Effect of Juglone against Pseudomonas syringae pv Actinidiae Planktonic Growth and Biofilm Formation
Abstract
:1. Introduction
2. Results
2.1. Antibacterial Activity of Juglone against P. syringae
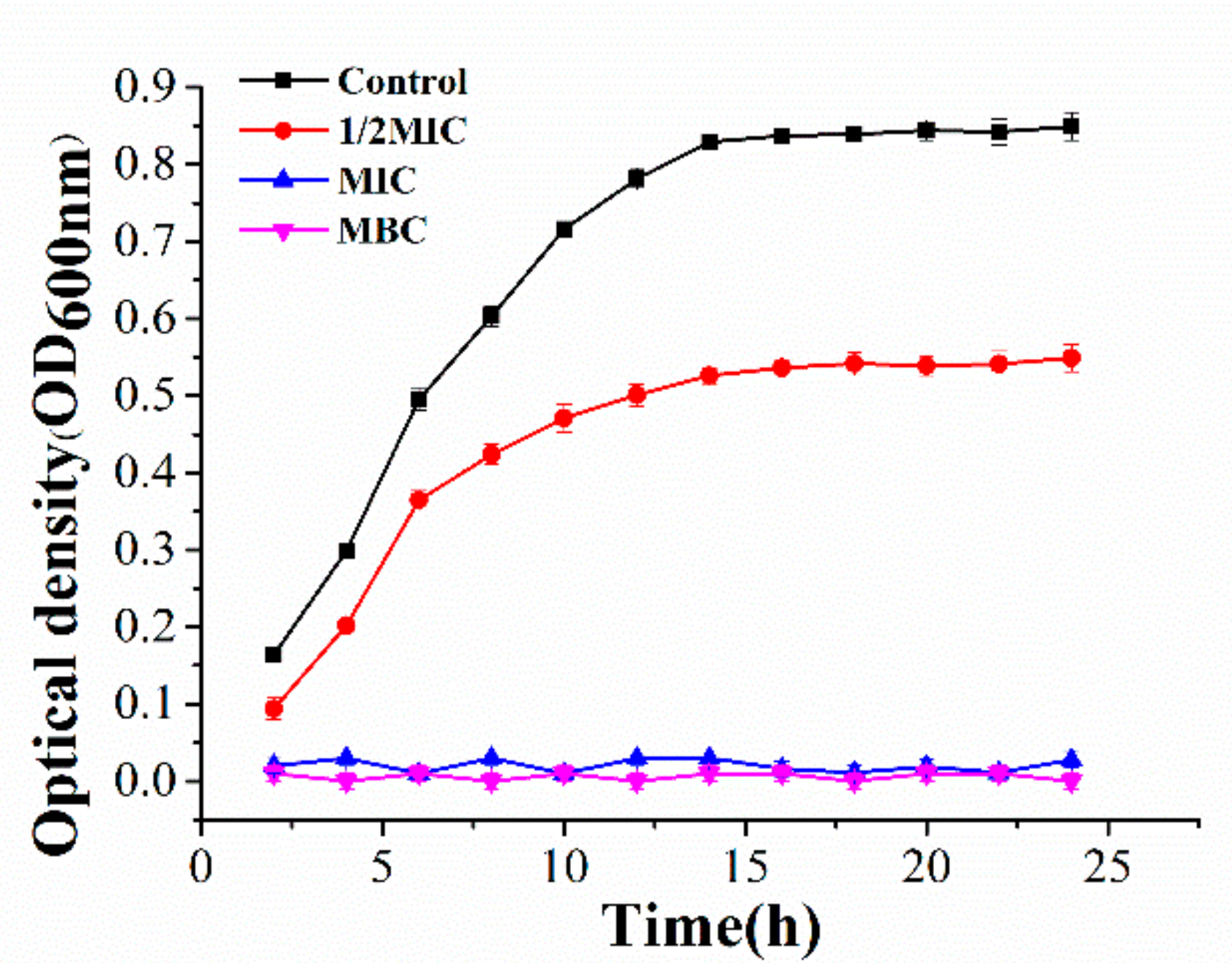
2.2. Effect of Juglone on the Vitality of P. syringae
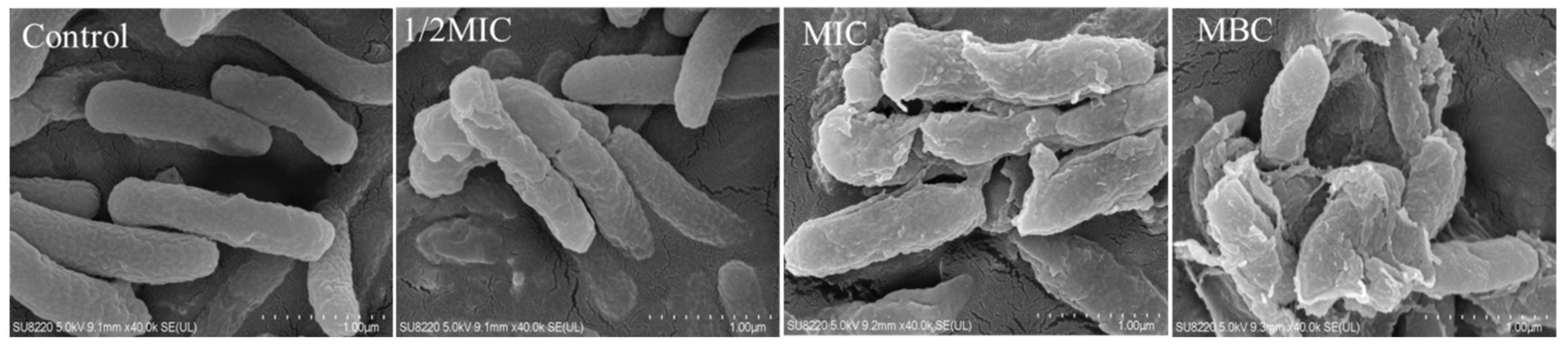
2.3. Effect of Juglone on the Cell Morphology of P. syringae
2.4. Juglong Induced Membrane Destruction in P. syringae Cells
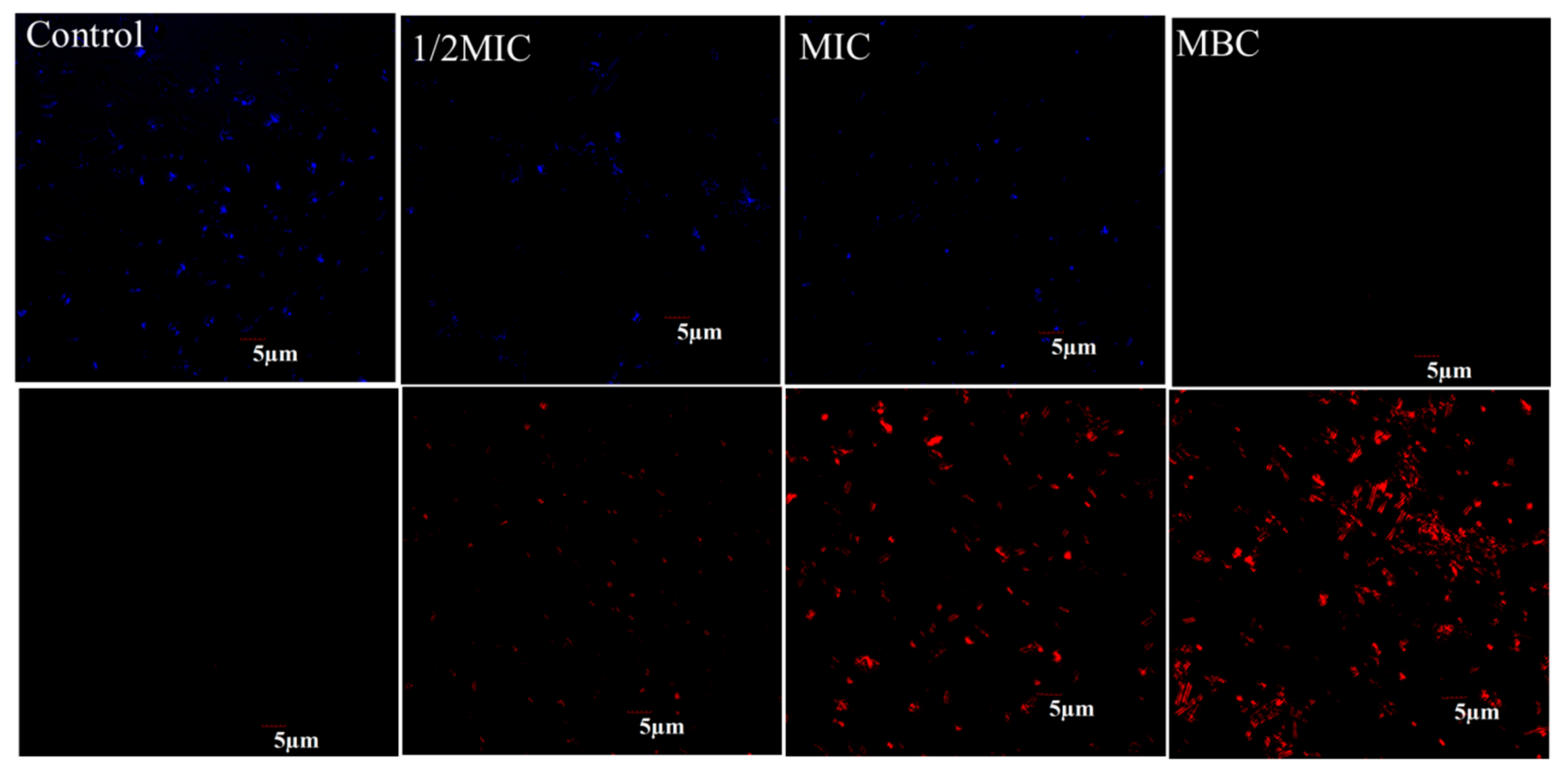
2.4.1. Effect of Juglone Induction on Cell Membrane Potential of P. syringae
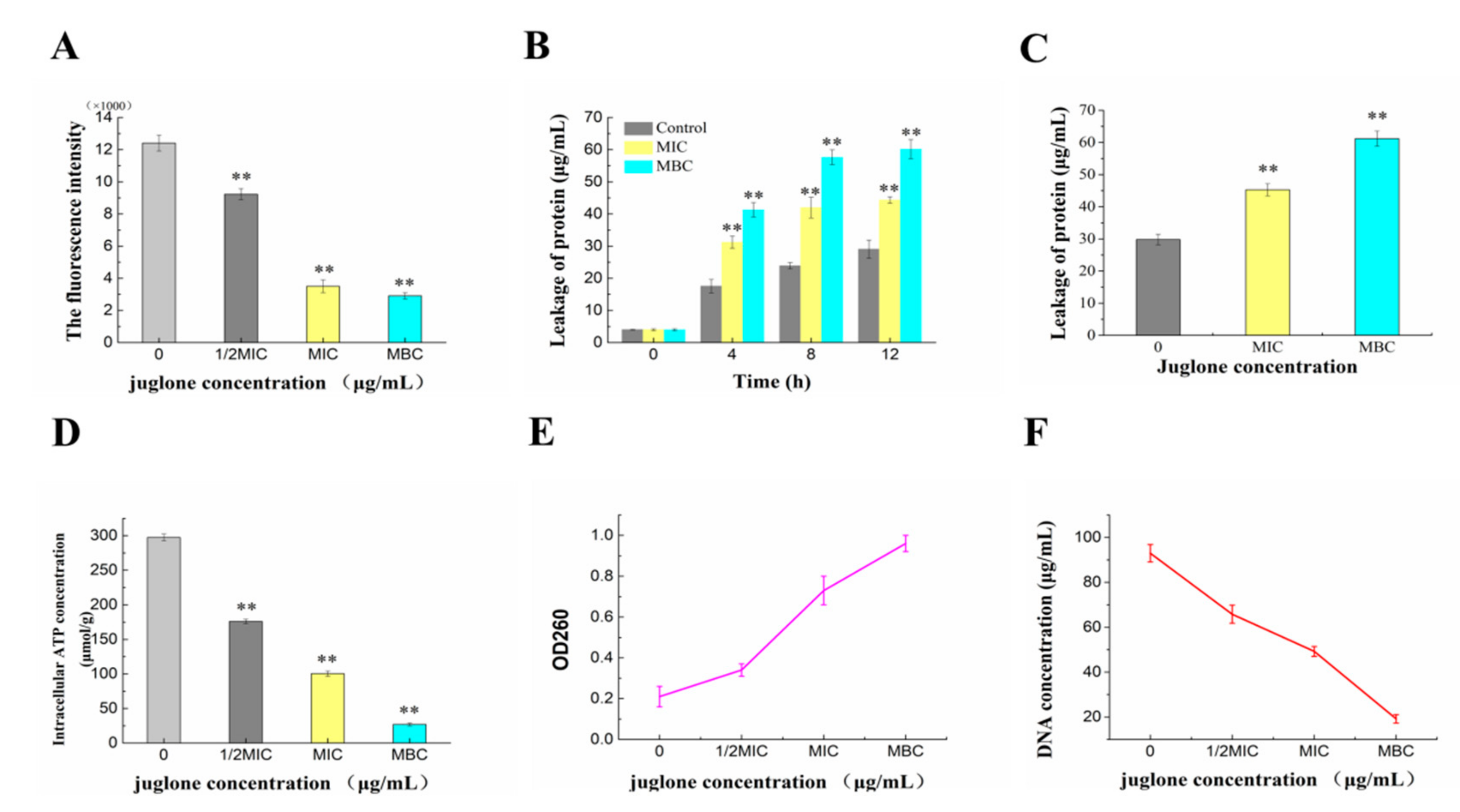
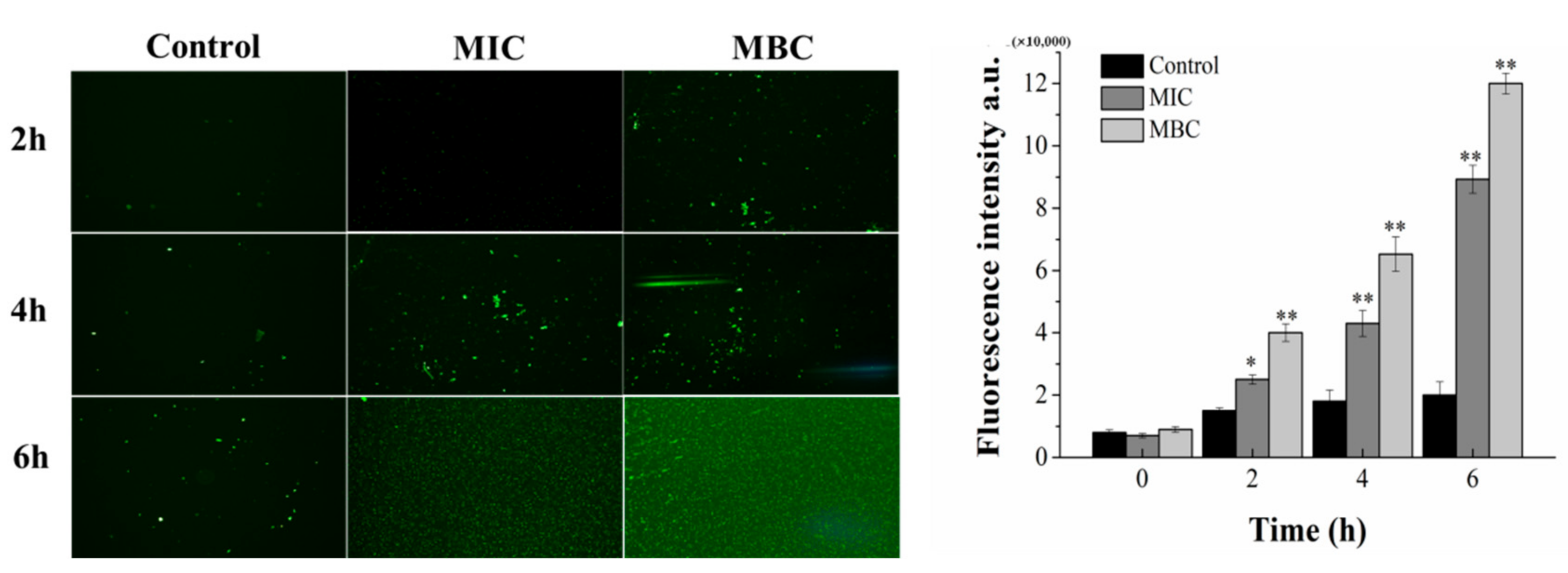
2.4.2. Effects of Juglone Induction on Intracellular Protein and Nucleic Acid Contents of P. syringae
2.4.3. Effect of Juginone Induction on Intracellular ATP Content of P. syringae
2.5. Intracellular Oxidative Stress Caused by Juglone
2.6. Effect of Juglone on DNA Structure
2.6.1. Fluorescence Spectra Analysis of DNA
2.6.2. CD Spectra Analysis
2.7. Effect of Juglone on P. syringae Biofilm Formation
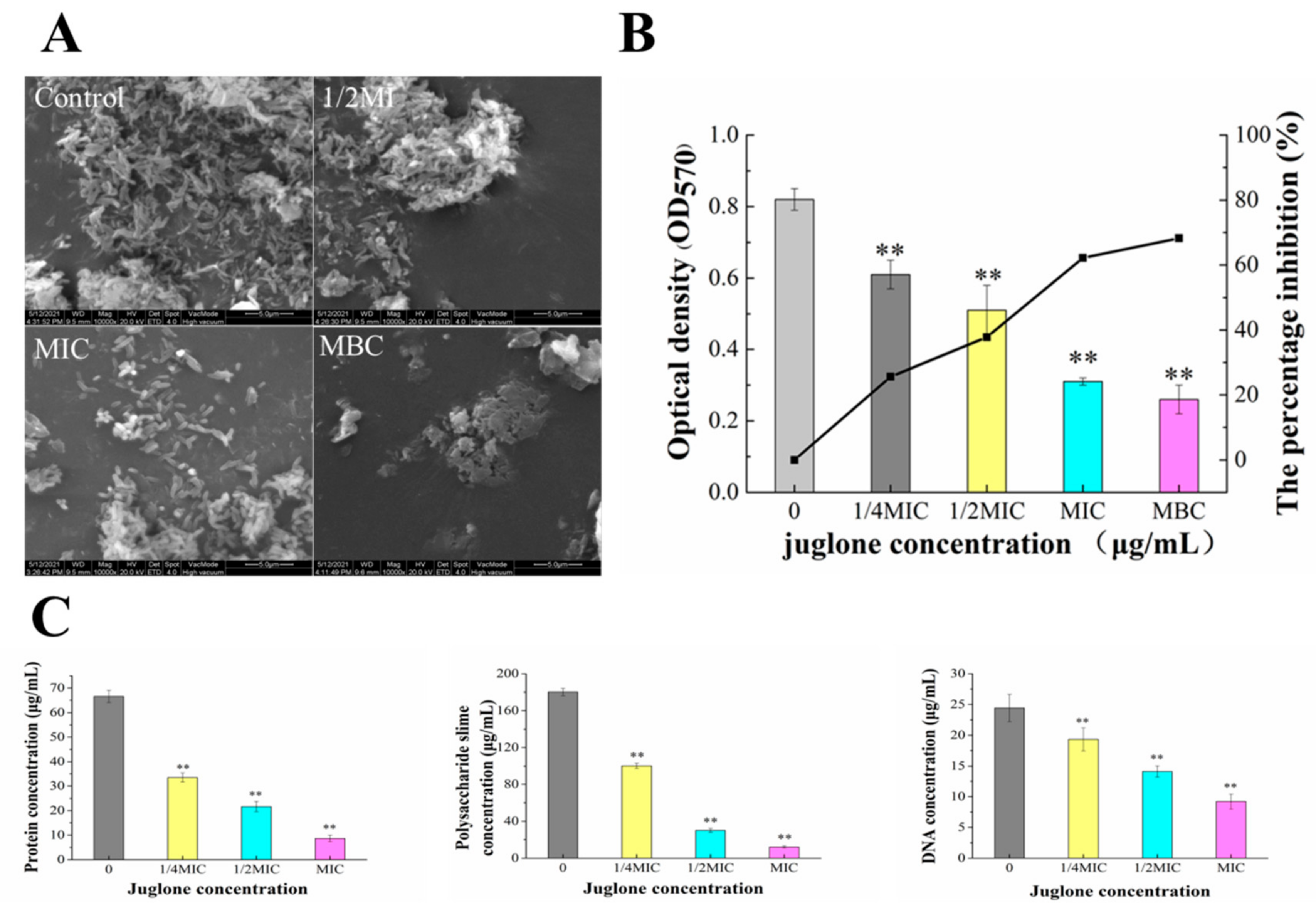
2.7.1. Determination of Fluorescence Intensity of Crystal Violet
2.7.2. Effect of Juglone Induction on Extracellular Polymer Content of P. syringae and ESEM Observation of Biofilm Formation
3. Discussion
4. Materails and Methods
4.1. Materials and Culture
4.2. Antibacterial Activity Assay of Juglone against P. syringae
4.2.1. Diameter of the Inhibitory Zone Assay
4.2.2. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration Assays
4.2.3. Growth Curve Assay
4.3. Antibacterial Mechanism of Juglone against P. syringae
4.3.1. Confocal Laser Scanning Microscope (CLSM) Assay
4.3.2. Field Emission Scanning Electron Microscope (FESEM) Observation and Analysis
4.3.3. Determination of Cell Membrane Potential (MP)
4.3.4. Determination of Intracellular and Extracellular Protein Content
4.3.5. Effects of Juglone on Intracellular and Extracellular DNA of P. syringae
4.3.6. Determination of Intracellular ATP Concentration
4.4. Reactive Oxygen Species (ROS) Assay
4.5. Antibacterial Mechanism on P. syringae Genomic DNA
4.5.1. Extraction of Genomic DNA of P. syringae and DNA Gel Electrophoresis
4.5.2. Circular Dichroic (CD) Spectrum Assay
4.5.3. Fluorescence Spectra Assay
4.6. Inhibition of Biofilm Formation by Juglone
4.6.1. Biofilm Formation and Quantitative Crystal Violet Assay
4.6.2. ESEM Assay
4.6.3. Determination of Main Extracellular Polymers Components
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassani, S. Chapter 3—A spotlight on the recent advances in bacterial plant diseases and their footprint on crop production. In Special Relativity: A Heuristic Approach; Elsevier: Amsterdam, The Netherlands, 2017; pp. 41–69. [Google Scholar]
- Pal, G.; Kumar, K.; Verma, A.; Verma, S.K. Application of bacterial biostimulants in promoting growth and disease prevention in crop plants. In Biostimulants for Crops from Seed Germination to Plant Development; Elsevier: Amsterdam, The Netherlands, 2021; pp. 393–410. [Google Scholar]
- Luti, S.; Campigli, S.; Ranaldi, F.; Paoli, P.; Pazzagli, L.; Marchi, G. Lscβ and lscγ, two novel levansucrases of Pseudomonas syringae pv. actinidiae biovar 3, the causal agent of bacterial canker of kiwifruit, show different enzymatic properties. Int. J. Biol. Macromol. 2021, 179, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Rangaswamy, V. Phosphorylation of CorS and CorR, regulatory proteins that modulate production of the phytotoxin coronatine in Pseudomonas syringae. FEMS Microbiol. Lett. 2000, 193, 13–18. [Google Scholar] [CrossRef]
- Montesinos, E.; Bardaji, E. Synthetic Antimicrobial Peptides as Agricultural Pesticides for Plant-Disease Control. Chem. Biodivers. 2008, 5, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; Sarojini, V. Pseudomonas syringaepv.actinidiae: Chemical control, resistance mechanisms and possible alternatives. Plant Pathol. 2013, 63, 1–11. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nat. Cell Biol. 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Griffin, K.; Gambley, C.; Brown, P.; Li, Y. Copper-tolerance in Pseudomonas syringae pv. tomato and Xanthomonas spp. and the control of diseases associated with these pathogens in tomato and pepper. A systematic literature review. Crop. Prot. 2017, 96, 144–150. [Google Scholar] [CrossRef]
- Vanneste, J.; Voyle, M. Genetic basis of copper resistance in New Zealand strains of Pseudomonas syringae. N. Z. Plant Prot. 2003, 56, 109–112. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.; Ferreres, F.; Bento, A.A.; Seabra, R.; Estevinho, M.L.M.F. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef]
- Wang, G.; Luo, Y.; Yang, J.; Hou, C.; Li, J. Inhibitory effects of polyphenols-enriched extracts from Debregeasia orientalis leaf against human cervical cancer in vitro & in vivo. Food Agric. Immunol. 2020, 31, 176–192. [Google Scholar] [CrossRef]
- Yu, H.; Zhong, Q.-Z.; Liu, T.-G.; Qiu, W.-Z.; Wu, B.-H.; Xu, Z.-K.; Wan, L.-S. Surface Deposition of Juglone/FeIII on Microporous Membranes for Oil/Water Separation and Dye Adsorption. Langmuir 2019, 35, 3643–3650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S.-Y. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Liu, T.; Kang, J.; Liu, L. Thymol as a critical component of Thymus vulgaris L. essential oil combats Pseudomonas aeruginosa by intercalating DNA and inactivating biofilm. LWT 2020, 136, 110354. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, X.; Fu, P. Spectroscopic studies on the interaction between carbaryl and calf thymus DNA with the use of ethidium bromide as a fluorescence probe. J. Photochem. Photobiol. B Biol. 2012, 108, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.-R.; Hu, Q.-P.; Zhang, H.; Xu, J.-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control. 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Xia, X.; Li, B.; Hung, Y.-C. Viability assay of E. coli O157: H7 treated with electrolyzed oxidizing water using flow cytometry. Food Control. 2018, 88, 47–53. [Google Scholar] [CrossRef]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Sarwar, T.; Rehman, S.U.; Husain, M.A.; Ishqi, H.; Tabish, M. Interaction of coumarin with calf thymus DNA: Deciphering the mode of binding by in vitro studies. Int. J. Biol. Macromol. 2015, 73, 9–16. [Google Scholar] [CrossRef]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 2000, 3, 3–8. [Google Scholar] [CrossRef]
- Ning, H.-Q.; Li, Y.-Q.; Tian, Q.-W.; Wang, Z.-S.; Mo, H.-Z. The apoptosis of Staphylococcus aureus induced by glycinin basic peptide through ROS oxidative stress response. LWT 2018, 99, 62–68. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Kang, S.C. Thymol disrupts the membrane integrity of Salmonella ser. typhimurium in vitro and recovers infected macrophages from oxidative stress in an ex vivo model. Res. Microbiol. 2014, 165, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and antibiofilm activities of peppermint essential oil against Staphylococcus aureus. LWT 2018, 101, 639–645. [Google Scholar] [CrossRef]
- Han, Q.; Yan, X.; Zhang, R.; Wang, G.; Zhang, Y. Juglone Inactivates Pseudomonas aeruginosa through Cell Membrane Damage, Biofilm Blockage, and Inhibition of Gene Expression. Molecules 2021, 26, 5854. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.-R.; Hu, Q.-P.; Feng, S.-S.; Li, W.-Q.; Xu, J.-G. Chemical Composition and Antibacterial Activity of the Essential Oil from Green Huajiao (Zanthoxylum schinifolium) against Selected Foodborne Pathogens. J. Agric. Food Chem. 2013, 61, 6044–6049. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Sun, Y.; Abdel-Samie, M.A.-S.; Lin, L. Antibacterial activity and mechanism of Chuzhou chrysanthemum essential oil. J. Funct. Foods 2018, 48, 159–166. [Google Scholar] [CrossRef]
- Kang, J.; Liu, L.; Liu, Y.; Wang, X. Ferulic Acid Inactivates Shigella flexneri through Cell Membrane Destructieon, Biofilm Retardation, and Altered Gene Expression. J. Agric. Food Chem. 2020, 68, 7121–7131. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control. 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Comas, J.; Vives-Rego, J. Assessment of the effects of gramicidin, formaldehyde, and surfactants on Esche-richia coli by flow cytometry using nucleic acid and membrane potential dyes. Cytom. Part A 2015, 29, 58–64. [Google Scholar] [CrossRef]
- Rhayour, K.; Bouchikhi, T.; Tantaoui-Elaraki, A.; Sendide, K.; Remmal, A. The Mechanism of Bactericidal Action of Oregano and Clove Essential Oils and of Their Phenolic Major Components on Escherichia coli and Bacillus subtilis. J. Essent. Oil Res. 2003, 15, 356–362. [Google Scholar] [CrossRef]
- Duan, F.; Xin, G.; Niu, H.; Huang, W. Chlorinated emodin as a natural antibacterial agent against drug-resistant bacteria through dual influence on bacterial cell membranes and DNA. Sci. Rep. 2017, 7, 12721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raza, H.; John, A.; Benedict, S. Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur. J. Pharmacol. 2011, 668, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Armenta, F.; Bernal-Mercado, A.; Rodriguez, M.R.T.; Gonzalez-Aguilar, G.; Lopez-Zavala, A.; Martínez-Téllez, M.A.; Oñate, M.A.H.; Ayala-Zavala, J. Quercetin reduces adhesion and inhibits biofilm development by Listeria monocytogenes by reducing the amount of extracellular proteins. Food Control. 2018, 90, 266–273. [Google Scholar] [CrossRef]

| Bacteria | DIZ(mm) | Concentration of Juglone (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | 60 | ||
| P. syringae | 22.3 ± 1.00 | ++ | ++ | + | + | + | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Q.; Feng, L.; Zhang, Y.; Zhang, R.; Wang, G.; Zhang, Y. Effect of Juglone against Pseudomonas syringae pv Actinidiae Planktonic Growth and Biofilm Formation. Molecules 2021, 26, 7580. https://doi.org/10.3390/molecules26247580
Han Q, Feng L, Zhang Y, Zhang R, Wang G, Zhang Y. Effect of Juglone against Pseudomonas syringae pv Actinidiae Planktonic Growth and Biofilm Formation. Molecules. 2021; 26(24):7580. https://doi.org/10.3390/molecules26247580
Chicago/Turabian StyleHan, Qiqi, Luoluo Feng, Yani Zhang, Runguang Zhang, Guoliang Wang, and Youlin Zhang. 2021. "Effect of Juglone against Pseudomonas syringae pv Actinidiae Planktonic Growth and Biofilm Formation" Molecules 26, no. 24: 7580. https://doi.org/10.3390/molecules26247580
APA StyleHan, Q., Feng, L., Zhang, Y., Zhang, R., Wang, G., & Zhang, Y. (2021). Effect of Juglone against Pseudomonas syringae pv Actinidiae Planktonic Growth and Biofilm Formation. Molecules, 26(24), 7580. https://doi.org/10.3390/molecules26247580






