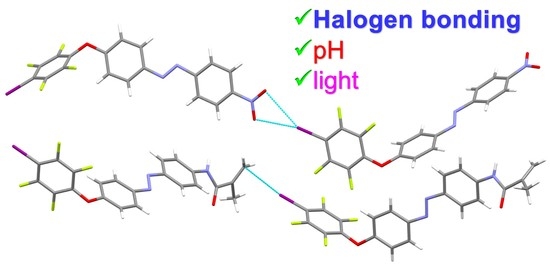
Stimuli Responsive Materials Supported by Orthogonal Hydrogen and Halogen Bonding or I···Alkene Interaction
Abstract
:1. Introduction
2. Results & Discussion
2.1. Synthesis of Halogen Bonded Azobenzenes
2.2. X-ray Structure Analysis of XB Azobenzenes
2.3. Halogen Bonding Properties
2.4. Photo-Responsive Properties
2.5. pH Sensitivity
3. Materials and Methods
3.1. Synthetic Procedures
3.2. X-ray Crystallography
3.3. 19F-NMR Titrations
3.4. UV–Vis Analysis of Azobenzenes
3.5. pH Sensitivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Chen, L.; Lim, K.H.; Gonuguntla, S.; Lim, K.W.; Pranantyo, D.; Yong, W.P.; Yam, W.J.T.; Low, Z.; Teo, W.J.; et al. The Pathway to Intelligence Using Stimuli-Responsive Materials as Building Blocks for Constructing Smart and Functional Systems. Adv. Mater. 2019, 31, 1804540. [Google Scholar] [CrossRef] [PubMed]
- McCune, J.A.; Mommer, S.; Parkins, C.C.; Scherman, O.A. Design Principles for Aqueous Interactive Materials: Lessons from Small Molecules and Stimuli-Responsive Systems. Adv. Mater. 2020, 32, 1906890. [Google Scholar] [CrossRef] [PubMed]
- Mrinalini, M.; Prasanthkumar, S. Recent Advances on Stimuli-Responsive Smart Materials and Their Applications. Chempluschem 2019, 84, 1103–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahim, M.A.; Jan, N.; Khan, S.; Shah, H.; Madni, A.; Khan, A.; Jabar, A.; Khan, S.; Elhissi, A.; Hussain, Z.; et al. Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers 2021, 13, 670. [Google Scholar] [CrossRef]
- Hu, L.; Shu, T.; Wan, Y.; Fang, C.; Gao, F.; Serpe, M.J. Recent Advances in Stimuli-Responsive Polymers for Sensing and Actuation. Mol. Syst. Des. Eng. 2021, 6, 108–121. [Google Scholar] [CrossRef]
- Gu, X.; Wang, J.; Liu, X.; Zhao, D.; Wang, Y.; Gao, H.; Wu, G. Temperature-Responsive Drug Delivery Systems Based on Polyaspartamides with Isopropylamine Pendant Groups. Soft Matter 2013, 9, 7267–7273. [Google Scholar] [CrossRef]
- Suedee, R.; Jantarat, C.; Lindner, W.; Viernstein, H.; Songkro, S.; Srichana, T. Development of a PH-Responsive Drug Delivery System for Enantioselective-Controlled Delivery of Racemic Drugs. J. Control. Release 2010, 142, 122–131. [Google Scholar] [CrossRef]
- Maggini, L.; Raquez, J.-M.; Marega, R.; Jensen Ahrens, J.; Pineux, F.; Meyer, F.; Dubois, P.; Bonifazi, D. Magnetic Poly(Vinylpyridine)-Coated Carbon Nanotubes: An Efficient Supramolecular Tool for Wastewater Purification. ChemSusChem 2013, 6, 367–373. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Zheng, Q.; Zhao, Q.; Zhang, H.; Ma, Y.; Fallon, J.K.; Fu, Q.; Haynes, M.T.; Lin, G.; et al. Disulfide Bond Bridge Insertion Turns Hydrophobic Anticancer Prodrugs into Self-Assembled Nanomedicines. Nano Lett. 2014, 14, 5577–5583. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.-N.; Li, J.-J.; Zhang, Q.; Luo, Z.-H. Light-Responsive Smart Surface with Controllable Wettability and Excellent Stability. Langmuir 2014, 30, 12236–12242. [Google Scholar] [CrossRef]
- Tao, Y.; Chan, H.F.; Shi, B.; Li, M.; Leong, K.W. Light: A Magical Tool for Controlled Drug Delivery. Adv. Funct. Mater. 2020, 30, 1–28. [Google Scholar] [CrossRef]
- Giles, L.W.; Faul, C.F.J.; Tabor, R.F. Azobenzene Isomerization in Condensed Matter: Lessons for the Design of Efficient Light-Responsive Soft-Matter Systems. Mater. Adv. 2021, 2, 4152–4164. [Google Scholar] [CrossRef]
- Merino, E. Synthesis of Azobenzenes: The Coloured Pieces of Molecular Materials. Chem. Soc. Rev. 2011, 40, 3835–3853. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in Different Classes of Azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Dong, M.; Babalhavaeji, A.; Samanta, S.; Beharry, A.A.; Woolley, G.A. Red-Shifting Azobenzene Photoswitches for in Vivo Use. Acc. Chem. Res. 2015, 48, 2662–2670. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen Bonding in Supramolecular Chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef]
- Bulfield, D.; Huber, S.M. Halogen Bonding in Organic Synthesis and Organocatalysis. Chem. A Eur. J. 2016, 22, 14434–14450. [Google Scholar] [CrossRef]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and Applications of Halogen Bonding in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef]
- Berger, G.; Frangville, P.; Meyer, F. Halogen Bonding for Molecular Recognition: New Developments in Materials and Biological Sciences. Chem. Commun. 2020, 56, 4970–4981. [Google Scholar] [CrossRef]
- Priimagi, A.; Cavallo, G.; Forni, A.; Gorynsztejn-Leben, M.; Kaivola, M.; Metrangolo, P.; Milani, R.; Shishido, A.; Pilati, T.; Resnati, G.; et al. Halogen Bonding versus Hydrogen Bonding in Driving Self-Assembly and Performance of Light-Responsive Supramolecular Polymers. Adv. Funct. Mater. 2012, 22, 2572–2579. [Google Scholar] [CrossRef] [Green Version]
- Saccone, M.; Dichiarante, V.; Forni, A.; Goulet-Hanssens, A.; Cavallo, G.; Vapaavuori, J.; Terraneo, G.; Barrett, C.J.; Resnati, G.; Metrangolo, P.; et al. Supramolecular Hierarchy among Halogen and Hydrogen Bond Donors in Light-Induced Surface Patterning. J. Mater. Chem. C 2015, 3, 759–768. [Google Scholar] [CrossRef] [Green Version]
- Bushuyev, O.S.; Corkery, T.C.; Barrett, C.J.; Friščić, T. Photo-Mechanical Azobenzene Cocrystals and in Situ X-Ray Diffraction Monitoring of Their Optically-Induced Crystal-to-Crystal Isomerisation. Chem. Sci. 2014, 5, 3158–3164. [Google Scholar] [CrossRef]
- Tong, X.; Qiu, Y.; Zhao, X.; Xiong, B.; Liao, R.; Peng, H.; Liao, Y.; Xie, X. Visible Light-Triggered Gel-to-Sol Transition in Halogen-Bond-Based Supramolecules. Soft Matter 2019, 15, 6411–6417. [Google Scholar] [CrossRef]
- Saccone, M.; Cavallo, G.; Metrangolo, P.; Resnati, G.; Priimagi, A. Halogen-Bonded Photoresponsive Materials. Top. Curr. Chem. 2015, 359, 147–166. [Google Scholar] [CrossRef]
- Berger, G.; Soubhye, J.; Meyer, F. Halogen Bonding in Polymer Science: From Crystal Engineering to Functional Supramolecular Polymers and Materials. Polym. Chem. 2015, 6, 3559–3580. [Google Scholar] [CrossRef]
- Chikh Alard, I.; Soubhye, J.; Berger, G.; Gelbcke, M.; Spassov, S.; Amighi, K.; Goole, J.; Meyer, F. Triple-Stimuli Responsive Polymers with Fine Tuneable Magnetic Responses. Polym. Chem. 2017, 8, 2450–2456. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, Y.; Jia, J.; Yu, W.; Qu, B.; Li, X.; Sun, X. H-Bonding and Charging Mediated Aggregation and Emission for Fluorescence Turn-on Detection of Hydrazine Hydrate. Chem. Commun. 2015, 51, 10656–10659. [Google Scholar] [CrossRef]
- Tothadi, S.; Desiraju, G.R. Designing Ternary Cocrystals with Hydrogen Bonds and Halogen Bonds. Chem. Commun. 2013, 49, 7791–7793. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van Der Waals Radii of Elements. Inorg. Chem. 2001, 37, 1031–1046. [Google Scholar] [CrossRef]
- Voth, A.R.; Khuu, P.; Oishi, K.; Ho, P.S. Halogen Bonds as Orthogonal Molecular Interactions to Hydrogen Bonds. Nat. Chem. 2009, 1, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Vasylyeva, V.; Nayak, S.K.; Terraneo, G.; Cavallo, G.; Metrangolo, P.; Resnati, G. Orthogonal Halogen and Hydrogen Bonds Involving a Peptide Bond Model. CrystEngComm 2014, 16, 8102–8105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Terwingen, S.; Brüx, D.; Wang, R.; Englert, U. Hydrogen-Bonded and Halogen-Bonded Orthogonal Interactions for the Chloride Anion of a Pyrazolium Salt. Molecules 2021, 26, 3982. [Google Scholar] [CrossRef] [PubMed]
- Thalladi, V.R.; Goud, B.S.; Hoy, V.J.; Allen, F.H.; Howard, J.A.K.; Desiraju, G.R. Supramolecular Synthons in Crystal Engineering. Structure Simplification, Synthon Robustness and Supramolecular Retrosynthesis. Chem. Commun. 1996, 401–402. [Google Scholar] [CrossRef]
- Lu, S.; Ng, S.V.H.; Lovato, K.; Ong, J.Y.; Poh, S.B.; Ng, X.Q.; Kürti, L.; Zhao, Y. Practical Access to Axially Chiral Sulfonamides and Biaryl Amino Phenols via Organocatalytic Atroposelective N-Alkylation. Nat. Commun. 2019, 10, 3061. [Google Scholar] [CrossRef] [Green Version]
- Shao, W.H.; Chen, B.Y.; Cheng, X.R.; Yuan, H.; Chen, H.; Chang, W.L.; Ye, J.; Lin, S.; Sun, Q.Y.; Zhang, W.D. Synthesis and Evaluation of New α-Methylene-γ-Lactone Carbamates with NO Production Inhibitory Effects in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Eur. J. Med. Chem. 2015, 93, 274–280. [Google Scholar] [CrossRef]
- Röse, P.; Emge, S.; Yoshida, J.I.; Hilt, G. Electrochemical Selenium- and Iodonium-Initiated Cyclisation of Hydroxy-Functionalised 1,4-Dienes. Beilstein J. Org. Chem. 2015, 11, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Tang, L.; Shen, C.; Li, H.; Wang, P.; Dong, K. Enantioselective Hydroesterificative Cyclization of 1,6-Enynes to Chiral γ-Lactams Bearing a Quaternary Carbon Stereocenter. Org. Lett. 2021, 23, 3561–3566. [Google Scholar] [CrossRef]
- Berger, G.; Soubhye, J.; van der Lee, A.; Vande Velde, C.; Wintjens, R.; Dubois, P.; Clément, S.; Meyer, F. Interplay between Halogen Bonding and Lone Pair-π Interactions: A Computational and Crystal Packing Study. Chempluschem 2014, 79, 552–558. [Google Scholar] [CrossRef]
- Berger, G.; Robeyns, K.; Soubhye, J.; Wintjens, R.; Meyer, F. Halogen Bonding in a Multi-Connected 1,2,2-Triiodo-Alkene Involving Geminal and/or Vicinal Iodines: A Crystallographic and DFT Study. CrystEngComm 2016, 18, 683–690. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Cariati, E.; Forni, A.; Biella, S.; Metrangolo, P.; Meyer, F.; Resnati, G.; Righetto, S.; Tordin, E.; Ugo, R. Tuning Second-Order NLO Responses through Halogen Bonding. Chem. Commun. 2007, 2590–2592. [Google Scholar] [CrossRef]
- Virkki, M.; Tuominen, O.; Forni, A.; Saccone, M.; Metrangolo, P.; Resnati, G.; Kauranen, M.; Priimagi, A. Halogen Bonding Enhances Nonlinear Optical Response in Poled Supramolecular Polymers. J. Mater. Chem. C 2015, 3, 3003–3006. [Google Scholar] [CrossRef] [Green Version]
- Baldrighi, M.; Metrangolo, P.; Meyer, F.; Pilati, T.; Proserpio, D.; Resnati, G.; Terraneo, G. Halogen-Bonded and Interpenetrated Networks through the Self-Assembly of Diiodoperfluoroarene and Tetrapyridyl Tectons. J. Fluor. Chem. 2010, 131, 1218–1224. [Google Scholar] [CrossRef]
- Kumar, V.; Pilati, T.; Terraneo, G.; Meyer, F.; Metrangolo, P.; Resnati, G. Halogen Bonded Borromean Networks by Design: Topology Invariance and Metric Tuning in a Library of Multi-Component Systems. Chem. Sci. 2017, 8, 1801–1810. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.Y.C.; Beer, P.D. Sigma-Hole Interactions in Anion Recognition. Chem 2018, 4, 731–783. [Google Scholar] [CrossRef]
- Machida, K.; Kim, B.-K.; Saito, Y.; Igarashi, K.; Uno, T. Resonance Raman Spectra of Acid-Base Indicators. I. p-Aminoazobenzene Derivatives. Bull. Chem. Soc. Jpn. 1974, 47, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Rigaku Oxford Diffraction. CrysAlisPro Software Systems; Rigaku Corporation: Oxford, UK, 2019. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT - Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]







| I–azo–NO2 | I-azo–NH–MMA | |
|---|---|---|
| Molecular formula | C18H8F4IN3O3 | C22H14F4IN3O2 |
| Formula weight (g·mol−1) | 517.17 | 555.26 |
| T (K) | 100(2) | 100(2) |
| Crystal system | triclinic | orthorhombic |
| Space group | P-1 (No. 2) | Pna21 (No. 33) |
| a (Å) | 5.65780(10) | 11.1393(2) |
| b (Å) | 9.9399(2) | 5.07170(10) |
| c (Å) | 32.4971(4) | 36.6524(7) |
| α (°) | 91.9240(10) | 90 |
| β (°) | 91.6640(10) | 90 |
| γ (°) | 105.8040(10) | 90 |
| V (Å3) | 1756.11(5) | 2070.68(7) |
| Z | 4 | 4 |
| ρcalc (g·cm−3) | 1.956 | 1.781 |
| μ (mm−1) | 14.975 | 12.712 |
| F (000) | 1000 | 1088 |
| 2θmax (°) | 147.59 | 147.34 |
| Measured reflections | 61712 | 7384 |
| Unique reflections | 6933 | 3466 |
| Observed reflections (I > 2σ(I)) | 6250 | 3128 |
| Parameters refined | 523 | 293 |
| R1 | 0.0250 | 0.0428 |
| wR2 | 0.0559 | 0.1109 |
| R1 (all data) | 0.0302 | 0.0485 |
| wR2 (all data) | 0.0584 | 0.1179 |
| Goodness-of-fit (GOF) | 1.030 | 1.054 |
| CCDC-entry | CCDC 2117642 | CCDC 2117643 |
| d (I···O), Å | d (I···O)/ΣvdWr, % | < (C–I···O), (H···O···I), ° | d (H···O), Å | d (H···F), Å |
|---|---|---|---|---|
| 3.348(2) (I1···O5) | ~4.5 | 149.13 (C–I1···O5) | 2.686 (H35···O6) | 2.571 (H29···F4) |
| 3.118(2) (I1···O6) | ~11 | 169.95 (C–I1···O6) | 2.444 (H15···O2) | 2.592 (H32···F1) |
| 3.308(2) (I2···O2) | ~5.5 | 154.63 (C–I2···O2) | 2.636 (H32···F2) | |
| 3.137(2) (I2···O3) | ~10.5 | 165.86 (C–I2···O3) | 2.656 (H17···F8) | |
| 100.54 (H15···O2···I2) | 2.574 (H18···F8) | |||
| 93.68 (H35···O6···I1) | 2.592 (H11···F7) | |||
| 2.570 (H14···F5) | ||||
| 2.452 (H9···F6) |
| Molar Ratio of Azo-Dye:TBACl | Volume of Azo-Dye Stock Solution (µL) | Volume of TBACl Stock Solution (µL) | Volume of CDCl3 (µL) | Total Volume (µL) |
|---|---|---|---|---|
| 1:0 | 200 | 0 | 500 | 700 |
| 1:1 | 200 | 50 | 450 | 700 |
| 1:2 | 200 | 100 | 400 | 700 |
| 1:5 | 200 | 250 | 250 | 700 |
| 1:10 | 200 | 500 | 0 | 700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frangville, P.; Kumar, S.; Gelbcke, M.; Van Hecke, K.; Meyer, F. Stimuli Responsive Materials Supported by Orthogonal Hydrogen and Halogen Bonding or I···Alkene Interaction. Molecules 2021, 26, 7586. https://doi.org/10.3390/molecules26247586
Frangville P, Kumar S, Gelbcke M, Van Hecke K, Meyer F. Stimuli Responsive Materials Supported by Orthogonal Hydrogen and Halogen Bonding or I···Alkene Interaction. Molecules. 2021; 26(24):7586. https://doi.org/10.3390/molecules26247586
Chicago/Turabian StyleFrangville, Pierre, Shiv Kumar, Michel Gelbcke, Kristof Van Hecke, and Franck Meyer. 2021. "Stimuli Responsive Materials Supported by Orthogonal Hydrogen and Halogen Bonding or I···Alkene Interaction" Molecules 26, no. 24: 7586. https://doi.org/10.3390/molecules26247586
APA StyleFrangville, P., Kumar, S., Gelbcke, M., Van Hecke, K., & Meyer, F. (2021). Stimuli Responsive Materials Supported by Orthogonal Hydrogen and Halogen Bonding or I···Alkene Interaction. Molecules, 26(24), 7586. https://doi.org/10.3390/molecules26247586