed chemical elements into three groups. The elements with significant impact in the formation of the first principal component (Mn, Fe, Cr, Ni, Cu, Mo, Sn, Sb and Co) have an increasing time trend in Struma sediment core (sampling site 1) and no trend (Co, Cr, Mn, Mo) or a decreasing one (Cu, Fe, Ni, Sb, Sn) at the Pchelina sampling site. The factor scores of the layers in the Pchelina sediment core (sampling site 3) increase until the late 1990s and at the end of the investigated period decreased to the levels between 1988 and 1994. The absence of an increasing trend in concentrations of the abovementioned elements at the reference point for Pchelina Reservoir (between the inflows of the two rivers—Struma River—anthropogenically affected and Svetlia River—anthropogenically unaffected) could be explained by the changed profile of the industry in the town of Pernik during the 1990s, since when mining and metallurgy have not been so dominant. These observations are supported by the calculated EFs and geoaccumulation indexes for the top layer at the Pchelina sampling site where only for Cu moderate enrichment is observed.
The chemical elements associated with the second principal component (Ce, Gd, La, Th, and Ti) have decreasing time trends in both sediment cores at the two river inflows and excluding Th in the Pchelina sediment core too. These elements have no anthropogenic origin and the moderate enrichment of Ce and Th at the Pchelina sampling site could be attributed to their geochemical immobility [
39]. The third group of chemical elements (Zn, Pb, Cd, Bi and U
nat) forming the third principal component have significant increasing trends in Struma and Pchelina sediment cores. This leads to the formation of two distinct groups of layers in the Pchelina sediment core before and after the 1990s (layers 11 and 12). The increased concentrations of these elements at the end of the investigated period have led to moderate enrichments for Pb and U
nat, and significant enrichments for Zn and Cd at the Pchelina sampling site. The respected geoaccumulation indexes confirm these observations with uncontaminated to moderately contaminated values for Pb and U
nat, and moderately contaminated ones for Zn and Cd. The results from the present study largely confirm the conclusions made by Meuser and co-authors in 2006 [
40] where the impact of industry located in the region of Pernik town results in increased concentrations of Pb, Cd, Cr, Cu and Zn.
The results of the conducted biotest Phytotoxkit F ™ reveal the low ecotoxicity of surface sediments, which is an indication that the concentrations of the elements classifying the Pchelina Reservoir samples as moderately contaminated has no significant ecotoxicological effect.
5. Conclusions
Based on the measurement of γ-activity of the technogenic 137Cs, an accumulation of an average of 15 cm of sediment was established for 34 years, at a sedimentation rate of an average of 0.44 cm/year.
The distribution of the concentrations of chemical elements in the sediment from the three sampling points (1—Pchelina Reservoir at the flow of Struma River, 2—Pchelina Reservoir at the flow of Svetlia River and 3—Pchelina Reservoir near the village of Radibosh) is presented. PCA of the data shows three main components, which describe nearly 80% of the variation of the data. The first main component (41.53% of the data variation) contains Mn, Fe, Cr, Ni, Cu, Mo, Sn, Sb and Co. The factor scores show that the concentrations of these elements decrease in the order Pchelina > Struma > Svetlia. All these elements have a positive time trend in the Struma sediment core, which is an indication that most of the elements in the Reservoir come through the anthropogenically affected river Struma. The second main component (22.16%) is formed by Ce, Gd, La, Th, and Ti, which decrease significantly with time in Svetlia and Struma. The third main component (15.44%) is formed by the elements Zn, Pb, Cd, Bi and Unat. The factor scores of the layers in the sedimentary cores show the anthropogenic origin of most of these elements. There is an increase over time in the sedimentary cores in Struma and Pchelina. The increase in the sedimentary layers of Pchelina is especially pronounced. In the first group (beginning of the studied period), there are contents comparable to the sediment core of Svetlia, while the contents of the elements in the second group are the highest for the three studied sediment cores.
To distinguish anthropogenic pollution from the natural content of elements in the sediment, enrichment factors (EF) have been calculated for which Fe concentrations have been used as a conservative element. Significant enrichment was observed only at the reference point (Pchelina), for Zn and Cd. In terms of Cd, the enrichment in Svetlia is moderate. The enrichment factor is 1.8 in the Struma, which shows significant contamination with this metal in the entire Reservoir. Except for these elements, moderate enrichment is observed for Cu, Pb, Ce and Th only in Pchelina, as well as for T1 and Unat in Svetlia and Pchelina. For most elements, the geoaccumulation index corresponds to an uncontaminated sample. In Svetlia, only for Cd, T1 and Unat the index corresponds to an uncontaminated to moderately contaminated sample. The index of geoaccumulation in the sediments of Struma corresponds to an uncontaminated to moderately contaminated sample for Fe, Cr, Mo and Sn and to a moderately contaminated sample for Sb and Cd. The geoaccumulation index for the sediments of Pchelina corresponds to an unpolluted to moderately contaminated sample for Pb, Tl and Unat (similar to the Svetlia sediments) and a moderately polluted sample for Zn and Cd (similar to the Svetlia sediments). Moderate contamination of the sediments of Pchelina Reservoir in all sampling points is from Cd.
The results of the Phytotoxkit F bioassay revealed the low ecotoxicity of Pchelina Reservoir surface sediments in terms of both seed germination (SG) and root growth (RG) of the plant species Sinapis alba.