Sorption and Textural Properties of Activated Carbon Derived from Charred Beech Wood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Textural Results
3.2. Sorption Results
3.3. Structural Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Lian, K.; Zhu, Y.; Li, W.; Dai, S.; Chen, C. Direct Synthesis of Thermoplastic Polyolefin Elastomers from Nickel-Catalyzed Ethylene Polymerization. Macromolecules 2017, 50, 6074–6080. [Google Scholar] [CrossRef]
- Sharma, S.; Biswal, B.K.; Kumari, D.; Bindra, P.; Kumar, S.; Stobdan, T.; Shanmugam, V. Ecofriendly Fruit Switches: Graphene Oxide-Based Wrapper for Programmed Fruit Preservative Delivery to Extend Shelf Life. ACS Appl. Mater. Interfaces 2018, 10, 18478–18488. [Google Scholar] [CrossRef]
- Nu, P.T.T.; Kobayashi, T.; Phuong, T.N.T. Nylon-6–Mordenite Composite Membranes for Adsorption of Ethylene Gas Released from Chiquita Bananas. Ind. Eng. Chem. Res. 2020, 59, 8212–8222. [Google Scholar] [CrossRef]
- Bailén, G.; Guillén, F.; Castillo, S.; Serrano, M.; Valero, D.; Martínez-Romero, D. Use of Activated Carbon inside Modified Atmosphere Packages to Maintain Tomato Fruit Quality during Cold Storage. J. Agric. Food Chem. 2006, 54, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Farcuh, M.; Li, B.; Rivero, R.M.; Shlizerman, L.; Sadka, A.; Blumwald, E. Sugar metabolism reprogramming in a non-climacteric bud mutant of a climacteric plum fruit during development on the tree. J. Exp. Bot. 2017, 68, 5813–5828. [Google Scholar] [CrossRef] [Green Version]
- Bapat, V.A.; Trivedi, P.K.; Ghosh, A.; Sane, V.A.; Ganapathi, T.R.; Nath, P. Ripening of fleshy fruit: Molecular insight and the role of ethylene. Biotechnol. Adv. 2010, 28, 94–107. [Google Scholar] [CrossRef]
- Forlani, S.; Masiero, S.; Mizzotti, C. Fruit ripening: The role of hormones, cell wall modifications, and their relationship with pathogens. J. Exp. Bot. 2019, 70, 2993–3006. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, D.; Wang, B.; Khan, I.; Ni, Y. Ethylene Control Technologies in Extending Postharvest Shelf Life of Climacteric Fruit. J. Agric. Food Chem. 2017, 65, 7308–7319. [Google Scholar] [CrossRef]
- Keller, N.; Ducamp, M.-N.; Robert, D.; Keller, V. Ethylene Removal and Fresh Product Storage: A Challenge at the Frontiers of Chemistry. Toward an Approach by Photocatalytic Oxidation. Chem. Rev. 2013, 113, 5029–5070. [Google Scholar] [CrossRef] [PubMed]
- Zgrzebnicki, M.; Nair, V.; Mitra, S.; Kałamaga, A.; Przepiórski, J.; Wrobel, R.J. N-doped activated carbon derived from furfuryl alcohol—Development of porosity, properties, and adsorption of carbon dioxide and ethene. Chem. Eng. J. 2022, 427, 131709. [Google Scholar] [CrossRef]
- Lee, H.-M.; Baek, J.; An, K.-H.; Park, S.-J.; Park, Y.-K.; Kim, B.-J. Effects of Pore Structure on n-Butane Adsorption Characteristics of Polymer-Based Activated Carbon. Ind. Eng. Chem. Res. 2019, 58, 736–741. [Google Scholar] [CrossRef]
- Lee, B.-H.; Lee, H.-M.; Chung, D.; Kim, B.-J. Effect of Mesopore Development on Butane Working Capacity of Biomass-Derived Activated Carbon for Automobile Canister. Nanomaterials 2021, 11, 673. [Google Scholar] [CrossRef] [PubMed]
- Ratanpara, A.; Shaw, A.; Thomas, M.; Patel, R.N.; Kim, M. Microfluidic analysis of seawater-based CO2 capture in an amine solution with nickel nanoparticle catalysts. J. CO2 Util. 2021, 53, 101712. [Google Scholar] [CrossRef]
- NI, J.; Wang, R.; Lin, J.; Wei, K. Ruthenium Ammonium Chloride as a Precursor for the Preparation of High Activity Catalysts for Ammonia Synthesis. Chin. J. Catal. 2009, 30, 185–190. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, X.; Zhang, Q.; Ren, X.; Yu, W.; Cai, H. Catalytic pyrolysis of wood-plastic composite waste over activated carbon catalyst for aromatics production: Effect of preparation process of activated carbon. Energy 2020, 212, 118983. [Google Scholar] [CrossRef]
- Phiri, J.; Dou, J.; Vuorinen, T.; Gane, P.A.C.; Maloney, T.C. Highly Porous Willow Wood-Derived Activated Carbon for High-Performance Supercapacitor Electrodes. ACS Omega 2019, 4, 18108–18117. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Nam, H.; Nam, H. Preparation of activated carbon from peanut shell with KOH activation and its application for H2S adsorption in confined space. J. Environ. Chem. Eng. 2020, 8, 103683. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, C.; Padilla-Ortega, E.; Robledo-Cabrera, A.; López-Valdivieso, A. Cr(VI) adsorption on activated carbon: Mechanisms, modeling and limitations in water treatment. J. Environ. Chem. Eng. 2020, 8, 104031. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Sapag, K.; Rodriguez-Reinoso, F. Tailoring biomass-based activated carbon for CH4 storage by combining chemical activation with H3PO4 or ZnCl2 and physical activation with CO. Carbon 2016, 110, 138–147. [Google Scholar] [CrossRef]
- Zhang, T.; Walawender, W.P.; Fan, L.; Fan, M.; Daugaard, D.; Brown, R. Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J. 2004, 105, 53–59. [Google Scholar] [CrossRef]
- Sun, K.; Jiang, J.C. Preparation and characterization of activated carbon from rubber-seed shell by physical activation with steam. Biomass Bioenergy 2010, 34, 539–544. [Google Scholar] [CrossRef]
- Kubota, M.; Hata, A.; Matsuda, H. Preparation of activated carbon from phenolic resin by KOH chemical activation under microwave heating. Carbon 2009, 47, 2805–2811. [Google Scholar] [CrossRef]
- Zubizarreta, L.; Arenillas, A.; Pirard, J.-P.; Pis, J.J.; Job, N. Tailoring the textural properties of activated carbon xerogels by chemical activation with KOH. Microporous Mesoporous Mater. 2008, 115, 480–490. [Google Scholar] [CrossRef]
- Prahas, D.; Kartika, Y.; Indraswati, N.; Ismadji, S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization. Chem. Eng. J. 2008, 140, 32–42. [Google Scholar] [CrossRef]
- Njoku, V.; Hameed, B. Preparation and characterization of activated carbon from corncob by chemical activation with H3PO4 for 2,4-dichlorophenoxyacetic acid adsorption. Chem. Eng. J. 2011, 173, 391–399. [Google Scholar] [CrossRef]
- Akash, B.; O’Brien, W. The production of activated carbon from a bituminous coal. Int. J. Energy Res. 1996, 20, 913–922. [Google Scholar] [CrossRef]
- Sriramoju, S.K.; Dash, P.S.; Majumdar, S. Meso-porous activated carbon from lignite waste and its application in methylene Blue adsorption and coke plant effluent treatment. J. Environ. Chem. Eng. 2021, 9, 104784. [Google Scholar] [CrossRef]
- Han, T.; Lu, X.; Sun, Y.; Jiang, J.; Yang, W.; Jönsson, P.G. Magnetic bio-activated carbon production from lignin via a streamlined process and its use in phosphate removal from aqueous solutions. Sci. Total. Environ. 2020, 708, 135069. [Google Scholar] [CrossRef]
- Tsubouchi, N.; Nishio, M.; Shinohara, Y.; Bud, J.; Mochizuki, Y. Production of activated carbon from peat by with natural soda ash and effect of nitrogen addition on the development of surface area. Fuel Process. Technol. 2018, 176, 76–84. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Rodriguez-Reinoso, F. Chemical versus physical activation of coconut shell: A comparative study. Microporous Mesoporous Mater. 2012, 152, 163–171. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Kaur, P.; Park, J.-S. From coconut shell biomass to oxygen reduction reaction catalyst: Tuning porosity and nitrogen doping. Renew. Sustain. Energy Rev. 2021, 147, 111173. [Google Scholar] [CrossRef]
- Olivares-Marín, M.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V. Preparation of activated carbon from cherry stones by physical activation in air. Influence of the chemical carbonisation with H2SO4. J. Anal. Appl. Pyrolysis 2012, 94, 131–137. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Sajjadi, S.-A.; Meknati, A.; Lima, E.C.; Dotto, G.L.; Mendoza-Castillo, D.I.; Anastopoulos, I.; Alakhras, F.; Unuabonah, E.I.; Singh, P.; Hosseini-Bandegharaei, A. A novel route for preparation of chemically activated carbon from pistachio wood for highly efficient Pb(II) sorption. J. Environ. Manag. 2019, 236, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, M.A.F.; Uemura, Y.; Yusup, S.; Elhassan, F.; Uddin, A.; Hiwada, A.; Demiya, M. Activated Carbon from Rubber Wood Sawdust by Carbon Dioxide Activation. Procedia Eng. 2016, 148, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Gęsikiewicz-Puchalska, A.; Zgrzebnicki, M.; Michalkiewicz, B.; Narkiewicz, U.; Morawski, A.; Wrobel, R. Improvement of CO2 uptake of activated carbons by treatment with mineral acids. Chem. Eng. J. 2017, 309, 159–171. [Google Scholar] [CrossRef]
- Yusop, M.; Ahmad, M.; Rosli, N.; Manaf, M. Adsorption of cationic methylene blue dye using microwave-assisted carbon derived from acacia wood: Optimization and batch studies. Arab. J. Chem. 2021, 14, 103122. [Google Scholar] [CrossRef]
- Gómez-Serrano, V.; Cuerda-Correa, E.M.; Fernández-González, C.; Franco, M.A.; Garcia, A.M. Preparation of activated carbons from chestnut wood by phosphoric acid-chemical activation. Study of microporosity and fractal dimension. Mater. Lett. 2005, 59, 846–853. [Google Scholar] [CrossRef]
- Caudullo, G.; de Rigo, D.; Durrant, T. Fagus sylvatica in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Durrant, T., Mauri, A., Eds.; EU Publications: Luxemburg, 2016; pp. 94–95. [Google Scholar]
- Gessler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees 2006, 21, 1–11. [Google Scholar] [CrossRef]
- Nadleśnicze Nadleśnictwo Klińska. Zarządzenie Nr 3/2020 Nadleśniczego Nadleśnictwa Kliniska w Sprawie Wprowadzenia Cen Detalicznych na Drewno; Nadleśnictwo Kliniska: Pucko, Poland, 2020.
- Ashrafi, M.N.; Asrami, H.S.; Rudgar, Z.V.; Far, M.G.; Heidari, A.; Rastbod, E.; Jafarzadeh, H.; Salehi, M.; Bari, E.; Ribera, J. Comparison of Physical and Mechanical Properties of Beech and Walnut Wood from Iran and Georgian Beech. Forests 2021, 12, 801. [Google Scholar] [CrossRef]
- Doczekalska, B.; Bartkowiak, M.; Zakrzewski, R. Modification of sawdust from pine and beech wood with the succinic anhydride. Holz Roh. Werkst. 2007, 65, 187–191. [Google Scholar] [CrossRef]
- Luedtke, J.; Amen, C.; Van Ofen, A.; Lehringer, C. 1C-PUR-bonded hardwoods for engineered wood products: Influence of selected processing parameters. Holz Roh. Werkst. 2015, 73, 167–178. [Google Scholar] [CrossRef]
- Zeng, K.; Gauthier, D.; Minh, D.P.; Weiss-Hortala, E.; Nzihou, A.; Flamant, G. Characterization of solar fuels obtained from beech wood solar pyrolysis. Fuel 2017, 188, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Silva, H.S.; Ruiz, S.V.; Granados, D.L.; Santángelo, J.M. Adsorption of mercury (II) from liquid solutions using modified activated carbons. Mater. Res. 2010, 13, 129–134. [Google Scholar] [CrossRef]
- Danish, M.; Hashim, R.; Ibrahim, M.N.M.; Sulaiman, O. Effect of acidic activating agents on surface area and surface functional groups of activated carbons produced from Acacia mangium wood. J. Anal. Appl. Pyrolysis 2013, 104, 418–425. [Google Scholar] [CrossRef]
- Ould-Idriss, A.; Stitou, M.; Cuerda-Correa, E.; Fernández-González, C.; Macías-García, A.; Alexandre-Franco, M.; Gómez-Serrano, V. Preparation of activated carbons from olive-tree wood revisited. I. Chemical activation with H3PO4. Fuel Process. Technol. 2011, 92, 261–265. [Google Scholar] [CrossRef]
- Guo, S.; Peng, J.; Li, W.; Yang, K.; Zhang, L.; Zhang, S.; Xia, H. Effects of CO2 activation on porous structures of coconut shell-based activated carbons. Appl. Surf. Sci. 2009, 255, 8443–8449. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Ma, Z.; Li, H.; Dong, Y.; Yang, H.; Yang, L.; Bai, L.; Wei, D.; Wang, W. A lignin dissolution-precipitation strategy for porous biomass carbon materials derived from cherry stones with excellent capacitance. J. Alloy. Compd. 2020, 832, 155029. [Google Scholar] [CrossRef]
- Liu, D.D.; Jia, B.Y.; Li, S.; Dong, L.J.; Gao, J.H.; Qin, Y.K. Effect of pyrolysis conditions on the improvement of the physicochemical structure of activated carbon obtained from Jixi bituminous coal. Asia Pac. J. Chem. Eng. 2019, 14, e2289. [Google Scholar] [CrossRef]
- Bratek, W.; Świątkowski, A.; Pakuła, M.; Biniak, S.; Bystrzejewski, M.; Szmigielski, R. Characteristics of activated carbon prepared from waste PET by carbon dioxide activation. J. Anal. Appl. Pyrolysis 2013, 100, 192–198. [Google Scholar] [CrossRef]
- Parshetti, G.; Chowdhury, S.; Balasubramanian, R. Biomass derived low-cost microporous adsorbents for efficient CO2 capture. Fuel 2015, 148, 246–254. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Cui, Z.; Fu, Z.; Yang, L.; Liu, G.; Li, M. Coffee grounds derived N enriched microporous activated carbons: Efficient adsorbent for post-combustion CO2 capture and conversion. J. Colloid Interface Sci. 2020, 578, 491–499. [Google Scholar] [CrossRef]
- Kaliszewski, M.; Zgrzebnicki, M.; Kałamaga, A.; Pinjara, S.; Wróbel, R. Commercial Kevlar derived activated carbons for CO2 and C2H4 sorption. Pol. J. Chem. Technol. 2021, 23, 81–87. [Google Scholar] [CrossRef]
- Do, D.; Ahmadpour, A.; King, B. Comparison of Equilibra and Kinetics of High surface Area Activated Carbon Produced from Different Precursors and by Different Chemical Treatmets. Ind. Eng. Chem. Res. 1998, 37, 1329–1334. [Google Scholar]
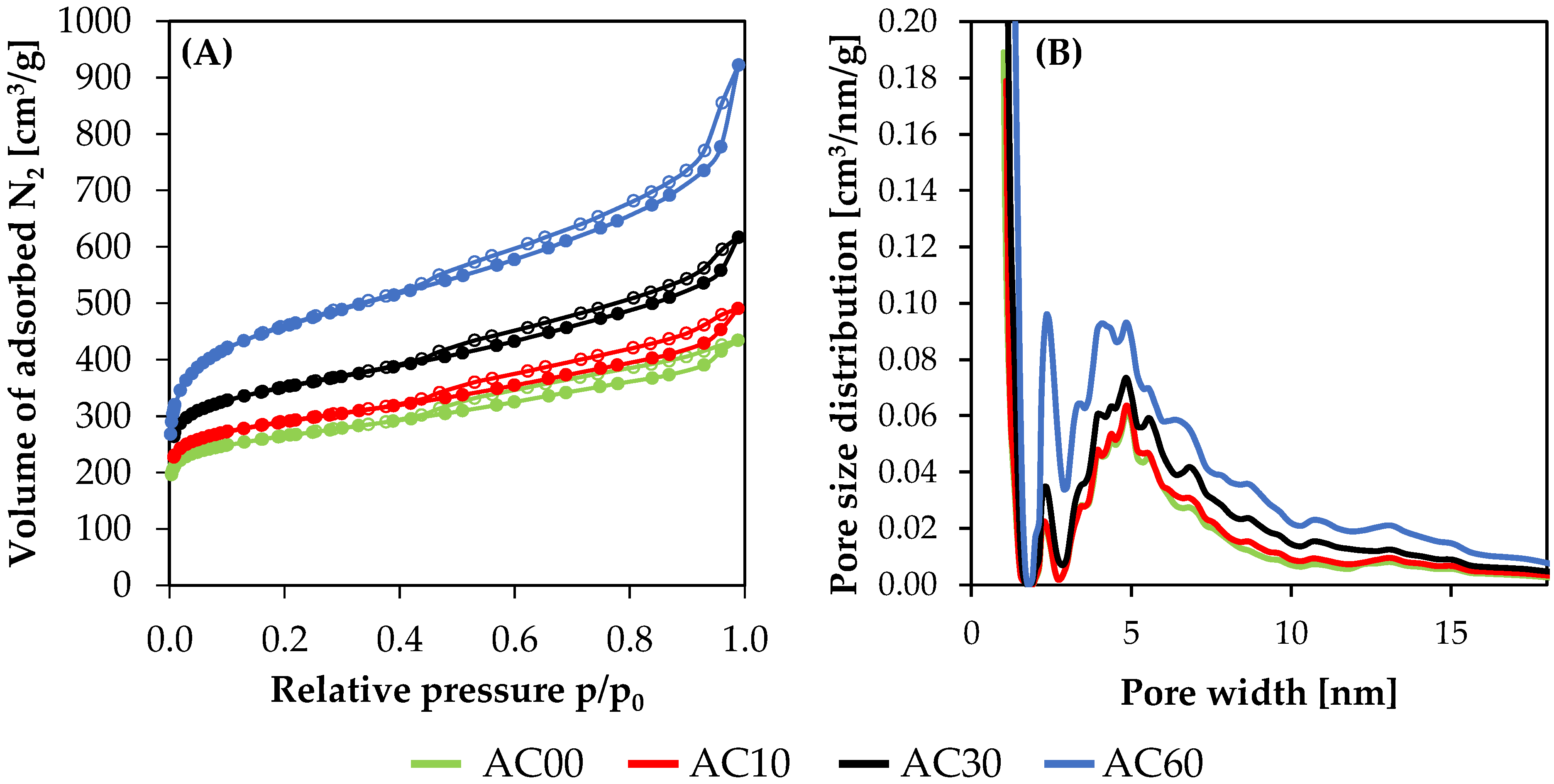
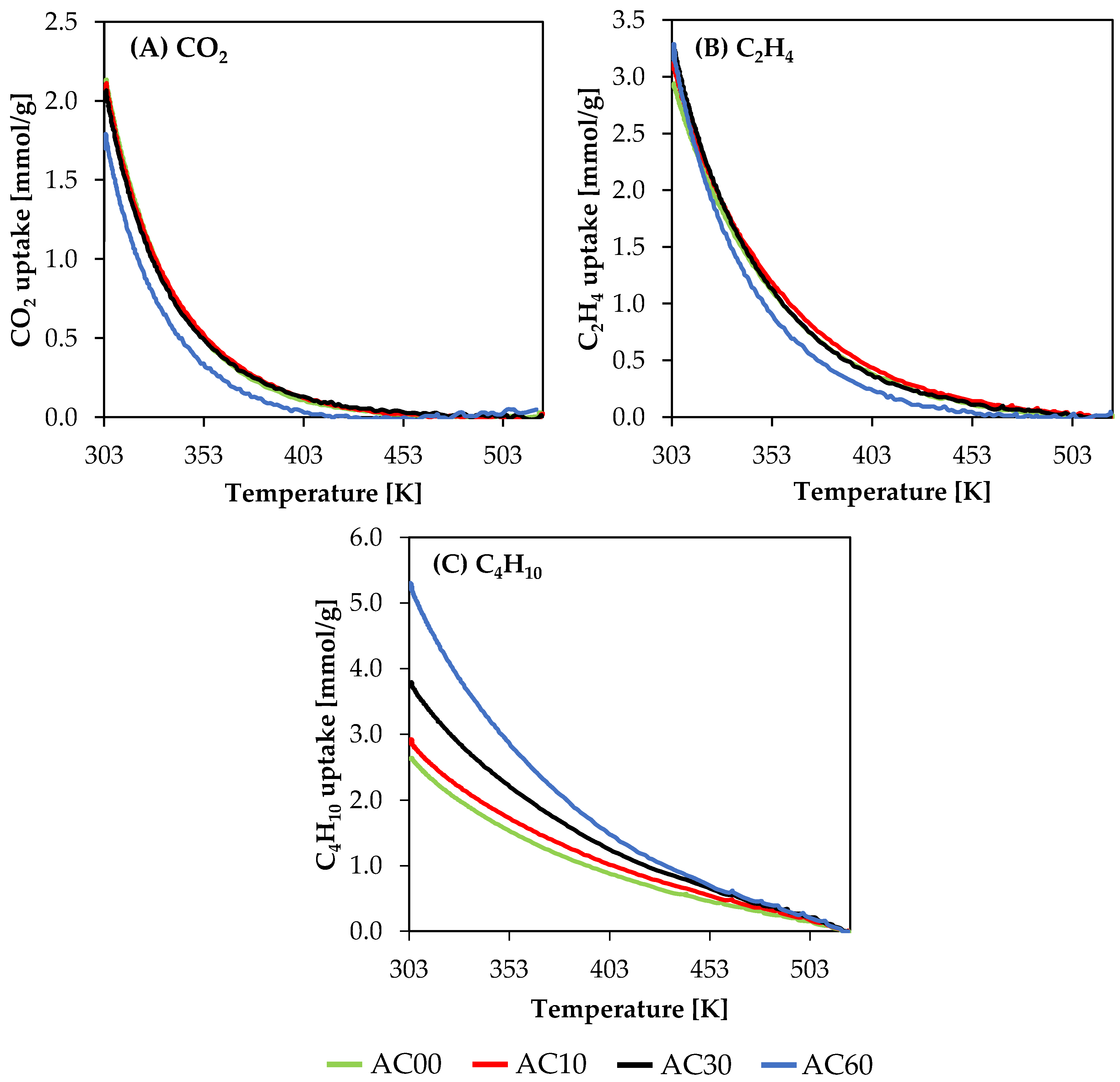
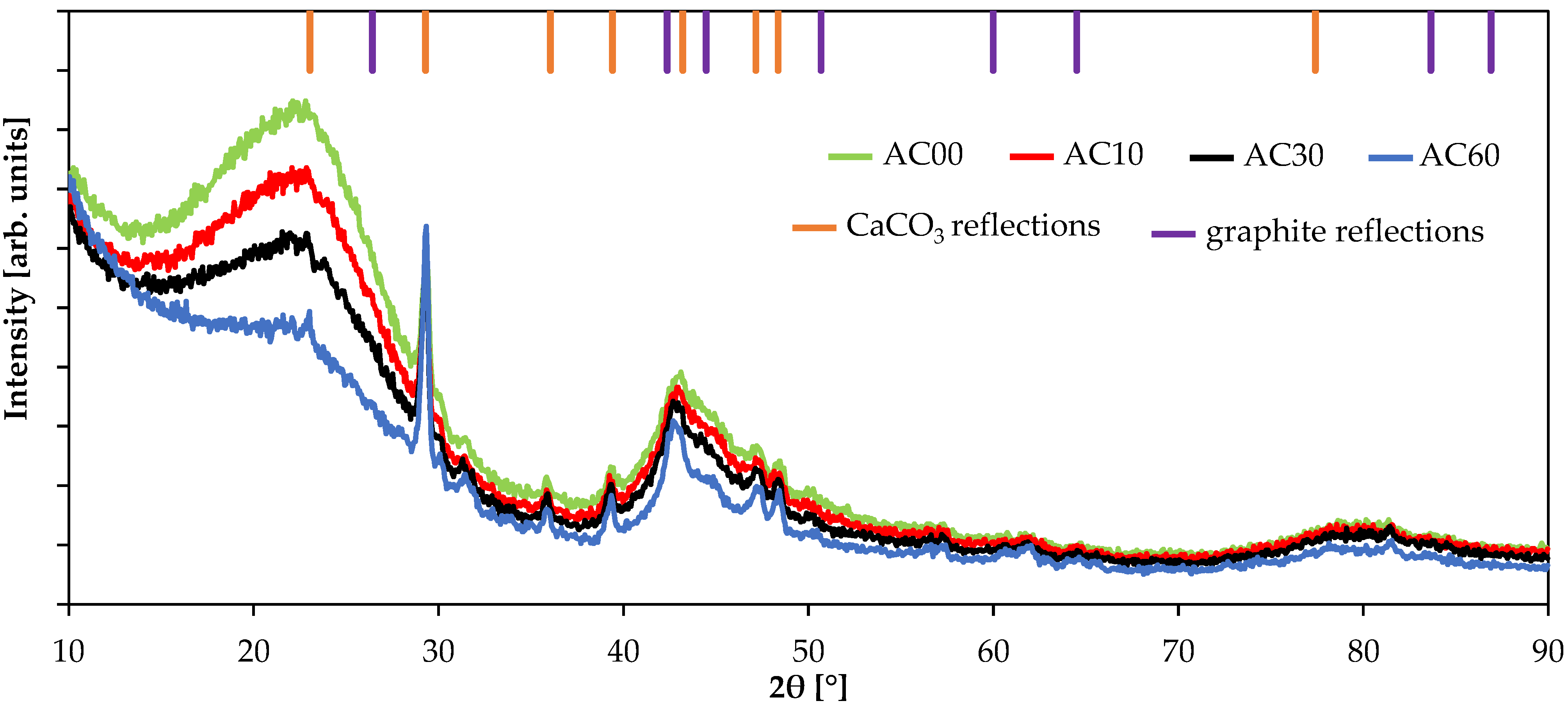
| Sample | Starting Material | SSA [m2/g] | Pore Volume [cm3/g] | Yield after Activation [%] | Ref. | ||
|---|---|---|---|---|---|---|---|
| Vtotal | Vmicro | Vmeso | |||||
| AC | Beech wood | 182 | 0.08 | 0.06 | 0.02 | - | This study |
| AC00 | Beech wood | 990 | 0.67 | 0.30 | 0.24 | 51 | |
| AC10 | Beech wood | 1087 | 0.76 | 0.33 | 0.26 | 45 | |
| AC30 | Beech wood | 1309 | 0.96 | 0.39 | 0.35 | 35 | |
| AC60 | Beech wood | 1695 | 1.43 | 0.46 | 0.54 | 20 | |
| AC | Eucalyptus wood | 701 | 0.51 | 0.26 | 0.25 | - | [48] |
| AC-H3PO4 | Acacia wood | 1039 | 0.55 | 0.34 | 0.18 | 46 | [49] |
| P1:3-500 | Chestnut wood | 783 | 0.29 | 0.28 | 0.01 | 37 | [39] |
| P55 | Olive-tree wood | 904 | 1.20 | 0.33 | 0.68 | 22 | [50] |
| 12 | Coconut shell | 1700 | 1.14 | 0.88 | - | 23 | [51] |
| CSC-SALT-800 | Cherry stones | 1200 | 0.63 | 0.45 | 0.12 | - | [52] |
| KJX-800-40-25.2 | Bituminous coal | 859 | 0.40 | 0.34 | - | 25 | [53] |
| Urea 1:3:2 | Peat | 1100 | 0.87 | 0.31 | 0.56 | 20 | [30] |
| 940-5 | PET | 1830 | - | 0.60 | 0.01 | 41 | [54] |
| Sample | Starting Material | Sorption Properties, 1 atm [mmol/g] | Ref. | |||
|---|---|---|---|---|---|---|
| CO2 | C2H4 | C4H10 | Temperature | |||
| AC | Beech wood | 1.3 | 1.4 | 0.8 | 303 K | This study |
| AC00 | Beech wood | 2.1 | 2.9 | 2.6 | 303 K | |
| AC10 | Beech wood | 2.1 | 3.1 | 2.9 | 303 K | |
| AC30 | Beech wood | 2.0 | 3.3 | 3.8 | 303 K | |
| AC60 | Beech wood | 1.7 | 3.2 | 5.2 | 303 K | |
| H250-800 | Palm fruit bunch | 3.7 | - | - | 298 K | [55] |
| CACs-2-800 | Coffee beans | 3.8 | - | - | 298 K | [56] |
| AC30 | Commercial kevlar | 1.7 | 3.1 | - | 303 K | [57] |
| AC-20 | PFA | 1.8 | 2.1 | - | 303 K | [11] |
| Coal:ZnCl2 | coal | - | - | 1.9 | 303 K | [58] |
| Sample | Stacking Height Lc [nm] | d(002) [nm] | N |
|---|---|---|---|
| AC00 | 1.07 | 0.393 | 2.72 |
| AC10 | 1.03 | 0.393 | 2.62 |
| AC30 | 1.08 | 0.392 | 2.76 |
| AC60 | 1.22 | 0.391 | 3.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zgrzebnicki, M.; Kałamaga, A.; Wrobel, R. Sorption and Textural Properties of Activated Carbon Derived from Charred Beech Wood. Molecules 2021, 26, 7604. https://doi.org/10.3390/molecules26247604
Zgrzebnicki M, Kałamaga A, Wrobel R. Sorption and Textural Properties of Activated Carbon Derived from Charred Beech Wood. Molecules. 2021; 26(24):7604. https://doi.org/10.3390/molecules26247604
Chicago/Turabian StyleZgrzebnicki, Michal, Agnieszka Kałamaga, and Rafal Wrobel. 2021. "Sorption and Textural Properties of Activated Carbon Derived from Charred Beech Wood" Molecules 26, no. 24: 7604. https://doi.org/10.3390/molecules26247604






