Chemical and Biological Characteristics of Oxytropis pseudoglandulosa Plant of Mongolian Origin
Abstract
:1. Introduction
2. Results
2.1. Spectrophotometric Determination of Total Phenolic and Flavonoid Content
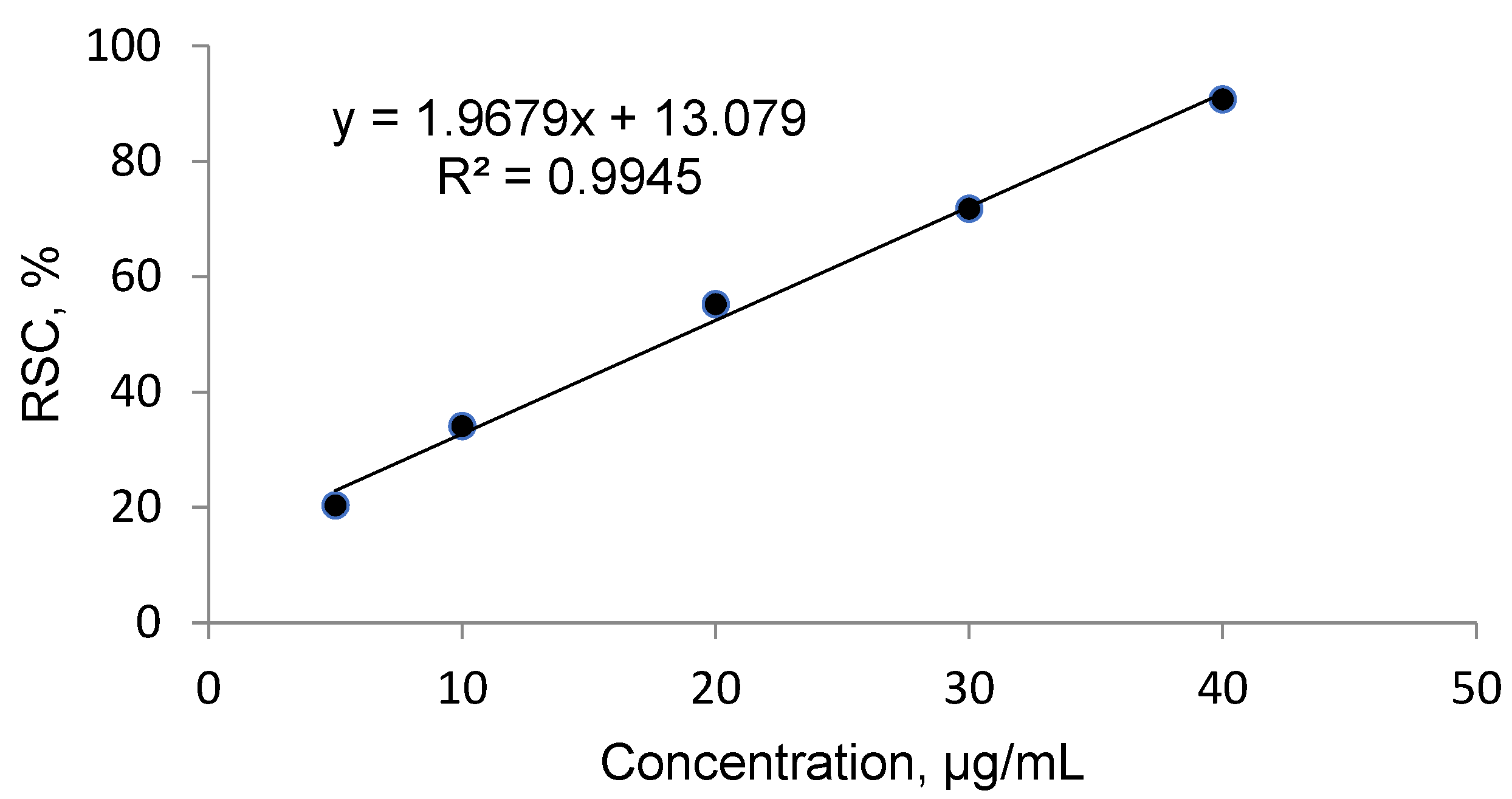
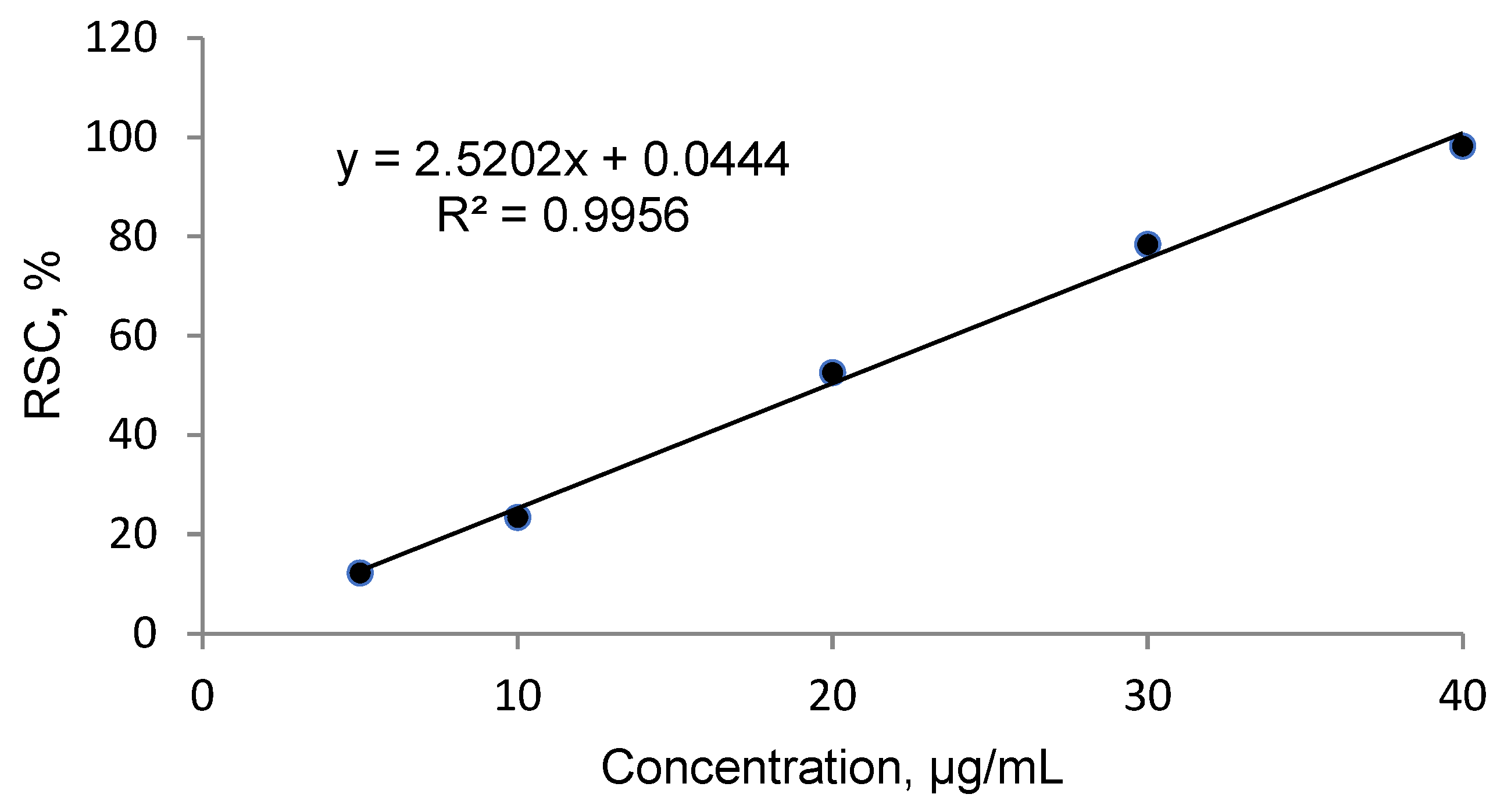
2.2. Antioxidant Activity
2.3. GC-MS Determination of Volatile Oil Composition
2.4. GC-MS Determination of Lipids
2.5. Antimicrobial Properties
2.6. Ingestive Allergenicitya
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Extraction of Phenolic and Flavonoid Compounds
4.3. Total Phenolic and Flavonoid Content
4.4. Free Radical Scavenging Activity and Calculation of the IC50
4.5. GC-MS Determination of Volatile oil Components
4.6. Extraction of Lipids
4.7. GC-MS Determination of Lipids
4.8. Antimicrobial Activity
4.9. Extraction of Food Allergens
4.10. Allergenic Protein Content Determination
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, J.-T.; Zhang, D.-G.; Lv, Z.-Y.; Huang, X.-H.; Liu, P.-J.; Yang, J.-N.; Yang, J.-Y.; Tojibaev, K.; Deng, T.; Sun, H. Oxytropis shennongjiaensis (Fabaceae), a New Species from Hubei, Central China. PhytoKeys 2020, 149, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Almerekova, S.; Mukhitdinov, N.; Abugalieva, S. Phylogenetic Study of the Endemic Species Oxytropis almaatensis (Fabaceae) Based on Nuclear Ribosomal DNA ITS Sequences. BMC Plant. Biol. 2017, 17, 173. [Google Scholar] [CrossRef] [Green Version]
- Li, M.X.; Lan, Z.H.; Wei, L.L.; Zhang, W.J.; Zhang, R.X.; Jia, Z.P. Phytochemical and Biological Studies of Plants from the Genus Oxytropis. Rec. Nat. Prod. 2012, 6, 1–20. [Google Scholar]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Kukula-Koch, W. Achillea Species as Sources of Active Phytochemicals for Dermatological and Cosmetic Applications. Oxid. Med. Cell. Longev. 2021, 2021, 6643827. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.; Park, H.-M.; Jung, W.-M.; Cha, W.-S.; Lee, D.; Chae, Y. Identification of Candidate Medicinal Herbs for Skincare via Data Mining of the Classic Donguibogam Text on Korean Medicine. Integr. Med. Res. 2020, 9, 100436. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Hayashi, S.; Craker, L. Culture, History and Applications of Medicinal and Aromatic Plants in Japan. In Aromatic and Medicinal Plants-Back to Nature; El-Shemy, H., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.-Y.; Litscher, G.; Gao, S.-H.; Zhou, S.-F.; Yu, Z.-L.; Chen, H.-Q.; Zhang, S.-F.; Tang, M.-K.; Sun, J.-N.; Ko, K.-M. Historical Perspective of Traditional Indigenous Medical Practices: The Current Renaissance and Conservation of Herbal Res.ources. Evid. Based Complement Altern. Med. 2014, 2014, 525340. [Google Scholar] [CrossRef]
- Milevskaya, V.V.; Prasad, S.; Temerdashev, Z.A. Extraction and Chromatographic Determination of Phenolic Compounds from Medicinal Herbs in the Lamiaceae and Hypericaceae Families: A Review. Microchem. J. 2018, 145, 1036–1049. [Google Scholar] [CrossRef]
- Jiang, H.; Zhan, W.Q.; Liu, X.; Jiang, S.X. Antioxidant Activities of Extracts and Flavonoid Compounds from Oxytropis falcate Bunge. Nat. Prod. Res. 2008, 22, 1650–1656. [Google Scholar] [CrossRef]
- Sarfaraz, D.; Rahimmalek, M.; Saeidi, G. Polyphenolic and Molecular Variation in Thymus Species Using HPLC and SRAP Analyses. Sci. Rep. 2021, 11, 5019. [Google Scholar] [CrossRef]
- Badral, D.; Mungunshagai, B.; Brantner, A.H.; Batkhuu, J. Phytochemical Studies and Antioxidant Activity of Saxifraga spinulosa Adams. Mong. Pharm. Pharm. 2014, 1, 11–15. [Google Scholar]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential Oils as Antimicrobials in Food Systems–A review. Food Control. 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Baroi, A.M.; Ortan, A. Selected Aspects Related to Medicinal and Aromatic Plants as Alternative Sources of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 1521. [Google Scholar] [CrossRef] [PubMed]
- Said, Z.; Garrouj, D.; Pagán, R.; Chabi, M.; Laglaoui, A.; Bakkali, M.; Zerrouk, M.H. Effect of Harvest Time on Yield, Chemical Composition, Antimicrobial and Antioxidant Activities of Thymus vulgaris and Mentha pulegium Essential Oils. Eur. J. Med. Plants 2015, 8, 69–77. [Google Scholar]
- Bashi, D.; Asili, J.; Mohtashami, S.; Farshchi, H. Composition of Essential Oil of Oxytropis kuchanensis from Iran. Chem. Nat. Compd. 2014, 50, 153–154. [Google Scholar]
- Jiang, H.; Zhan, W.Q.; Liu, X.; Jiang, S.X. Chemical Composition and Antioxidant Activity of the Essential Oil From Oxytropis falcate Bunge. J. Essent. Oil. Res. 2009, 21, 300–302. [Google Scholar] [CrossRef]
- Chen, C.; Liu, F.; Zhao, J.; Chen, T.; Li, Y.; Zhang, D. Efficient Separation of Five Flavonoids from Oxytropis falcata Bunge by High-Speed Counter-Current Chromatography and Their Anticancer Activity. Acta. Chromatogr. 2020, 32, 189–193. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-Inflammatory Property of n-Hexadecanoic Acid: Structural Evidence and Kinetic Assessment. Chem. Biol. Drug. Des. 2012, 80, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudah, M.; Al-Jaber, H.; Mayyas, A.S.; Orabi, S.; Zarga, M.A. Chemical Compositions of Essential Oil from the Jordanian Medicinal Plant Dittrichia viscosa. Jordan J. Chem. 2010, 5, 343–348. [Google Scholar]
- Khleifat, K.M.; Matar, S.A.; Jaafreh, M.; Qaralleh, H.; Al-limoun, M.O.; Alsharafa, K.Y. Essential Oil of Centaurea damascena Aerial Parts, Antibacterial and Synergistic Effect. J. Essent. Oil-Bear Plants 2019, 22, 356–367. [Google Scholar] [CrossRef]
- Tao, C.; Wu, J.; Liu, Y.; Liu, M.; Yang, R.; Lv, Z. Antimicrobial activities of bamboo (Phyllostachys heterocycla cv. Pubescens) leaf essential oil and its major components. Eur. Food Res. Technol. 2018, 244, 881–891. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, A. A Floral Fragrance, Methyl Benzoate, is An Efficient Green Pesticide. Sci. Rep. 2017, 7, 42168. [Google Scholar] [CrossRef] [PubMed]
- Ninkovic, V.; Markovic, D.; Rensing, M. Plant Volatiles as Cues and Signals in Plant Communication. Plant Cell Environ. 2021, 44, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Mallavarapu, G.R.; Gurudutt, K.N.; Syamasundar, K.V. Chapter 99-Ylang–Ylang (Cananga odorata) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 865–873. [Google Scholar]
- Briggs, M.A.; Petersen, K.S.; Kris-Etherton, P.M. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Hu, J.R.; Zhan, W.Q.; Liu, X. Screening for Fractions of Oxytropis falcata Bunge with Antibacterial Activity. Nat. Prod. Res. 2009, 23, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Ao, W.; Huo, W.; Wang, X.; Wang, H. Study on Screening for Antibacterial Active Site of Oxytropis myriophylla in Vitro. Chin. J. Inf. Tradit. Chin. Med. 2016, 23, 51–54. [Google Scholar]
- Zhang, M.R.; Jiang, K.; Yang, J.L.; Shi, Y.P. Flavonoids as Key Bioactive Components of Oxytropis falcata Bunge, a Traditional Anti-Inflammatory and Analgesic Tibetan Medicine. Nat. Prod. Res. 2020, 34, 3335–3352. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hu, J.; Zhang, L. Antibacterial Activity of Alkaloids Extract from Oxytropis falcata Bunge Against 9 Pathogens. Chin. J. Inf. Tradit. Chin. Med. 2014, 12, 53–55. [Google Scholar]
- Hofer, H.; Asam, C.; Hauser, M.; Nagl, B.; Laimer, J.; Himly, M.; Briza, P.; Ebner, C.; Lang, R.; Hawranek, T.; et al. Tackling Bet v 1 and associated food allergies with a single hybrid protein. J. Allergy. Clin. Immunol. 2017, 140, 525–533.e10. [Google Scholar] [CrossRef] [Green Version]
- Ziemianin, M.; Waga, J.; Czarnobilska, E.; Myszkowska, D. Changes in qualitative and quantitative traits of birch (Betula pendula) pollen allergenic proteins in relation to the pollution contamination. Environ. Sci. Pollut. Res. 2021, 28, 39952–39965. [Google Scholar] [CrossRef]
- Aninowski, M.; Leszczyńska, J. The Determination of Potentially Allergenicity of Selected Herbs. Biotech. Food Sci. 2019, 83, 3–11. [Google Scholar]
- Patel, J.B.; Cockerill, F.R.; Bradford, P.A. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard; CLSI: Wayne, PA, USA, 2015; Volume 35, No. 2; pp. 15–48. [Google Scholar]

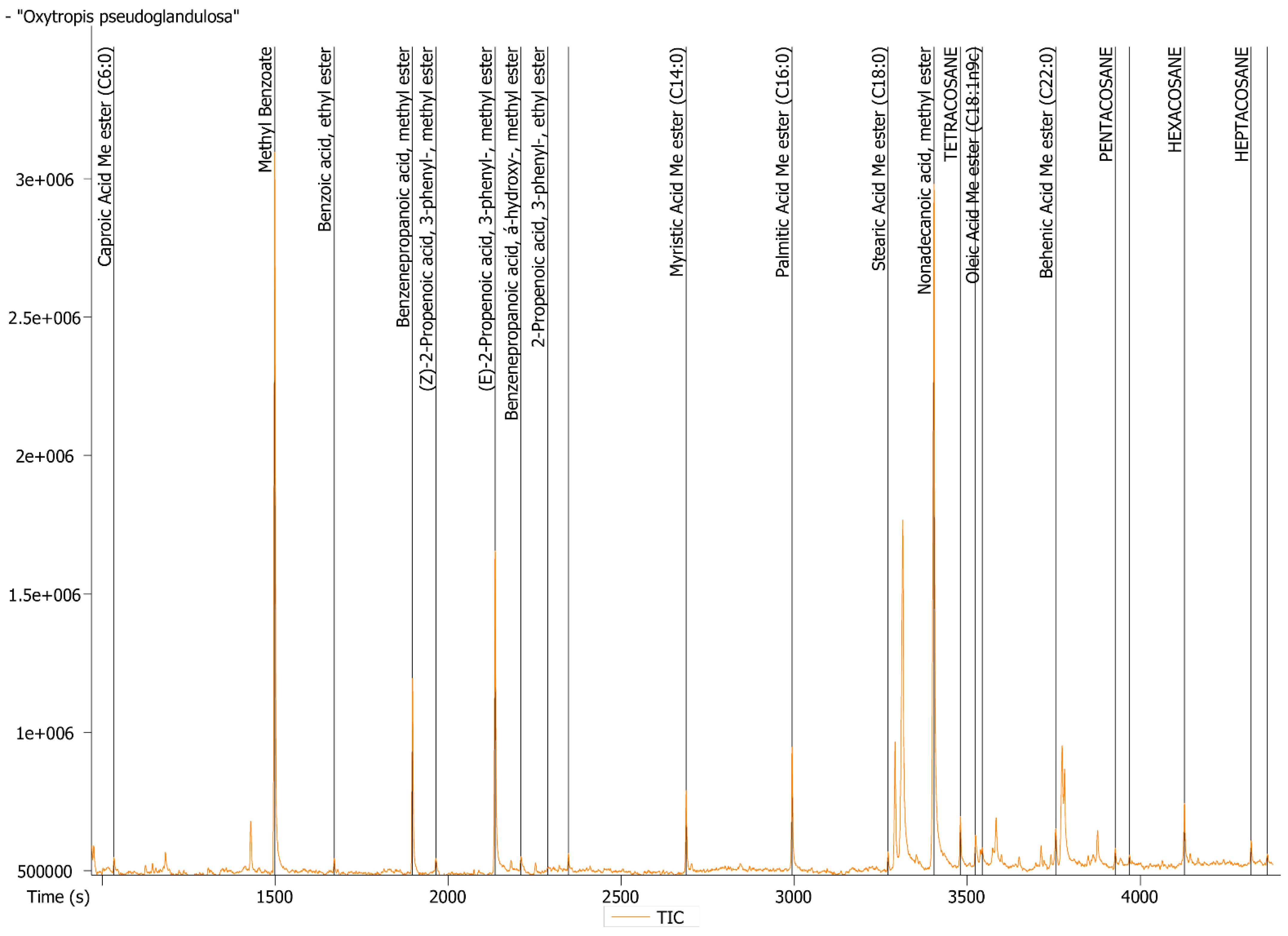
| Compound | Total, % | Compound | Total, % |
|---|---|---|---|
| Oct-1-en-3-one | 3.20 | (Z)-Hex-3-enyl cinnamate | 0.75 |
| Limonene | 0.62 | Methyl hexadecanoate | 0.34 |
| Dec-1-en-3-one | 2.05 | Sclareoloxide (Cis-B/C) | 1.76 |
| Linalool | 1.09 | Hexadecanoic acid | 13.13 |
| Vitispirane | 1.01 | Kaur-16-ene | 1.00 |
| Trans-beta-Ionone | 0.69 | Methyl linoleate | 0.91 |
| α-Bulnesene | 4.31 | Phytol | 3.48 |
| Elemicin | 4.40 | Prasterone | 4.33 |
| Nerolidol | 0.82 | Tricosane | 5.55 |
| Fokienol | 11.46 | Tetracosane | 0.70 |
| Pogostol | 9.85 | Pentacosane | 3.05 |
| Bulnesol | 0.77 | Hexacosane | 0.83 |
| Tetradecanoic acid | 2.78 | Nonacosane | 1.32 |
| Hexahydrofarnesyl acetone | 2.57 | ||
| Total identified | 82.77 |
| Compound | Total, % | Compound | Total, % |
|---|---|---|---|
| Caproic acid (C6:0) | 0.79 | Benzenepropanoic acid, α-hydroxy- | 1.32 |
| Lauric acid (C12:0) | 0.78 | (Z)-prop-2-enoic acid, 3-phenyl- | 1.19 |
| Myristic acid (C14:0) | 4.02 | (E)-prop-2-enoic acid, 3-phenyl- | 18.55 |
| Palmitic acid (C16:0) | 6.77 | Propenoic acid, 3-phenyl-, ethyl | 0.11 |
| Stearic acid (C18:0) | 0.84 | Methyl benzoate | 40.69 |
| Arachidic acid (C20:0) | 2.09 | Benzoic acid, ethyl | 0.82 |
| Behenic acid (C22:0) | 2.46 | Tetracosane | 1.27 |
| Lignoceric acid (C24:0) | 0.45 | Pentacosane | 1.15 |
| Octacosanoicacid | 0.53 | Hexacosane | 3.75 |
| Oleic acid (C18:1) | 1.28 | Heptacosane | 1.17 |
| Benzenepropanoic acid | 9.97 |
| Gram+ | MIC, %(v/v) | Gram- | MIC, %(v/v) | Fungi | MIC, %(v/v) |
|---|---|---|---|---|---|
| M. flavus | 4.162 | P. fluorescens | 4.162 | S. cerevisiae | 0.260 |
| S. aureus | 4.162 | P. aeruginosa | 8.325 | C. vini | 1.040 |
| B. subtilis | 2.081 | E. coli | 8.325 | A. niger | 4.162 |
| S. epidermidis | 4.162 | E. aerogenes | 8.325 | P. expansum | 4.162 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narangerel, T.; Bonikowski, R.; Jastrząbek, K.; Kunicka-Styczyńska, A.; Plucińska, A.; Śmigielski, K.; Majak, I.; Bartos, A.; Leszczyńska, J. Chemical and Biological Characteristics of Oxytropis pseudoglandulosa Plant of Mongolian Origin. Molecules 2021, 26, 7573. https://doi.org/10.3390/molecules26247573
Narangerel T, Bonikowski R, Jastrząbek K, Kunicka-Styczyńska A, Plucińska A, Śmigielski K, Majak I, Bartos A, Leszczyńska J. Chemical and Biological Characteristics of Oxytropis pseudoglandulosa Plant of Mongolian Origin. Molecules. 2021; 26(24):7573. https://doi.org/10.3390/molecules26247573
Chicago/Turabian StyleNarangerel, Tuya, Radosław Bonikowski, Konrad Jastrząbek, Alina Kunicka-Styczyńska, Aleksandra Plucińska, Krzysztof Śmigielski, Iwona Majak, Adrian Bartos, and Joanna Leszczyńska. 2021. "Chemical and Biological Characteristics of Oxytropis pseudoglandulosa Plant of Mongolian Origin" Molecules 26, no. 24: 7573. https://doi.org/10.3390/molecules26247573






