Heterogeneous Photocatalysis of Metronidazole in Aquatic Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Preparation of the Standard Solutions
2.3. Performance of the Photodegradation Experiments
2.4. Total Organic Carbon Determination
2.5. Instrumentation and Analytical Conditions
2.6. Method Validation
2.7. Determination of Metronidazole Degradation Products
3. Results and Discussion
3.1. Developing Chromatographic Conditions
3.2. Validation of the Analytical Method
3.3. Selection of Heterogenic Photocatalysis Conditions
3.3.1. Selection of the Photocatalyst
3.3.2. Influence of the Catalyst Amount on the Process of Catalysis
3.3.3. Effect of Irradiance Intensity on the Efficiency of Photocatalysis Process
3.3.4. TOC Studies for Post-Reaction Mixtures
3.4. Identification of Degradation Products in Post-Reaction Mixtures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Englande, A.J.; Krenkel, P.; Shamas, J. Wastewater Treatment & Water Reclamation. In Reference Module in Earth Systems and Environmental Sciences; Elsevier Inc.: Philadelphia, PA, USA, 2015; pp. 1–31. [Google Scholar]
- Radha, K.V.; Sirisha, K. Electrochemical Oxidation Processes. In Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology; Academic Press: Cambridge, MA, USA, 2018; pp. 359–373. [Google Scholar]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef] [PubMed]
- García-Galán, M.J.; Anfruns, A.; Gonzalez-Olmos, R.; Rodriguez-Mozaz, S.; Comas, J. Advanced oxidation of the antibiotic sulfapyridine by UV/H2O2: Characterization of its transformation products and ecotoxicological implications. Chemosphere 2016, 147, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Voigt, M.; Bartels, I.; Nickisch-Hartfiel, A.; Jaeger, M. Elimination of macrolides in water bodies using photochemical oxidation. AIMS Environ. Sci. 2018, 5, 372–388. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, K.; Shah, M.P. Advanced oxidation processes for complex wastewater treatment. In Advanced Oxidation Processes for Effluent Treatment Plants; Elsevier Inc.: Philadelphia, PA, USA, 2021; pp. 1–31. [Google Scholar]
- Wang, S.; Wang, J. Comparative study on sulfamethoxazole degradation by Fenton and Fe(II)-activated persulfate process. RSC Adv. 2017, 7, 48670–48677. [Google Scholar] [CrossRef] [Green Version]
- Psutka, J.M.; Dion-Fortier, A.; Dieckmann, T.; Campbell, J.L.; Segura, P.A.; Hopkins, W.S. Identifying Fenton-Reacted Trimethoprim Transformation Products Using Differential Mobility Spectrometry. Anal. Chem. 2018, 90, 5352–5357. [Google Scholar] [CrossRef]
- Pichat, P. Photocatalysis and Water Purification; Willey-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Pichat, P. Photocatalysis: Fundamentals Materials and Potential; MDPI: Basel, Switzerland, 2016. [Google Scholar] [CrossRef] [Green Version]
- Colmenares, J.C.; Xu, Y.J. Heterogeneous Photocatalysis: From Fundamentals to Green Application; Springer: Berlin, Germany, 2016. [Google Scholar]
- Schneider, J.; Bahnemann, D.; Ye, J.; Puma, G.L.; Dionysiou, D.D. Photocatalysis: Hundamentals and Perspectives; RSC: London, UK, 2016. [Google Scholar]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Augugliaro, V.; Bellardita, M.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 224–245. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Vaya, D. Photocatalytic activity of TiO2 nanomaterial. J. Chil. Chem. Soc. 2017, 62, 3683–3690. [Google Scholar] [CrossRef] [Green Version]
- Oros-Ruiz, S.; Zanella, R.; Prado, B. Photocatalytic degradation of trimethoprim by metallic nanoparticles supported on TiO2-P25. J. Hazard. Mater. 2013, 263, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Wang, Y.J.; Sun, R.J.; Zhou, D.M. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 2013, 92, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Nam, S.N.; Son, J.; Oh, J. Tungsten Trioxide (WO3)-assisted Photocatalytic Degradation of Amoxicillin by Simulated Solar Irradiation. Sci. Rep. 2019, 9, 9349. [Google Scholar] [CrossRef] [PubMed]
- Alalm, G.M.; Ookawara, S.; Fukushi, D.; Sato, A.; Tawfik, A. Improved WO3 photocatalytic efficiency using ZrO2 and Ru for the degradation of carbofuran and ampicillin. J. Hazard. Mater. 2016, 302, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.; Ibrahim, M.G.; Gar, M.; Fujii, M. MIL-53 (Al)/ZnO coated plates with high photocatalytic activity for extended degradation of trimethoprim via novel photocatalytic reactor. Sep. Purif. Technol. 2020, 249, 117173. [Google Scholar] [CrossRef]
- Boopathy, G.; Gangasalam, A.; Mahalingam, A. Photocatalytic removal of organic pollutants and self-cleaning performance of PES membrane incorporated sulfonated graphene oxide/ZnO nanocomposite. J. Chem. Technol. Biotechnol. 2020, 95, 3012–3023. [Google Scholar] [CrossRef]
- Sekar, K.; Chuaicham, C.; Balijapalli, U.; Li, W.; Wilson, K.; Lee, F.A.; Sasaki, K. Surfactant- and template-free hydrothermal assembly of Cu2O visible light photocatalysts for trimethoprim degradation. Appl. Catal. B Environ. 2021, 284, 119741. [Google Scholar] [CrossRef]
- Liu, S.H.; Lu, J.S. Facet-dependent cuprous oxide nanocrystals decorated with graphene as durable photocatalysts under visible light. Nanomaterials 2018, 8, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Gu, S.; Zhao, Y.; Zhou, G.; Li, W. BiVO4, Bi2WO6 and Bi2MoO6 photocatalysis: A brief review. J. Mater. Sci. Technol. 2020, 56, 45–68. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Gu, S.; Liu, X.; Li, H.; Ren, C.; Ma, X.; Zhou, H. Efficient ytterbium-doped Bi2WO6 photocatalysts. Synthesis, the formation of oxygen vacancies and boosted superoxide yield for enhanced visible-light photocatalytic activity. J. Alloy. Compd. 2021, 851, 156935. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Sufian, S. Hybrid 2D/3D g-C3N4/BiVO4 photocatalyst decorated with RGO for boosted photoelectrocatalytic hydrogen production from natural lake water and photocatalytic degradation of antibiotics. J. Mol. Liq. 2020, 314, 113530. [Google Scholar] [CrossRef]
- Nong, L.X.; Nguyen, V.H.; Bach, L.G.; Tran, T.V.; Nguyen, T.D. Photocatalytic activity for degradation of sulfamethoxazole by Bivo4 using visible light. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 042016. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Fu, Y.; Dionysiou, D.D. Degradation kinetics and mechanism of oxytetracycline by hydroxyl radical-based advanced oxidation processes. Chem. Eng. J. 2016, 284, 1317–1327. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Xu, Z.; Niu, J.; Hou, L.A. Electrochemical degradation of enrofloxacin by lead dioxide anode: Kinetics, mechanism and toxicity evaluation. Chem. Eng. J. 2017, 326, 911–920. [Google Scholar] [CrossRef]
- Lecours, M.-A.; Eysseric, E.; Yargeau, V.; Lessard, J.; Brisard, G.; Segura, P. Electrochemistry-High Resolution Mass Spectrometry to Study Oxidation Products of Trimethoprim. Environments 2018, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Pretali, L.; Fasani, E.; Albini, A. Photochemical degradation of marbofloxacin and enrofloxacin in natural waters. Environ. Sci. Technol. 2010, 44, 4564–4569. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.L.; Fu, C.C.; Juang, R.S. Removal of metronidazole and amoxicillin mixtures by UV/TiO2 photocatalysis: An insight into degradation pathways and performance improvement. Environ. Sci. Pollut. Res. 2019, 26, 11846–11855. [Google Scholar] [CrossRef]
- Berkani, M.; Smaali, A.; Kadmi, Y.; Almomani, F.; Vasseghian, Y.; Lakhdari, N.; Alyane, M. Photocatalytic degradation of Penicillin G in aqueous solutions: Kinetic, degradation pathway, and microbioassays assessment. J. Hazard. Mater. 2022, 421, 126719. [Google Scholar] [CrossRef]
- Balakrishna, K.; Rath, A.; Praveenkumarreddy, Y.; Guruge, K.S.; Subedi, B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol. Environ. Saf. 2017, 137, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Vosough, M.; Rashvand, M.; Esfahani, H.M.; Kargosha, K.; Salemi, A. Direct analysis of six antibiotics in wastewater samples using rapid high-performance liquid chromatography coupled with diode array detector: A chemometric study towards green analytical chemistry. Talanta 2015, 135, 7–17. [Google Scholar] [CrossRef]
- Velasco-Garduño, O.; González-Blanco, G.; Fajardo-Ortiz, M.d.C.; Beristain-Cardoso, R. Influence of metronidazole on activated sludge activity. Environ. Technol. 2020, 42, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Felis, E.; Sochacki, A.; Magiera, S. Degradation of benzotriazole and benzothiazole in treatment wetlands and by artificial sunlight. Water Res. 2016, 104, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kaushik, G.; Thotakura, N.; Raza, K.; Sharma, N.; Nimesh, S. Enhancement effects of process optimization technique while elucidating the degradation pathways of drugs present in pharmaceutical industry wastewater using Micrococcus yunnanensis. Chemosphere 2020, 238, 124689. [Google Scholar] [CrossRef]
- Tong, L.; Pérez, S.; Gonçalves, C.; Alpendurada, F.; Wang, Y.; Barceló, D. Kinetic and mechanistic studies of the photolysis of metronidazole in simulated aqueous environmental matrices using a mass spectrometric approach. Anal. Bioanal. Chem. 2011, 399, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.L.; Fu, C.C.; Juang, R.S. Effects of water matrix components on degradation efficiency and pathways of antibiotic metronidazole by UV/TiO2 photocatalysis. J. Mol. Liq. 2019, 276, 32–38. [Google Scholar] [CrossRef]
- Tran, M.L.; Nguyen, C.H.; Fu, C.C.; Juang, R.S. Hybridizing Ag-Doped ZnO nanoparticles with graphite as potential photocatalysts for enhanced removal of metronidazole antibiotic from water. J. Environ. Manag. 2019, 252, 109611. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. Multi-residue method for the determination of basic/neutral pharmaceuticals and illicit drugs in surface water by solid-phase extraction and ultra performance liquid chromatography-positive electrospray ionisation tandem mass spectrometry. J. Chromatogr. A 2007, 1161, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Bajkacz, S.; Felis, E.; Kycia-Słocka, E.; Harnisz, M.; Korzeniewska, E. Development of a new SLE-SPE-HPLC-MS/MS method for the determination of selected antibiotics and their transformation products in anthropogenically altered solid environmental matrices. Sci. Total Environ. 2020, 726, 138071. [Google Scholar] [CrossRef] [PubMed]
- Bodzek, M.; Rajca, M. Photocatalysis in the treatment and disinfection of water. Part I. Theoretical backgrounds. Ecol. Chem. Eng. S 2012, 19, 489–512. [Google Scholar] [CrossRef]
- Hamidi, F.; Aslani, F. TiO2-based photocatalytic cementitious composites: Materials, properties, influential parameters, and assessment techniques. Nanomaterials 2019, 9, 1444. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Du, Y.; Bai, Y.; An, J.; Cai, X.; Chen, Y.; Wang, P. Facile Formation of Anatase/Rutile TiO2 Nanocomposites with Enhanced Photocatalytic Activity. Molecules 2019, 24, 2996. [Google Scholar] [CrossRef] [Green Version]
- Kunz, L.Y.; Hong, J.; Riscoe, A.R.; Majumdar, A.; Cargnello, M. Reducing instability in dispersed powder photocatalysis derived from variable dispersion, metallic co-catalyst morphology, and light fluctuations. J. Photochem. Photobiol. 2020, 2, 100004. [Google Scholar] [CrossRef]
- Palominos, R.A.; Mondaca, M.A.; Giraldo, A.; Peñuela, G.; Pérez-Moya, M.; Mansilla, H.D. Photocatalytic oxidation of the antibiotic tetracycline on TiO2 and ZnO suspensions. Catal. Today 2009, 144, 100–105. [Google Scholar] [CrossRef]
- Ratanatawanate, C.; Tao, Y.; Balkus, K.J. Photocatalytic activity of PbS quantum dot/TiO2 nanotube composites. J. Phys. Chem. C 2009, 113, 10755–10760. [Google Scholar] [CrossRef]
- Nordin, R.; Latiff, N.; Yusof, R.; Nawawi, W.I.; Salihin, M.Z.; Ishak, Z.A.M. Effect of several commercial rubbers as substrates for zinc oxide in the photocatalytic degradation of methylene blue under visible irradiation. Express Polym. Lett. 2020, 14, 838–847. [Google Scholar] [CrossRef]
- Hart, J.N.; Cutini, M.; Allan, N.L. Band gap modification of ZnO and ZnS through solid solution formation for applications in photocatalysis. Energy Procedia 2014, 60, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Sambur, J.B.; Novet, T.; Parkinson, B.A. Multiple exciton collection in a sensitized photovoltaic system. Science 2010, 330, 63–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Wang, C.; Thompson, R.L.; Ohodnicki, P.; Baltrus, J.; Matranga, C. Size-dependent photocatalytic reduction of CO2 with PbS quantum dot sensitized TiO2 heterostructured photocatalysts. J. Mater. Chem. 2011, 21, 13452–13457. [Google Scholar] [CrossRef]
- Brahimi, R.; Bessekhouad, Y.; Bouguelia, A.; Trari, M. Improvement of eosin visible light degradation using PbS-sensititized TiO2. J. Photochem. Photobiol. A Chem. 2008, 194, 173–180. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, I.; Shah, T.; Park, S.Y. Synthesis, characterization and photocatalytic activity of silver nanoparticles/amidoxime-modified polyacrylonitrile nanofibers. Fibers Polym. 2015, 16, 1870–1875. [Google Scholar] [CrossRef]
- Beheshti, F.; Tehrani, R.M.A.; Khadir, A. Sulfamethoxazole removal by photocatalytic degradation utilizing TiO2 and WO3 nanoparticles as catalysts: Analysis of various operational parameters. Int. J. Environ. Sci. Technol. 2019, 16, 7987–7996. [Google Scholar] [CrossRef]
- Pellegrino, F.; Pellutiè, L.; Sordello, F.; Minero, C.; Ortel, E.; Hodoroaba, V.D.; Maurino, V. Influence of agglomeration and aggregation on the photocatalytic activity of TiO2 nanoparticles. Appl. Catal. B Environ. 2017, 216, 80–87. [Google Scholar] [CrossRef]
- Curti, M.; Bahnemann, D.W.; Mendive, C.B. Mechanisms in Heterogeneous Photocatalysis: Titania under UV and Visible Light Illumination; Elsevier Inc.: Philadelphia, PA, USA, 2016. [Google Scholar]
- Matuszko, D. Wpływ Zachmurzenia na Usłonecznienie i Całkowite Promieniowanie Słoneczne na Przykładzie Krakowskiej Serii Pomiarów; Wydawnictwo Uniwersytetu Jagiellońskiego: Kraków, Polska, 2009. [Google Scholar]
- Global Solar Atlas 2.0 The World Bank. Available online: https://solargis.com/maps-and-gis-data/download/poland (accessed on 12 December 2021).
- Enesca, A.; Isac, L. The influence of light irradiation on the photocatalytic degradation of organic pollutants. Materials 2020, 13, 2494. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Z.; Fang, F.; Yifeng, E.; Zhao, G. Novel visible-light-induced BiOCl/g-C3N4 photocatalyst for efficient degradation of metronidazole. Inorg. Chem. Commun. 2021, 132, 108820. [Google Scholar] [CrossRef]
- Salimi, M.; Esrafili, A.; Sobhi, H.R.; Behbahani, M.; Gholami, M.; Farzadkia, M.; Jafari, A.J.; Kalantary, R.R. Photocatalytic Degradation of Metronidazole Using D-g-C3N4-Bi5O7I Composites Under Visible Light Irradiation: Degradation Product, and Mechanisms. ChemistrySelect 2019, 4, 10288–10295. [Google Scholar] [CrossRef]
- Wilkins, B.J.; Moore, D.E. Common products from gamma-radiolysis a n d ultraviolet photolysis of metronidazole. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 2006, 36, 547–550. [Google Scholar]
- Ammar, H.B.; Brahim, M.B.; Abdelhédi, R.; Samet, Y. Enhanced degradation of metronidazole by sunlight via photo-Fenton process under gradual addition of hydrogen peroxide. J. Mol. Catal. A Chem. 2016, 420, 222–227. [Google Scholar] [CrossRef]
- Ammar, H.B.; Brahim, M.B.; Abdelhédi, R.; Samet, Y. Green electrochemical process for metronidazole degradation at BDD anode in aqueous solutions via direct and indirect oxidation. Sep. Purif. Technol. 2016, 157, 9–16. [Google Scholar] [CrossRef]
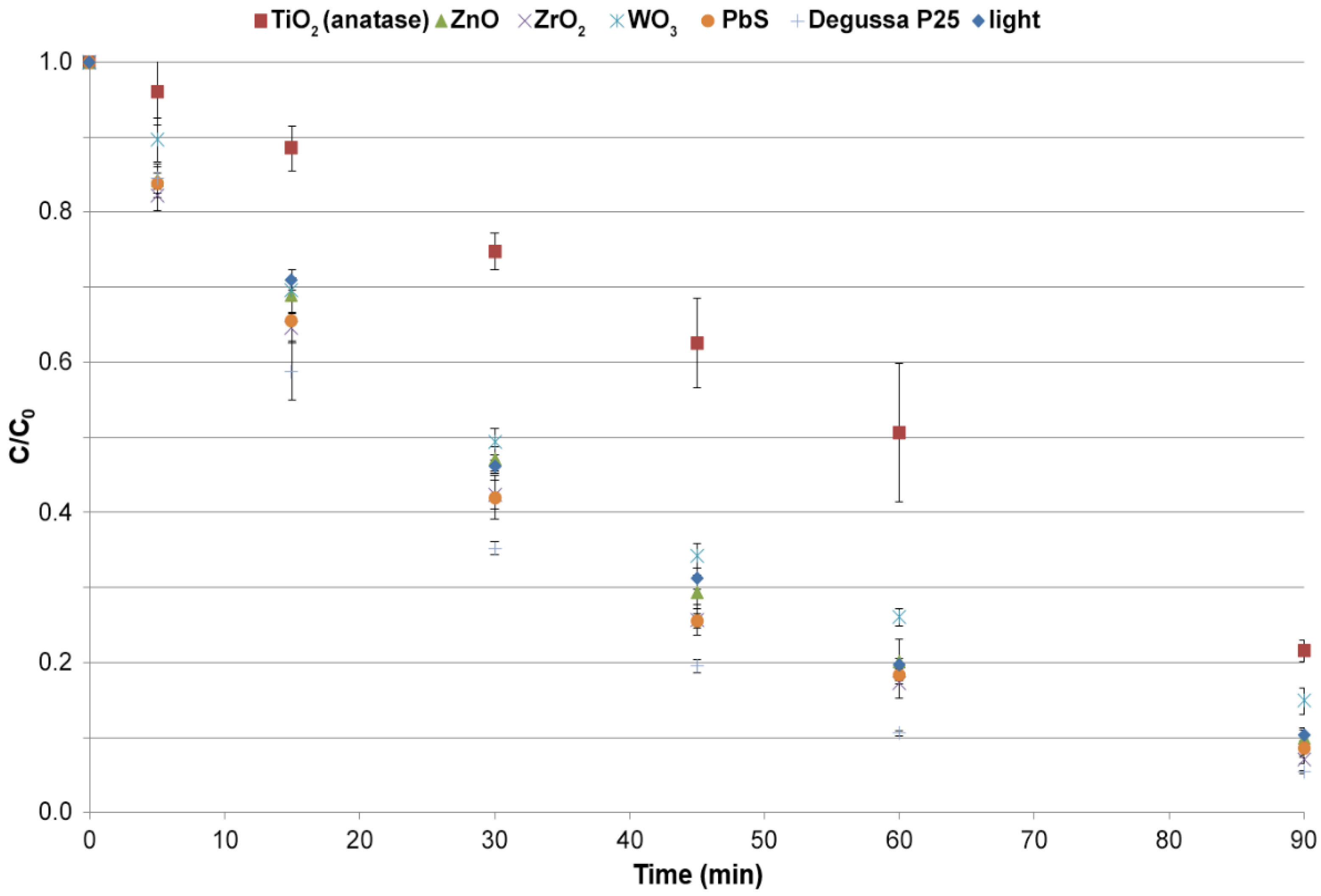
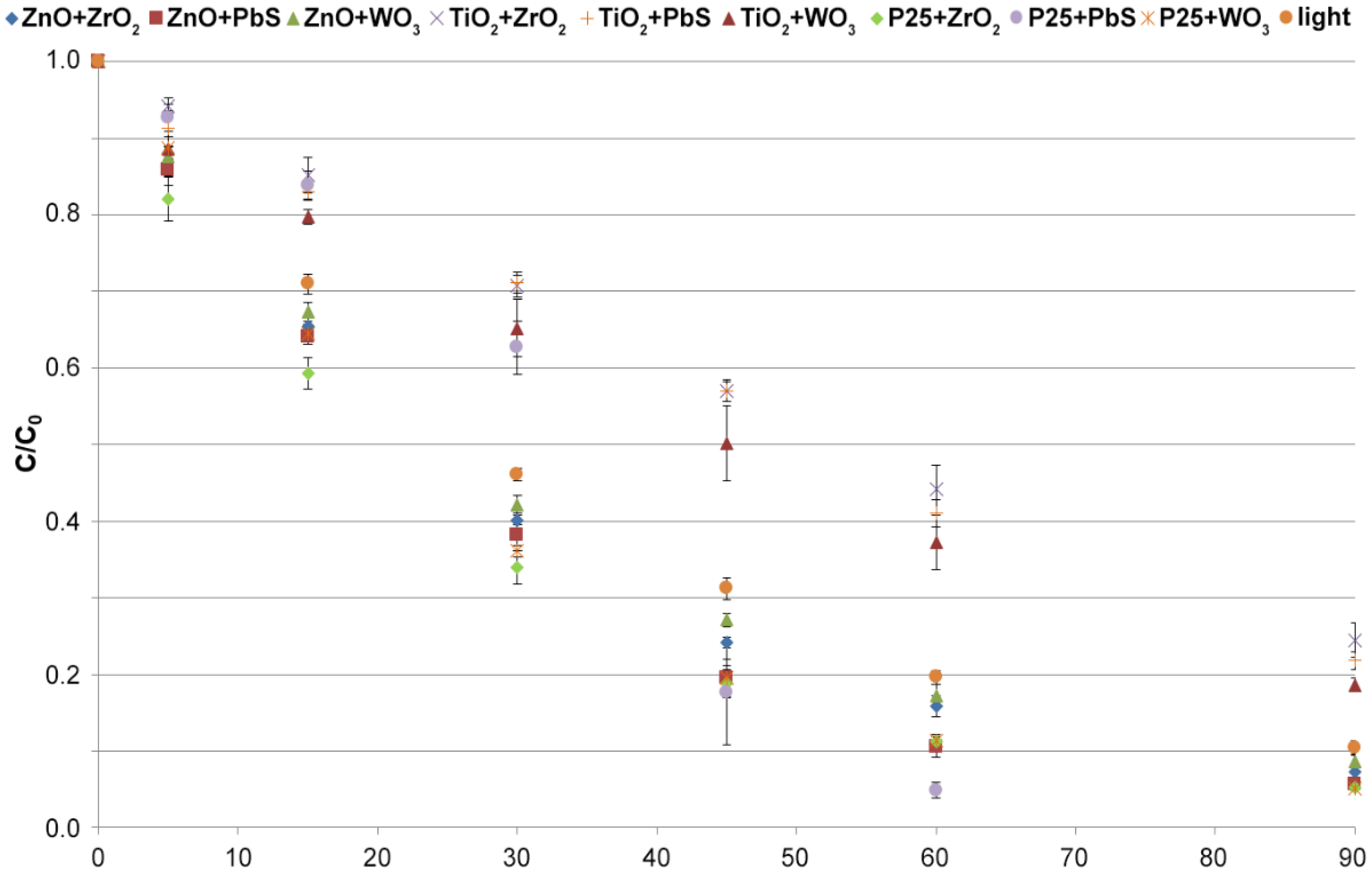
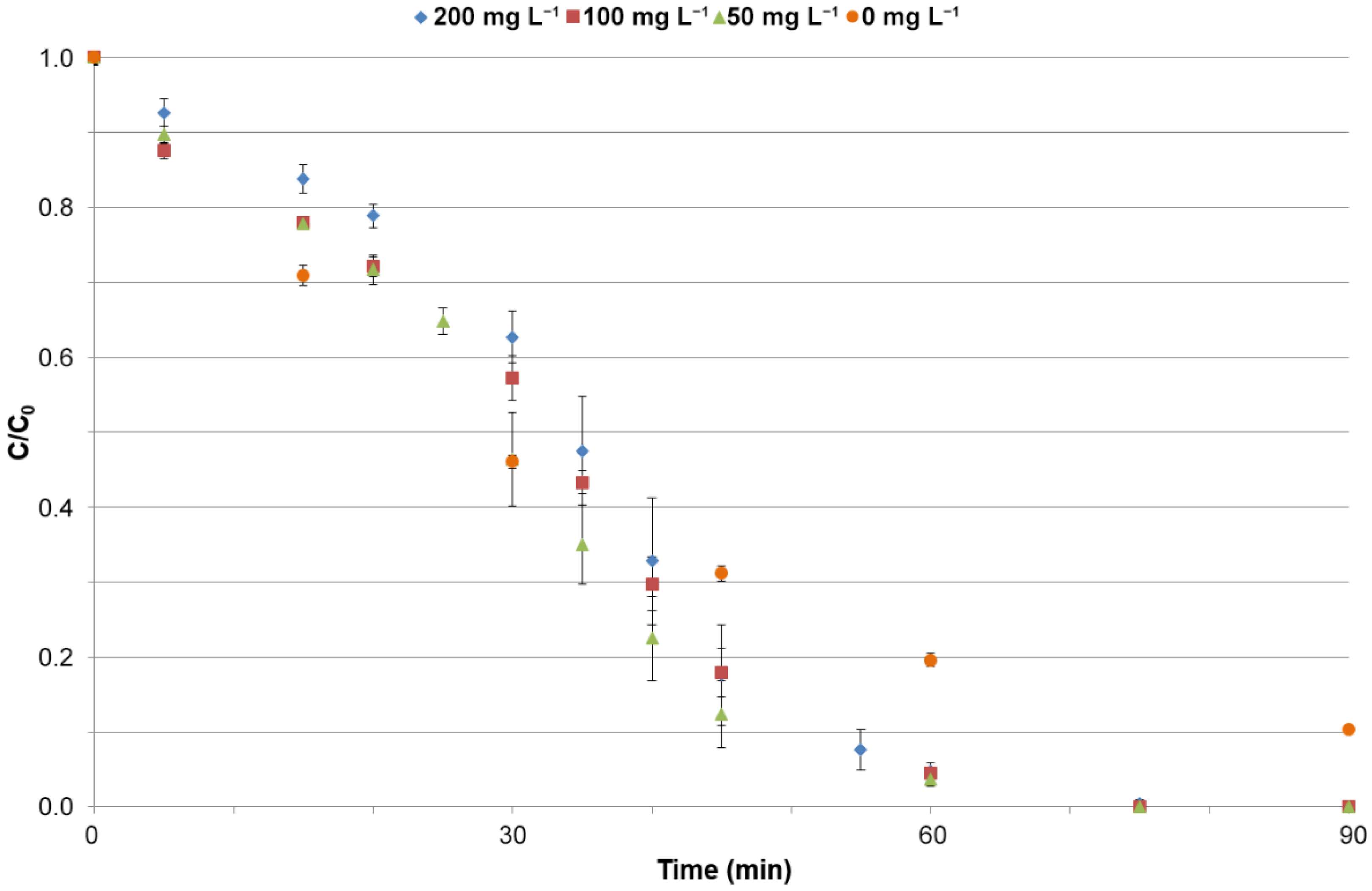

| Catalyst | k (1 min−1) | R2 |
|---|---|---|
| TiO2 + ZrO2 | 0.0154 | 0.985 |
| TiO2 | 0.0160 | 0.934 |
| TiO2 + PbS | 0.0164 | 0.976 |
| TiO2 + WO3 | 0.0181 | 0.985 |
| WO3 | 0.0215 | 0.997 |
| light | 0.0245 | 0.996 |
| ZnO | 0.0259 | 0.998 |
| PbS | 0.0275 | 0.997 |
| ZnO + WO3 | 0.0278 | 0.997 |
| ZrO2 | 0.0294 | 0.999 |
| ZnO + ZrO2 | 0.0298 | 0.998 |
| Degussa P25 | 0.0337 | 0.991 |
| P25 + ZrO2 | 0.0337 | 0.994 |
| ZnO + PbS | 0.0338 | 0.987 |
| P25 + WO3 | 0.0344 | 0.994 |
| P25 + PbS | k1 = 0.0114 (0–20 min) k2 = 0.0889 (30–90 min) | 0.991 (0–20 min) 0.993 (30–90 min) |
| Time (min) | Removal Efficiency (%) | |||
|---|---|---|---|---|
| 250 W m−2 | 500 W m−2 | 750 W m−2 | 1000 W m−2 | |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 |
| 5 | 6.75 | 10.2 | 11.6 | 14.3 |
| 15 | 15.6 | 22.1 | 30.4 | 52.1 |
| 20 | 17.6 | 28.3 | 43.3 | 71.5 |
| 30 | 28.4 | 53.6 | 69.5 | 95.0 |
| 35 | 30.6 | 65.0 | 82.7 | 100 |
| 40 | 35.1 | 77.5 | 91.3 | 100 |
| 45 | 41.3 | 87.7 | 94.1 | 100 |
| 60 | 61.6 | 96.1 | 100 | 100 |
| 75 | 75.2 | 100 | 100 | 100 |
| 90 | 86.3 | 100 | 100 | 100 |
| Sample | Time (min) | TOC (mg L−1) | SD (mg L−1) | Mineralization Degree (%) |
|---|---|---|---|---|
| without light and catalyst | 90 | 28.20 | 0.23 | 0.0 |
| light | 90 | 18.25 | 0.02 | 37.1 |
| TiO2 | 90 | 13.65 | 0.03 | 51.5 |
| ZnO | 90 | 11.63 | 0.04 | 58.7 |
| WO3 | 90 | 11.03 | 0.29 | 60.8 |
| PbS | 90 | 10.53 | 0.03 | 62.6 |
| ZrO2 | 90 | 10.79 | 0.03 | 61.7 |
| Degussa P25 | 90 | 10.70 | 0.10 | 62.0 |
| P25 + PbS | 35 | 22.48 | 0.06 | 20.2 |
| P25 + PbS | 45 | 17.23 | 0.02 | 38.8 |
| P25 + PbS | 60 | 14.78 | 0.13 | 47.5 |
| P25 + PbS | 90 | 8.35 | 0.14 | 70.3 |
| P25 + ZrO2 | 90 | 11.48 | 0.11 | 59.2 |
| P25 + WO3 | 90 | 11.71 | 0.02 | 58.4 |
| ZnO + WO3 | 90 | 18.02 | 1.04 | 36.0 |
| ZnO + ZrO2 | 90 | 14.91 | 0.14 | 47.0 |
| ZnO + PbS | 90 | 15.31 | 0.06 | 45.6 |
| TiO2 + WO3 | 90 | 13.17 | 0.03 | 53.2 |
| TiO2 + ZrO2 | 90 | 14.06 | 0.03 | 50.1 |
| TiO2 + PbS | 90 | 12.51 | 0.01 | 55.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stando, K.; Kasprzyk, P.; Felis, E.; Bajkacz, S. Heterogeneous Photocatalysis of Metronidazole in Aquatic Samples. Molecules 2021, 26, 7612. https://doi.org/10.3390/molecules26247612
Stando K, Kasprzyk P, Felis E, Bajkacz S. Heterogeneous Photocatalysis of Metronidazole in Aquatic Samples. Molecules. 2021; 26(24):7612. https://doi.org/10.3390/molecules26247612
Chicago/Turabian StyleStando, Klaudia, Patrycja Kasprzyk, Ewa Felis, and Sylwia Bajkacz. 2021. "Heterogeneous Photocatalysis of Metronidazole in Aquatic Samples" Molecules 26, no. 24: 7612. https://doi.org/10.3390/molecules26247612
APA StyleStando, K., Kasprzyk, P., Felis, E., & Bajkacz, S. (2021). Heterogeneous Photocatalysis of Metronidazole in Aquatic Samples. Molecules, 26(24), 7612. https://doi.org/10.3390/molecules26247612






