Spectroscopic Evidence for Photooxidation of Tocopherols in n-Hexane
Abstract
1. Introduction
2. Results and Discussion
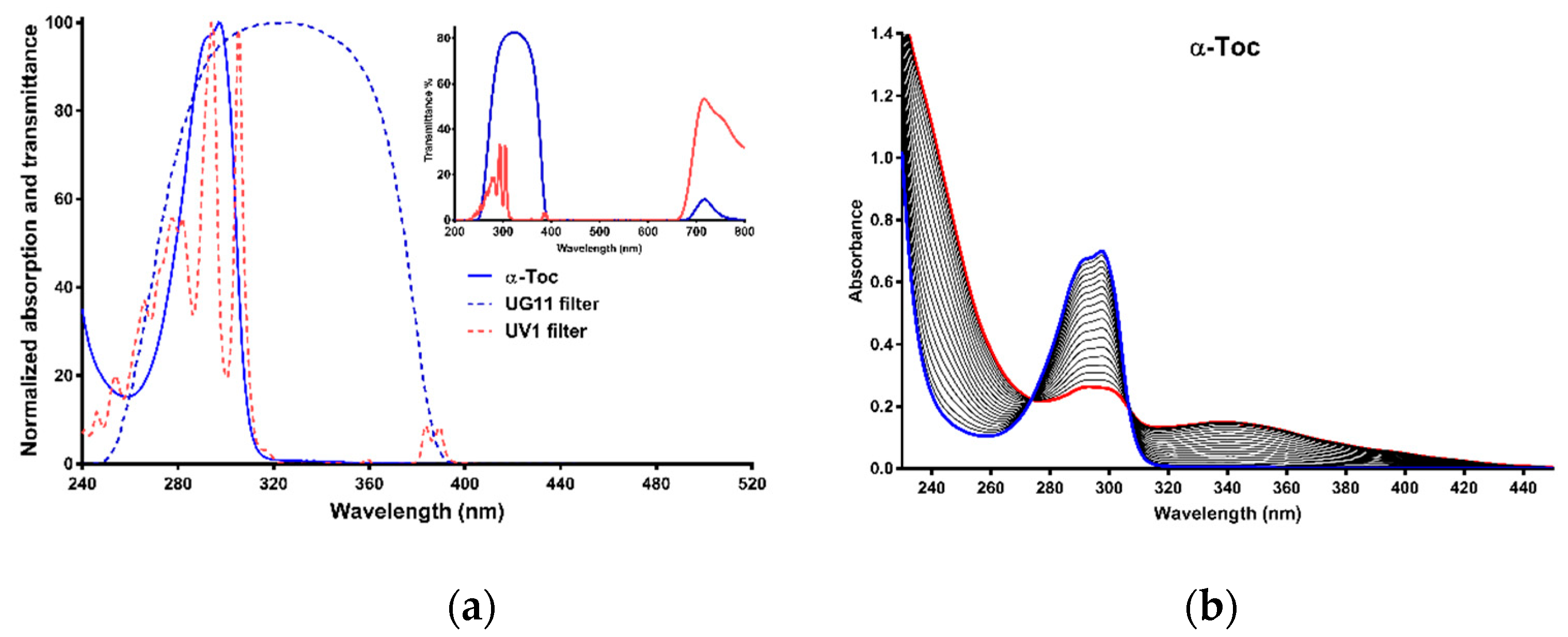
2.1. Absorption Spectra
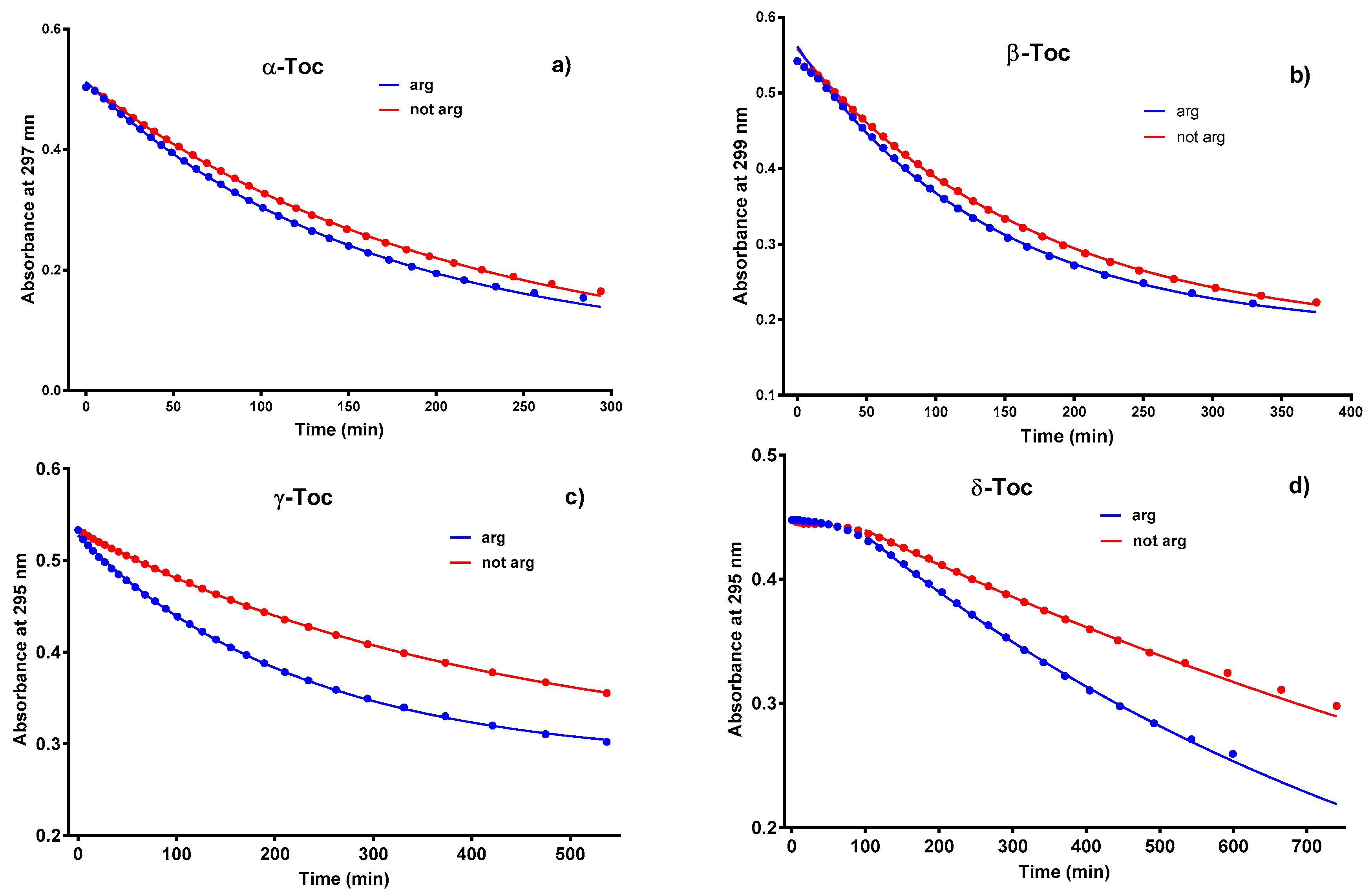
2.2. Rate Constants from Absorption Spectra
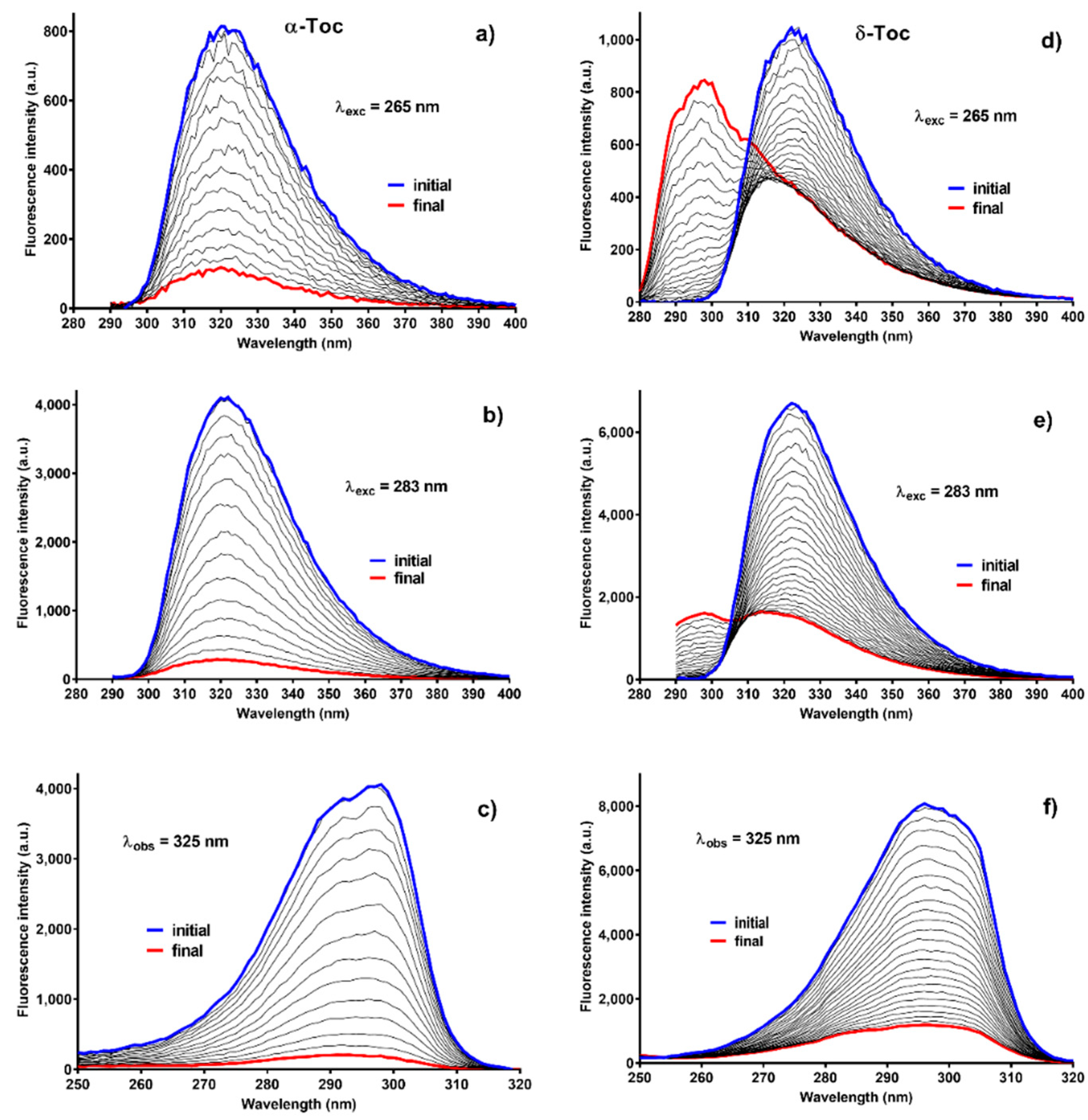
2.3. Fluorescence
2.4. Fluorescence of C-T Complexes
2.5. Fluorescence Lifetimes
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Deoxygenation
3.2.2. Samples Irradiation
3.2.3. Apparatus
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| C-T | Charge-transfer complexes |
| DAS | Decay-associated spectra |
| FP | “first product” |
| Toc | Tocopherols |
| TQ | Tocopherylquinone |
| TRES | Time resolved emission spectra |
References
- Burton, G.W.; Traber, M.G. Vitamin E: Antioxidant activity, biokinetics and bioavailability. Annu. Rev. Nutr. 1990, 10, 357–382. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Lipid Oxidation; The Oily Press Ltd.: Dundee, UK, 1998. [Google Scholar]
- Kramer, K.A.; Liebler, D.C. UVB Induced Photooxidation of Vitamin E. Chem. Res. Toxicol. 1997, 10, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.L.; Csallany, A.S. Separation of α-Tocopherol and Its Oxidation Products by High Performance Liquid Chromatography. Lipids 1988, 23, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.L.; Csallany, A.S. α-Tocopherol Oxidation Mediated by Superoxide Anion (O2-). I. Reactions in Aprotic and Protic Conditions. Lipids 1992, 27, 195–200. [Google Scholar] [CrossRef]
- Kramer-Stickland, K.A.; Krol, E.S.; Liebler, D.C. UV-B-Induced Photooxidation of Vitamin E in Mouse Skin. Chem. Res. Toxicol. 1999, 12, 187–191. [Google Scholar] [CrossRef]
- Krol, E.S.; Kramer-Stickland, K.A.; Liebler, D.C. Photoprotective Actions of Topically Applied Vitamin E. Drug Metab. Rev. 2000, 32, 413–420. [Google Scholar] [CrossRef]
- Krol, E.S.; Escalante, D.D.J.; Liebler, D.C. Mechanisms of Dimer and Trimer Formation from Ultraviolet-Irradiated α-Tocopherol. Lipids 2001, 36, 49–55. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.-A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Suarna, C.; Southwell-Keely, P.T. Effect of Alcohols on the Oxidation of the Vitamin E Model Compound, 2,2,5,7,8-Pentamethyl-6-chromanol. Lipids 1989, 24, 56–60. [Google Scholar] [CrossRef]
- Suarna, C.; Baca, M.; Southwell-Keely, P.T. Oxidation of the α-Tocopherol Model Compound 2,2,5,7,8-Pentamethyl-6-chromanol in the Presence of Alcohols. Lipids 1992, 27, 447–453. [Google Scholar] [CrossRef]
- Crisostomo, A.G.; Moreno, R.B.; Navaratnam, S.; Wilkinson, J.A.; Bisby, R.H. Generation of superoxide and singlet oxygen from α-tocopherolquinone and analogues. Free Radic. Res. 2007, 41, 730–737. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cho, E.; Min, D.B. Mechanism and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Inokami, Y.; Tokumura, A.; Terao, J.; Suzuki, A. Singlet oxygen scavenging by α-tocopherol and β-carotene: Kinetic studies in phospholipid membranes and ethanol solution. BioFactors 1998, 7, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Foote, C.S. Chemistry of oxygen. XXVI: Photooxygenation of phenols. Photochem. Photobiol. 1978, 27, 683–693. [Google Scholar] [CrossRef]
- Yamauchi, R.; Matsushita, S. Quenching effect of tocopherols on the methyl linoleate photooxidation and their oxidation products. Agric. Biol. Chem. Tokyo 1977, 41, 1425–1430. [Google Scholar]
- Fukuzawa, K.; Matsuura, K.; Tokumura, A.; Suzuki, A.; Terao, J. Kinetics and dynamics of singlet oxygen scavenging by α-tocopherol in phospholipid model membranes. Free Radic. Biol. Med. 1997, 22, 923–930. [Google Scholar] [CrossRef]
- Murkovic, M.; Wiltschko, D.; Pfannhauser, W. Formation of alpha-tocopherolquinone and alphatocopherolquinone epoxides in plant oil. Fett 1997, 99, 165–169. [Google Scholar] [CrossRef]
- Kruk, J.; Holländer-Czytko, H.; Oettmeier, W.; Trebst, A. Tocopherol as singlet oxygen scavenger in photosystem II. J. Plant Physiol. 2005, 162, 749–757. [Google Scholar] [CrossRef]
- Trebst, A. Function of β-carotene and tocopherol in photosystem II. Z. Naturforsch. 2003, 58, 609–620. [Google Scholar] [CrossRef]
- Smyk, B. Singlet oxygen autoxidation of vegetable oils: Evidences for lack of synergy between β-carotene and tocopherols. Food Chem. 2015, 182, 209–216. [Google Scholar] [CrossRef]
- Cornwell, D.G.; Williams, M.V.; Wani, A.A.; Wani, G.; Shen, E.; Jones, K.H. Mutagenicity of tocopheryl quinones: Evolutionary advantage of selective accumulation of dietary alpha-tocopherol. Nutr. Cancer 2002, 43, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, E. Recent advances in vitamin E metabolism and deficiency. Eur. J. Pediatrics 2006, 165, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.M.; MacKenzie, D.A.; Olguin, S.L.; Scariano, J.K.; Rabinowitz, I.; Thompson, T.A. Antioxidants Abrogate Alpha-Tocopherylquinone-Mediated Down-Regulation of the Androgen Receptor in Androgen-Responsive Prostate Cancer Cells. PLoS ONE 2016, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, R.; Thomas, B.; Wang, X.; Ma, J.; Jones, K.H.; Hatcher, P.G.; Cornwell, D.G. Tocopherol metabolism using thermochemolysis: Chemical and biological properties of gamma-tocopherol, gamma-carboxyethyl-hydroxychroman, and their quinones. Chem. Res. Toxicol. 2005, 18, 1018–1025. [Google Scholar] [CrossRef]
- Liebler, D.C.; Burr, J.A.; Philips, L.; Ham, A.J.L. Gas Chromatography–Mass Spectrometry Analysis of Vitamin E and Its Oxidation Products. Anal. Biochem. 1996, 236, 27–34. [Google Scholar] [CrossRef]
- Szymańska, R.; Kruk, J. γ-Tocopherol dominates in young leaves of runner bean (Phaseolus coccineus) under a variety of growing conditions: The possible functions of γ-tocopherol. Phytochemistry 2008, 69, 2142–2148. [Google Scholar] [CrossRef]
- Clough, R.L.; Yee, B.G.; Foote, C.S. Chemistry of Singlet Oxygen. 30. The Unstable Primary Product of Tocopherol Photooxidation. J. Am. Chem. Soc. 1979, 101, 683–686. [Google Scholar] [CrossRef]
- Grams, G.W.; Eskins, K. Dye-Sensitized Photooxidation of Tocopherols. Correlation between Singlet Oxygen Reactivity and Vitamin E Activity. Biochemistry 1972, 11, 606–608. [Google Scholar] [CrossRef]
- Kobayashi, N.; DellaPenna, D. Tocopherol metabolism, oxidation and recycling under high light stress in Arabidopsis. Plant J. 2008, 55, 607–618. [Google Scholar] [CrossRef]
- Cooney, R.V.; Franke, A.A.; Harwood, P.J.; Hatch-Pigott, V.; Custer, L.J.; Mordan, L.J. γ-Tocopherol detoxification of nitrogen dioxide: Superiority to α-tocopherol. Proc. Natl. Acad. Sci. USA 1993, 90, 1771–1775. [Google Scholar] [CrossRef]
- Kruk, J.; Strzałka, K. Charge-transfer complexes of plastoquinone and α-tocopherol quinone in unsaturated fatty acids and temperature dependences of their absorption spectra. Chem. Phys. Lipids 1991, 58, 27–33. [Google Scholar] [CrossRef]
- Foster, R. Organic Charge-Transfer Complexes; Academic Press: London, UK; New York, NY, USA, 1969. [Google Scholar]
- Kruk, J. Charge-transfer complexes of plastoquinone and a-tocopherol quinone in vitro. Biophysi. Chem. 1988, 30, 143–149. [Google Scholar] [CrossRef]
- Jadżyn, J.; Żywucki, B.; Prałat, K. Self-Association of α-Tocopherol in non-polar media. Acta Phys. Pol. 1985, 68, 833–840. [Google Scholar]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2116#section=3D-Conformer (accessed on 16 September 2020).
- Neunert, G.; Szwengiel, A.; Walejkoc, P.; Witkowski, S.; Polewski, K. Photostability of alpha-tocopherol ester derivatives in solutions and liposomes. Spectroscopic and LC–MS studies. J. Photochem. Photobiol. 2016, 160, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Feng, X.; Zhou, C.; Liu, Y.; Yu, X.; Zhao, G. A Phase Regulation Strategy of Ammonium Ions Perovskite Nanocrystals from 1D Orthomorphic NH4PbI3 to 3D Cubic Phase by Cs Ions to Notedly Enhance Photoluminescence. Angew. Chem. Int. Ed. 2019, 58, 11642–11646. [Google Scholar] [CrossRef]
- Maroncelli, M.; Fleming, G.R. Picosecond solvation dynamics of coumarin 153: The importance of molecular aspects of solvation. J. Chem. Phys. 1987, 86, 6221–6239. [Google Scholar] [CrossRef]
- Kasparek, A.; Smyk, B. A new approach to the old problem: Inner filter effect type I and II in fluorescence. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 198, 297–303. [Google Scholar] [CrossRef]

| Fluorescence | |||
|---|---|---|---|
| λexc = 283 nm k × 103 [min−1] | λobs = 325 nm k × 103 [min−1] | ||
| α-Toc | arg | 8.86 ± 0.52 | 9.31 ± 0.57 |
| non-arg | 5.89 ± 0.47 | 6.20 ± 0.73 | |
| β-Toc | arg | 5.69 ± 0.18 | 6.20 ± 0.14 |
| non-arg | 4.63 ± 0.11 | 4.80 ± 0.11 | |
| γ-Toc | arg | 13.10 ± 0.15 | 12.18 ± 0.14 |
| non-arg | 7.21 ± 0.84 | 7.38 ± 0.18 | |
| δ-Toc | arg | 9.30 ± 0.15 | 7.73 ± 0.05 |
| non-arg | 3.36 ± 0.32 | 1.27 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smyk, B. Spectroscopic Evidence for Photooxidation of Tocopherols in n-Hexane. Molecules 2021, 26, 571. https://doi.org/10.3390/molecules26030571
Smyk B. Spectroscopic Evidence for Photooxidation of Tocopherols in n-Hexane. Molecules. 2021; 26(3):571. https://doi.org/10.3390/molecules26030571
Chicago/Turabian StyleSmyk, Bogdan. 2021. "Spectroscopic Evidence for Photooxidation of Tocopherols in n-Hexane" Molecules 26, no. 3: 571. https://doi.org/10.3390/molecules26030571
APA StyleSmyk, B. (2021). Spectroscopic Evidence for Photooxidation of Tocopherols in n-Hexane. Molecules, 26(3), 571. https://doi.org/10.3390/molecules26030571






