An Overview of the Potential Therapeutic Applications of Essential Oils
Abstract
:1. Introduction
2. Essential Oil Research
2.1. AMR and Antimicrobial activity
2.1.1. Antibacterial Activity of EO
2.1.2. Antifungal Activity of EO
2.1.3. Antiviral Activity of EO
2.2. Synergistic Activity in EO
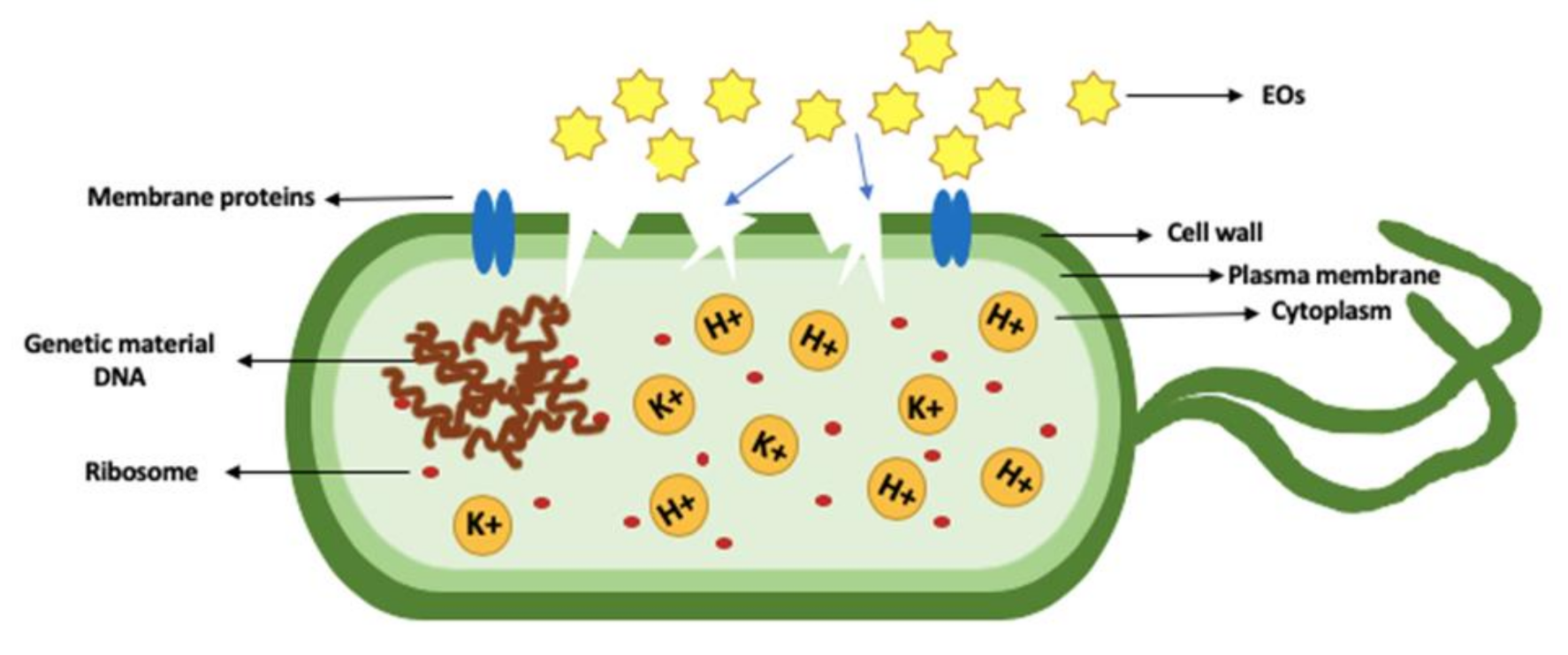
2.3. Mode of Action of EO Compounds on Pathogenic Bacteria
3. Recent Approaches
3.1. Genomics Perspective
3.2. Proteomics Perspective
4. Limitations in Essential Oils Research
5. Future Strategies and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Durand, G.A.; Raoult, D.; Dubourg, G. Antibiotic Discovery: History, Methods and Perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial Resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsan, M.; Schoemaker, L.; Eggleston, K.; Kammili, N.; Kolli, P.; Bhattacharya, J. Out-of-Pocket Health Expenditures and Antimicrobial Resistance in Low-Income and Middle-Income Countries: An Economic Analysis. Lancet Infect. Dis. Lond. 2015, 15, 1203–1210. [Google Scholar] [CrossRef] [Green Version]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartley, P.S.; Domitrovic, T.N.; Moretto, V.T.; Santos, C.S.; Ponce-Terashima, R.; Reis, M.G.; Barbosa, L.M.; Blanton, R.E.; Bonomo, R.A.; Perez, F. Antibiotic Resistance in Enterobacteriaceae from Surface Waters in Urban Brazil Highlights the Risks of Poor Sanitation. Am. J. Trop. Med. Hyg. 2019, 100, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- CDC. The Biggest Antibiotic-Resistant Threats in the U.S. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 18 March 2020).
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [Green Version]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of Antimicrobial Resistance in the Environment and Its Relevance to Environmental Regulators. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Ventola, C.L. The Antibiotic Resistance Crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Luyt, C.-E.; Bréchot, N.; Trouillet, J.-L.; Chastre, J. Antibiotic Stewardship in the Intensive Care Unit. Crit. Care 2014, 18, 480. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Webster, T.J. Bacteria Antibiotic Resistance: New Challenges and Opportunities for Implant-Associated Orthopaedic Infections. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2018, 36, 22–32. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO|Antimicrobial Resistance: Global Report on Surveillance 2014; World Health Organization: Geneva, Switzerland, 2014; p. 257. [Google Scholar]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular Mechanisms of Antibiotic Resistance. Nat. Rev. Microbiol. Lond. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.; Docherty, K.; Gill, S.; Baker, K.; Teachout, J.; Vonhof, M. Antibiotic Resistant Bacteria Are Widespread in Songbirds across Rural and Urban Environments. Sci. Total Environ. 2018, 627, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Díaz, F.; Fernández-López, C.; Lurz, R.; Bravo, A.; Espinosa, M. Crosstalk between Vertical and Horizontal Gene Transfer: Plasmid Replication Control by a Conjugative Relaxase. Nucleic Acids Res. 2017, 45, 7774–7785. [Google Scholar] [CrossRef] [PubMed]
- Read, A.F.; Woods, R.J. Antibiotic Resistance Management. Evol. Med. Public Health 2014, 2014, 147. [Google Scholar] [CrossRef]
- Yang, S.-K.; Low, L.-Y.; Yap, P.S.-X.; Yusoff, K.; Mai, C.-W.; Lai, K.-S.; Lim, S.-H.E. Plant-Derived Antimicrobials: Insights into Mitigation of Antimicrobial Resistance. Rec. Nat. Prod. 2018, 12, 295–396. [Google Scholar] [CrossRef]
- Antifungal Resistance|Fungal Diseases|CDC. Available online: https://www.cdc.gov/fungal/antifungal-resistance.html (accessed on 2 November 2020).
- Antifungal Resistance in Candida|Fungal Diseases|CDC. Available online: https://www.cdc.gov/fungal/diseases/candidiasis/antifungal-resistant.html (accessed on 2 November 2020).
- Antifungal Resistance in Aspergillus|Fungal Diseases|CDC. Available online: https://www.cdc.gov/fungal/diseases/aspergillosis/antifungal-resistant.html (accessed on 2 November 2020).
- Kumar, M.; Mazumder, P.; Mohapatra, S.; Kumar Thakur, A.; Dhangar, K.; Taki, K.; Mukherjee, S.; Kumar Patel, A.; Bhattacharya, P.; Mohapatra, P.; et al. A Chronicle of SARS-CoV-2: Seasonality, Environmental Fate, Transport, Inactivation, and Antiviral Drug Resistance. J. Hazard. Mater. 2020, 124043. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid.-Based Complement. Altern. Med. ECAM 2016, 2016. [Google Scholar] [CrossRef]
- Solórzano-Santos, F.; Miranda-Novales, M.G. Essential Oils from Aromatic Herbs as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef]
- Aljaafari, M.; Alhosani, M.S.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. Essential Oils: Partnering with Antibiotics. In Essential—Oils of Nature; IntechOpen: London, UK, 2019. [Google Scholar]
- Srivastava, A.K.; Singh, V.K. Biological Action of Essential Oils (Terpenes). Biomedscidirect 2019, 10, 6854–6859. [Google Scholar]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus Vulgaris in Water Deficit Stress Response. Mol. Basel Switz. 2020, 25, 1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K.; Rather, M.A.; Kumar Jha, A.; Shashank, A.; Singhal, S.; Sharma, M.; Pathak, U.; Sharma, D.; Mastinu, A. Artocarpus Lakoocha Roxb. and Artocarpus Heterophyllus Lam. Flowers: New Sources of Bioactive Compounds. Plants 2020, 9, 1329. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, S.; Ali, S.; Valadabadi, R.; Pouryousef, M.; Saifzadeh, S.; Zakrin, H.; Mastinu, A. Quantitative and Qualitative Evaluation of Sorghum Bicolor L. under Intercropping with Legumes and Different Weed Control Methods. Horticulturae 2020, 6, 78. [Google Scholar] [CrossRef]
- Lee, M.Y. Essential Oils as Repellents against Arthropods. BioMed Res. Int. 2018, 2018, e6860271. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Memo, M.; Mastinu, A. Plant Behaviour: An Evolutionary Response to the Environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, H.; Morquecho-Contreras, A. Chemical Plant Defense against Herbivores. Herbivores 2017. [Google Scholar] [CrossRef]
- Tölke, E.D.; do Capelli, N.V.; Pastori, T.; Alencar, A.C.; Cole, T.C.H.; Demarco, D. Diversity of Floral Glands and Their Secretions in Pollinator Attraction. In Co-Evolution of Secondary Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2020; pp. 709–754. ISBN 978-3-319-96397-6. [Google Scholar]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Stephane, F.F.Y.; Jules, B.K.J. Terpenoids as Important Bioactive Constituents of Essential Oils. Essent. Oils Bioact. Compd. New Perspect. Appl. 2020. [Google Scholar] [CrossRef]
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic Plants: Are They New Cannabinoidergic Modulators? Planta 2019, 249, 1681–1694. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Chapter 11—Terpenoids. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 233–266. ISBN 978-0-12-802104-0. [Google Scholar]
- Lima, P.S.S.; Lucchese, A.M.; Araújo-Filho, H.G.; Menezes, P.P.; Araújo, A.A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S. Inclusion of Terpenes in Cyclodextrins: Preparation, Characterization and Pharmacological Approaches. Carbohydr. Polym. 2016, 151, 965–987. [Google Scholar] [CrossRef]
- Dias, N.; Dias, M.C.; Cavaleiro, C.; Sousa, M.C.; Lima, N.; Machado, M. Oxygenated Monoterpenes-Rich Volatile Oils as Potential Antifungal Agents for Dermatophytes. Nat. Prod. Res. 2017, 31, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ouazzou, A.; Cherrat, L.; Espina, L.; Lorán, S.; Rota, C.; Pagán, R. The Antimicrobial Activity of Hydrophobic Essential Oil Constituents Acting Alone or in Combined Processes of Food Preservation. Innov. Food Sci. Emerg. Technol. 2011, 12, 320–329. [Google Scholar] [CrossRef]
- Stefanakis, M.K.; Touloupakis, E.; Anastasopoulos, E.; Ghanotakis, D.; Katerinopoulos, H.E.; Makridis, P. Antibacterial Activity of Essential Oils from Plants of the Genus Origanum. Food Control 2013, 34, 539–546. [Google Scholar] [CrossRef]
- Böhme, K.; Barros-Velázquez, J.; Calo-Mata, P.; Aubourg, S.P. Antibacterial, Antiviral and Antifungal Activity of Essential Oils: Mechanisms and Applications. In Antimicrobial Compounds: Current Strategies and New Alternatives; Villa, T.G., Veiga-Crespo, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 51–81. ISBN 978-3-642-40444-3. [Google Scholar]
- Fokou, J.B.H.; Dongmo, P.M.J.; Boyom, F.F. Essential Oil’s Chemical Composition and Pharmacological Properties. In Essential Oils—Oils of Nature; IntechOpen: London, UK, 2020. [Google Scholar]
- Nazzaro, F.; Florinda, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of Essential Oils in Food Safety: Antimicrobial and Antioxidant Applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Wang, T.-H.; Hsia, S.-M.; Wu, C.-H.; Ko, S.-Y.; Chen, M.Y.; Shih, Y.-H.; Shieh, T.-M.; Chuang, L.-C.; Wu, C.-Y. Evaluation of the Antibacterial Potential of Liquid and Vapor Phase Phenolic Essential Oil Compounds against Oral Microorganisms. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Griensven, L.J.L.D. van Antibacterial Effects of the Essential Oils of Commonly Consumed Medicinal Herbs Using an In Vitro Model. Molecules 2010, 15, 7532. [Google Scholar] [CrossRef] [Green Version]
- Kovács, J.K.; Felső, P.; Makszin, L.; Pápai, Z.; Horváth, G.; Ábrahám, H.; Palkovics, T.; Böszörményi, A.; Emődy, L.; Schneider, G. Antimicrobial and Virulence-Modulating Effects of Clove Essential Oil on the Foodborne Pathogen Campylobacter Jejuni. Appl. Environ. Microbiol. 2016, 82, 6158–6166. [Google Scholar] [CrossRef] [Green Version]
- Siddiqua, S.; Anusha, B.A.; Ashwini, L.S.; Negi, P.S. Antibacterial Activity of Cinnamaldehyde and Clove Oil: Effect on Selected Foodborne Pathogens in Model Food Systems and Watermelon Juice. J. Food Sci. Technol. 2015, 52, 5834–5841. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia Coli and Staphylococcus Aureus. Evid. Based Complement. Alternat. Med. 2015, 2015, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the Antimicrobial Activity and Cytotoxicity of Different Components of Natural Origin Present in Essential Oils. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, L.C. Antimicrobial activity of secondary metabolites and lectins from plants. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology, 1st ed.; FORMATEX: Badajoz, Spain, 2010; pp. 396–406. ISBN 978-84-614-6194-3. [Google Scholar]
- Reyes-Jurado, F.; López-Malo, A.; Palou, E. Antimicrobial Activity of Individual and Combined Essential Oils against Foodborne Pathogenic Bacteria—ProQuest. Available online: http://search.proquest.com/docview/1759178700?pq-origsite=summon (accessed on 1 April 2020).
- Liu, X.; Ma, Z.; Zhang, J.; Yang, L. Antifungal Compounds against Candida Infections from Traditional Chinese Medicine. BioMed Res. Int. 2017, 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The Antibacterial and Antifungal Activity of Six Essential Oils and Their Cyto/Genotoxicity to Human HEL 12469 Cells. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertas, A.; Garbusińska, A.; Szliszka, E.; Jureczko, A.; Kowalska, M.; Król, W. The Influence of Tea Tree Oil (Melaleuca Alternifolia) on Fluconazole Activity against Fluconazole-Resistant Candida Albicans Strains. BioMed Res. Int. 2015, 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Bahuguna, A.; Kumar, P.; Bajpai, V.K.; Kang, S.C. Antimicrobial Potential of Carvacrol against Uropathogenic Escherichia Coli via Membrane Disruption, Depolarization, and Reactive Oxygen Species Generation. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and Antifungal Activities of Thymol: A Brief Review of the Literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Gnanamani, A.; Mandal, A. A Potential Antibacterial Agent Embelin, a Natural Benzoquinone Extracted from Embelia Ribes. Biol. Med. 2011, 3, 1–7. [Google Scholar]
- Mihai, A.L.; Popa, M.E. In Vitro Activity of Natural Antimicrobial Compounds against Aspergillus Strains. Agric. Agric. Sci. Procedia 2015, 6, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Lai, W.-L.; Chuang, H.-S.; Lee, M.-H.; Wei, C.-L.; Lin, C.-F.; Tsai, Y.-C. Inhibition of Herpes Simplex Virus Type 1 by Thymol-Related Monoterpenoids. Planta Med. 2012, 78, 1636–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for Antiviral Activities of Isolated Compounds from Essential Oils. Evid. Based Complement. Alternat. Med. 2011, 2011, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahizan, N.A.; Yang, S.-K.; Moo, C.-L.; Song, A.A.-L.; Chong, C.-M.; Chong, C.-W.; Abushelaibi, A.; Lim, S.-H.E.; Lai, K.-S. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V. Antimicrobial Activity of Essential Oils. Int. J. FOOD Ferment. Technol. 2011, 1, 161–172. [Google Scholar]
- Raut, J.S.; Karuppayil, S.M. A Status Review on the Medicinal Properties of Essential Oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Abdollahzadeh, E.; Rezaei, M.; Hosseini, H. Antibacterial Activity of Plant Essential Oils and Extracts: The Role of Thyme Essential Oil, Nisin, and Their Combination to Control Listeria Monocytogenes Inoculated in Minced Fish Meat. Food Control 2014, 35, 177–183. [Google Scholar] [CrossRef]
- Dutra, T.V.; Castro, J.C.; Menezes, J.L.; Ramos, T.R.; do Prado, I.N.; Machinski, M.; Mikcha, J.M.G.; de Abreu Filho, B.A. Bioactivity of Oregano (Origanum Vulgare) Essential Oil against Alicyclobacillus Spp. Ind. Crops Prod. 2019, 129, 345–349. [Google Scholar] [CrossRef]
- LeBel, G.; Haas, B.; Adam, A.-A.; Veilleux, M.-P.; Lagha, A.B.; Grenier, D. Effect of Cinnamon (Cinnamomum Verum) Bark Essential Oil on the Halitosis-Associated Bacterium Solobacterium Moorei and in Vitro Cytotoxicity. Arch. Oral Biol. 2017, 83, 97–104. [Google Scholar] [CrossRef]
- Lorenzo-Leal, A.C.; Palou, E.; López-Malo, A. Evaluation of the Efficiency of Allspice, Thyme and Rosemary Essential Oils on Two Foodborne Pathogens in in-Vitro and on Alfalfa Seeds, and Their Effect on Sensory Characteristics of the Sprouts. Int. J. Food Microbiol. 2019, 295, 19–24. [Google Scholar] [CrossRef]
- Mohamed, M.S.M.; Abdallah, A.A.; Shalaby, A.M.; Mahran, M.H. Potential Alternative Treatment of Ocular Bacterial Infections by Oil Derived from Syzygium Aromaticum Flower (Clove). Curr. Eye Res. 2018, 43, 873–881. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.-N.; Tang, G.-Y.; Li, H.-B. Antibacterial and Antifungal Activities of Spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.-K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Akseer, R.; Lim, S.-H.E.; Lai, K.-S. Disruption of KPC-Producing Klebsiella Pneumoniae Membrane via Induction of Oxidative Stress by Cinnamon Bark (Cinnamomum verum J. Presl) Essential Oil. PLoS ONE 2019, 14, e0214326. [Google Scholar] [CrossRef] [Green Version]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Potential Application of Spice and Herb Extracts as Natural Preservatives in Cheese. J. Med. Food 2010, 14, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Ehsani, A.; Hosseini Jazani, N.; Aliakbarlu, J.; Mahmoudi, R. Chemical Composition and in Vitro Antibacterial Activity of Essential Oil and Methanol Extract of Echinophora Platyloba D.C against Some of Food-Borne Pathogenic Bacteria. Vet. Res. Forum 2013, 4, 123–127. [Google Scholar]
- Hussain, A.I.; Anwar, F.; Chatha, S.A.S.; Jabbar, A.; Mahboob, S.; Nigam, P.S. Rosmarinus Officinalis Essential Oil: Antiproliferative, Antioxidant and Antibacterial Activities. Braz. J. Microbiol. 2010, 41, 1070–1078. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. Antibacterial Activity and Anticancer Activity of Rosmarinus officinalis L. Essential Oil Compared to That of Its Main Components. Molecules 2012, 17, 2704–2713. [Google Scholar] [CrossRef] [Green Version]
- Helal, I.M.; El-Bessoumy, A.; Al-Bataineh, E.; Joseph, M.R.P.; Rajagopalan, P.; Chandramoorthy, H.C.; Ben Hadj Ahmed, S. Antimicrobial Efficiency of Essential Oils from Traditional Medicinal Plants of Asir Region, Saudi Arabia, over Drug Resistant Isolates. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of Antifungal and Anti-Aflatoxigenic Properties of Essential Oil Derived from Turmeric (Curcuma longa L.) on Aspergillus Flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef]
- Rahman, A.; Al-Reza, S.M.; Kang, S.C. Antifungal Activity of Essential Oil and Extracts of Piper Chaba Hunter against Phytopathogenic Fungi. JAOCS J. Am. Oil Chem. Soc. Champaign 2011, 88, 573–579. [Google Scholar] [CrossRef]
- Schroder, T.; Gaskin, S.; Ross, K.; Whiley, H. Antifungal Activity of Essential Oils against Fungi Isolated from Air. Int. J. Occup. Environ. Health 2017, 23, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ye-Lin, S.; Tang, Y.-J.; Wen-Wen, Z. Antifungal Activity of Essential Oil Compounds (Geraniol and Citral) and Inhibitory Mechanisms on Grain Pathogens (Aspergillus flavus and Aspergillus ochraceus). Mol. Basel 2018, 23, 2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Huang, B.; Wang, Y. Chemical Composition and Antifungal Activity of Essential Oil from Cicuta Virosa L. Var. Latisecta Celak. Int. J. Food Microbiol. 2011, 145, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Singh, P.; Kedia, A.; Dubey, N.K. Assessment of Some Essential Oils as Food Preservatives Based on Antifungal, Antiaflatoxin, Antioxidant Activities and in Vivo Efficacy in Food System. Food Res. Int. 2012, 49, 201–208. [Google Scholar] [CrossRef]
- Ksouri, S.; Djebir, S.; Bentorki, A.A.; Gouri, A.; Hadef, Y.; Benakhla, A. Antifungal Activity of Essential Oils Extract from Origanum floribundum Munby, Rosmarinus officinalis L. and Thymus ciliatus Desf. against Candida albicans Isolated from Bovine Clinical Mastitis. J. Mycol. Médicale 2017, 27, 245–249. [Google Scholar] [CrossRef]
- Vieira, P.R.N.; de Morais, S.M.; Bezerra, F.H.Q.; Travassos Ferreira, P.A.; Oliveira, Í.R.; Silva, M.G.V. Chemical Composition and Antifungal Activity of Essential Oils from Ocimum Species. Ind. Crops Prod. 2014, 55, 267–271. [Google Scholar] [CrossRef]
- Hu, F.; Tu, X.-F.; Thakur, K.; Hu, F.; Li, X.-L.; Zhang, Y.-S.; Zhang, J.-G.; Wei, Z.-J. Comparison of Antifungal Activity of Essential Oils from Different Plants against Three Fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef]
- Benabdelkader, T.; Zitouni, A.; Guitton, Y.; Jullien, F.; Maitre, D.; Casabianca, H.; Legendre, L.; Kameli, A. Essential Oils from Wild Populations of Algerian Lavandula stoechas L.: Composition, Chemical Variability, and in Vitro Biological Properties. Chem. Biodivers. 2011, 8, 937–953. [Google Scholar] [CrossRef]
- Scalas, D.; Mandras, N.; Roana, J.; Tardugno, R.; Cuffini, A.M.; Ghisetti, V.; Benvenuti, S.; Tullio, V. Use of Pinus sylvestris L. (Pinaceae), Origanum vulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) Essential Oils and Their Main Components to Enhance Itraconazole Activity against Azole Susceptible/Not-Susceptible Cryptococcus neoformans Strains. BMC Complement. Altern. Med. 2018, 18, 143. [Google Scholar] [CrossRef] [Green Version]
- Gelzleichter, T.R. Chapter 7—Early Characterization of Biosimilar Therapeutics. In Nonclinical Development of Novel Biologics, Biosimilars, Vaccines and Specialty Biologics; Plitnick, L.M., Herzyk, D.J., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 185–210. ISBN 978-0-12-394810-6. [Google Scholar]
- Akhtar, M.S. Antimicrobial Activity of Essential Oils Extracted from Medicinal Plants against the Pathogenic Microorganisms: A Review. Issues Biol. Sci. Pharm. Res. 2014, 2, 1–7. [Google Scholar]
- Fung, J.; Seto, W.-K.; Lai, C.-L.; Yuen, M.-F. Extrahepatic Effects of Nucleoside and Nucleotide Analogues in Chronic Hepatitis B Treatment. J. Gastroenterol. Hepatol. 2014, 29, 428–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayaaslan, B.; Guner, R. Adverse Effects of Oral Antiviral Therapy in Chronic Hepatitis B. World J. Hepatol. 2017, 9, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Orhan, İ.E.; ÖzçeliK, B.; Kartal, M.; Kan, Y. Antimicrobial and Antiviral Effects of Essential Oils from Selected Umbelliferae and Labiatae Plants and Individual Essential Oil Components. Enzym. Inhib. Act. Nat. Compd. 2012, 36, 239–246. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents against Drug-Resistant Microbial Pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P. Essential Oils for the Treatment of Herpes Simplex Virus Infections. Chemotherapy 2019, 64, 1–7. [Google Scholar] [CrossRef]
- Gavanji, S.; Sayedipour, S.S.; Larki, B.; Bakhtari, A. Antiviral Activity of Some Plant Oils against Herpes Simplex Virus Type 1 in Vero Cell Culture. J. Acute Med. 2015, 5, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Bektaş, E.; Daferera, D.; Sökmen, M.; Serdar, G.; Erturk, M.; Polissiou, M.; Sökmen, A. In Vitro Antimicrobial, Antioxidant, and Antiviral Activities of the Essential Oil and Various Extracts from Thymus Nummularis M. Bieb. Indian J. Tradit. Knowl. 2016, 15, 403–410. [Google Scholar]
- Moo, C.-L.; Yang, S.-K.; Osman, M.-A.; Yuswan, M.H.; Loh, J.-Y.; Lim, W.-M.; Lim, S.-H.-E.; Lai, K.-S. Antibacterial Activity and Mode of Action of β-Caryophyllene on Bacillus Cereus. Pol. J. Microbiol. 2020, 69, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Pájaro-Castro, N.; Flechas, M.C.; Ocazionez, R.; Stashenko, E.; Olivero-Verbel, J. Potential Interaction of Components from Essential Oils with Dengue Virus Proteins. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2015, 14, 141–155. [Google Scholar]
- Roy, S.; Chaurvedi, P.; Chowdhary, A. Evaluation of Antiviral Activity of Essential Oil of Trachyspermum Ammi against Japanese Encephalitis Virus. Pharmacogn. Res. 2015, 7, 263–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, L.; Laird, K. Synchronous Application of Antibiotics and Essential Oils: Dual Mechanisms of Action as a Potential Solution to Antibiotic Resistance. Crit. Rev. Microbiol. 2018, 44, 414–435. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-K.; Yusoff, K.; Mai, C.-W.; Lim, W.-M.; Yap, W.-S.; Lim, S.-H.E.; Lai, K.-S. Additivity vs Synergism: Investigation of the Additive Interaction of Cinnamon Bark Oil and Meropenem in Combinatory Therapy. Molecules 2017, 22, 1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, P.S.X.; Lim, S.H.E.; Hu, C.P.; Yiap, B.C. Combination of Essential Oils and Antibiotics Reduce Antibiotic Resistance in Plasmid-Conferred Multidrug Resistant Bacteria. Phytomedicine 2013, 20, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-K.; Yap, P.S.-X.; Krishnan, T.; Yusoff, K.; Chan, K.-G.; Yap, W.-S.; Lai, K.-S.; Lim, S.-H.E. Mode of Action: Synergistic Interaction of Peppermint (Mentha x Piperita L. Carl) Essential Oil and Meropenem against Plasmid-Mediated Resistant E. Coli. Rec. Nat. Prod. 2018, 12, 582–594. [Google Scholar] [CrossRef]
- Stević, T.; Berić, T.; Šavikin, K.; Soković, M.; Gođevac, D.; Dimkić, I.; Stanković, S. Antifungal Activity of Selected Essential Oils against Fungi Isolated from Medicinal Plant. Ind. Crops. Prod. 2014, 55, 116–122. [Google Scholar] [CrossRef]
- Hossain, F.; Follett, P.; Dang Vu, K.; Harich, M.; Salmieri, S.; Lacroix, M. Evidence for Synergistic Activity of Plant-Derived Essential Oils against Fungal Pathogens of Food. Food Microbiol. 2016, 53, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.B.; Vadhana, P.; Bhardwaj, M.; Or, V.K.; Sinha, D.K.; Singh, S.V. Comparative Antimicrobial Activity of Tea Tree Oil (Melaleuca Oil) and Common Topical Antimicrobials against Bacteria Associated With Wound and Topical Infections. Pharm. Anal. Acta 2016, 7, 1–9. [Google Scholar] [CrossRef]
- D’Arrigo, M.; Ginestra, G.; Mandalari, G.; Furneri, P.M.; Bisignano, G. Synergism and Postantibiotic Effect of Tobramycin and Melaleuca Alternifolia (Tea Tree) Oil against Staphylococcus Aureus and Escherichia Coli. Phytomedicine 2010, 17, 317–322. [Google Scholar] [CrossRef]
- Chovanová, R.; Mezovská, J.; Vaverková, Š.; Mikulášová, M. The Inhibition the Tet(K) Efflux Pump of Tetracycline Resistant Staphylococcus Epidermidis by Essential Oils from Three Salvia Species. Lett. Appl. Microbiol. 2015, 61, 58–62. [Google Scholar] [CrossRef]
- Alexa, E.; Sumalan, R.M.; Danciu, C.; Obistioiu, D.; Negrea, M.; Poiana, M.-A.; Rus, C.; Radulov, I.; Pop, G.; Dehelean, C. Synergistic Antifungal, Allelopatic and Anti-Proliferative Potential of Salvia officinalis L., and Thymus vulgaris L. Essential Oils. Mol. Basel Switz. 2018, 23, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tullio, V.; Roana, J.; Scalas, D.; Mandras, N. Evaluation of the Antifungal Activity of Mentha x Piperita (Lamiaceae) of Pancalieri (Turin, Italy) Essential Oil and Its Synergistic Interaction with Azoles. Mol. Basel Switz. 2019, 24, 3148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essid, R.; Hammami, M.; Gharbi, D.; Karkouch, I.; Hamouda, T.B.; Elkahoui, S.; Limam, F.; Tabbene, O. Antifungal Mechanism of the Combination of Cinnamomum Verum and Pelargonium Graveolens Essential Oils with Fluconazole against Pathogenic Candida Strains. Appl. Microbiol. Biotechnol. 2017, 101, 6993–7006. [Google Scholar] [CrossRef] [PubMed]
- Pourghanbari, G.; Nili, H.; Moattari, A.; Mohammadi, A.; Iraji, A. Antiviral Activity of the Oseltamivir and Melissa Officinalis L. Essential Oil against Avian Influenza A Virus (H9N2). VirusDisease 2016, 27, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimnoi, N.; Reuk-ngam, N.; Chuysinuan, P.; Khlaychan, P.; Khunnawutmanotham, N.; Chokchaichamnankit, D.; Thamniyom, W.; Klayraung, S.; Mahidol, C.; Techasakul, S. Characterization of Essential Oil from Ocimum Gratissimum Leaves: Antibacterial and Mode of Action against Selected Gastroenteritis Pathogens. Microb. Pathog. 2018, 118, 290–300. [Google Scholar] [CrossRef]
- Cho, T.J.; Park, S.M.; Yu, H.; Seo, G.H.; Kim, H.W.; Kim, S.A.; Rhee, M.S. Recent Advances in the Application of Antibacterial Complexes Using Essential Oils. Molecules 2020, 25, 1752. [Google Scholar] [CrossRef]
- Yang, S.-K.; Yusoff, K.; Thomas, W.; Akseer, R.; Alhosani, M.S.; Abushelaibi, A.; Lim, S.-H.-E.; Lai, K.-S. Lavender Essential Oil Induces Oxidative Stress Which Modifies the Bacterial Membrane Permeability of Carbapenemase Producing Klebsiella Pneumoniae. Sci. Rep. 2020, 10, 819. [Google Scholar] [CrossRef]
- Lai, P.-J.; Ng, E.-V.; Yang, S.-K.; Moo, C.-L.; Low, W.Y.; Yap, P.S.-X.; Lim, S.-H.E.; Lai, K.-S. Transcriptomic Analysis of Multi-Drug Resistant Escherichia Coli K-12 Strain in Response to Lavandula Angustifolia Essential Oil. 3 Biotech 2020, 10, 313. [Google Scholar] [CrossRef]
- Yang, S.-K.; Yusoff, K.; Ajat, M.; Yap, W.-S.; Lim, S.-H.-E.; Lai, K.-S. Antimicrobial Activity and Mode of Action of Terpene Linalyl Anthranilate against Carbapenemase-Producing Klebsiella Pneumoniae. J. Pharm. Anal. 2020. [Google Scholar] [CrossRef]
- Limaverde, P.W.; Campina, F.F.; da Cunha, F.A.B.; Crispim, F.D.; Figueredo, F.G.; Lima, L.F.; de Datiane, M.; Oliveira-Tintino, C.; de Matos, Y.M.L.S.; Morais-Braga, M.F.B.; et al. Inhibition of the TetK Efflux-Pump by the Essential Oil of Chenopodium Ambrosioides L. and α-Terpinene against Staphylococcus Aureus IS-58. Food Chem. Toxicol. 2017, 109, 957–961. [Google Scholar] [CrossRef]
- Moo, C.-L.; Yang, S.-K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Lim, S.H.E.; Lai, K.-S. Mechanisms of Antimicrobial Resistance (AMR) and Alternative Approaches to Overcome AMR. Curr. Drug Discov. Technol. 2019. [Google Scholar] [CrossRef]
- Mikulášová, M.; Chovanová, R.; Vaverková, Š. Synergism between Antibiotics and Plant Extracts or Essential Oils with Efflux Pump Inhibitory Activity in Coping with Multidrug-Resistant Staphylococci. Phytochem. Rev. Dordr. 2016, 15, 651–662. [Google Scholar] [CrossRef]
- Johny, A.K.; Hoagland, T.; Venkitanarayanan, K. Effect of Subinhibitory Concentrations of Plant-Derived Molecules in Increasing the Sensitivity of Multidrug-Resistant Salmonella Enterica Serovar Typhimurium DT104 to Antibiotics. Foodborne Pathog. Dis. 2010, 7, 1165–1170. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Bouyahya, A.; Abrini, J.; Dakka, N.; Bakri, Y. Essential Oils of Origanum Compactum Increase Membrane Permeability, Disturb Cell Membrane Integrity, and Suppress Quorum-Sensing Phenotype in Bacteria. J. Pharm. Anal. 2019, 9, 301–311. [Google Scholar] [CrossRef]
- Mempin, R.; Tran, H.; Chen, C.; Gong, H.; Kim Ho, K.; Lu, S. Release of Extracellular ATP by Bacteria during Growth. BMC Microbiol. 2013, 13, 301. [Google Scholar] [CrossRef] [Green Version]
- Nowotarska, S.W.; Nowotarski, K.; Grant, I.R.; Elliott, C.T.; Friedman, M.; Situ, C. Mechanisms of Antimicrobial Action of Cinnamon and Oregano Oils, Cinnamaldehyde, Carvacrol, 2,5-Dihydroxybenzaldehyde, and 2-Hydroxy-5-Methoxybenzaldehyde against Mycobacterium Avium Subsp. Paratuberculosis (Map). Foods Basel 2017, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- van Alphen, L.B.; Burt, S.A.; Veenendaal, A.K.J.; Bleumink-Pluym, N.M.C.; van Putten, J.P.M. The Natural Antimicrobial Carvacrol Inhibits Campylobacter Jejuni Motility and Infection of Epithelial Cells. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Ayaz, M.; Subhan, F.; Sadiq, A.; Ullah, F.; Ahmed, J.; Sewell, R.D.E. Cellular Efflux Transporters and the Potential Role of Natural Products in Combating Efflux Mediated Drug Resistance. Front. Biosci. 2017, 22, 732–756. [Google Scholar] [CrossRef]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic Interactions of Phytochemicals with Antimicrobial Agents: Potential Strategy to Counteract Drug Resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef]
- Han, X.; Parker, T.L. Lemongrass (Cymbopogon Flexuosus) Essential Oil Demonstrated Anti-Inflammatory Effect in Pre-Inflamed Human Dermal Fibroblasts. Biochim. Open 2017, 4, 107–111. [Google Scholar] [CrossRef]
- Fields, F.R.; Lee, S.W.; McConnell, M.J. Using Bacterial Genomes and Essential Genes for the Development of New Antibiotics. Biochem. Pharmacol. 2017, 134, 74–86. [Google Scholar] [CrossRef]
- Pieta, L.; Escudero, F.L.G.; Jacobus, A.P.; Cheiran, K.P.; Gross, J.; Moya, M.L.E.; Soares, G.L.G.; Margis, R.; Frazzon, A.P.G.; Frazzon, J. Comparative Transcriptomic Analysis of Listeria Monocytogenes Reveals Upregulation of Stress Genes and Downregulation of Virulence Genes in Response to Essential Oil Extracted from Baccharis Psiadioides. Ann. Microbiol. 2017, 67, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Kot, B.; Sytykiewicz, H.; Sprawka, I.; Witeska, M. Effect of Trans-Cinnamaldehyde on Methicillin-Resistant Staphylococcus Aureus Biofilm Formation: Metabolic Activity Assessment and Analysis of the Biofilm-Associated Genes Expression. Int. J. Mol. Sci. 2020, 21, 102. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Llarena, F.J.; Bou, G. Proteomics As a Tool for Studying Bacterial Virulence and Antimicrobial Resistance. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Magdeldin, S. Gel Electrophoresis: Principles and Basics; BoD—Books on Demand: Norderstedt, Germany, 2012; ISBN 978-953-51-0458-2. [Google Scholar]
- Magdeldin, S.; Enany, S.; Yoshida, Y.; Xu, B.; Zhang, Y.; Zureena, Z.; Lokamani, I.; Yaoita, E.; Yamamoto, T. Basics and Recent Advances of Two Dimensional- Polyacrylamide Gel Electrophoresis. Clin. Proteom. 2014, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Nomura, F. Proteome-Based Bacterial Identification Using Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry (MALDI-TOF MS): A Revolutionary Shift in Clinical Diagnostic Microbiology. Biochim. Biophys. Acta BBA—Proteins Proteom. 2015, 1854, 528–537. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF Mass Spectrometry: An Emerging Technology for Microbial Identification and Diagnosis. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Božik, M.; Cejnar, P.; Šašková, M.; Nový, P.; Maršík, P.; Klouček, P. Stress Response of Escherichia Coli to Essential Oil Components—Insights on Low-Molecular-Weight Proteins from MALDI-TOF. Sci. Rep. Nat. Publ. Group Lond. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Feucherolles, M.; Poppert, S.; Utzinger, J.; Becker, S.L. MALDI-TOF Mass Spectrometry as a Diagnostic Tool in Human and Veterinary Helminthology: A Systematic Review. Parasit. Vectors 2019, 12, 245. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Mamone, G.; Ferranti, P.; Ercolini, D.; Mauriello, G. Changes in the Proteome of Salmonella Enterica Serovar Thompson as Stress Adaptation to Sublethal Concentrations of Thymol. Proteomics 2010, 10, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Tachikawa, M.; Obuchi, W.; Hoshi, Y.; Tomioka, Y.; Ohtsuki, S.; Terasaki, T. A Study Protocol for Quantitative Targeted Absolute Proteomics (QTAP) by LC-MS/MS: Application for Inter-Strain Differences in Protein Expression Levels of Transporters, Receptors, Claudin-5, and Marker Proteins at the Blood–Brain Barrier in DdY, FVB, and C57BL/6J Mice. Fluids Barriers CNS 2013, 10, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, S.; Villas-Bôas, S.G.; Ferreira, E.C.; Rocha, I. Metabolic Footprint Analysis of Recombinant Escherichia Coli Strains during Fed-Batch Fermentations. Mol. Biosyst. 2011, 7, 899–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, T.B.; Pinto, M.F.S.; Ribeiro, S.M.; de Lima, L.A.; Viana, J.C.; Júnior, N.G.; de Cândido, E.S.; Dias, S.C.; Franco, O.L. Bacterial Resistance Mechanism: What Proteomics Can Elucidate. FASEB J. 2013, 27, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; Laird, K.; Wilson, P.B. Structure-Activity Modelling of Essential Oils, Their Components, and Key Molecular Parameters and Descriptors. Mol. Cell. Probes 2018, 38, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiley, H.; Gaskin, S.; Schroder, T.; Ross, K. Antifungal Properties of Essential Oils for Improvement of Indoor Air Quality: A Review. Rev. Environ. Health Tel. Aviv. 2018, 33, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A. Essential Oil as Antimicrobial Agents: Efficacy, Stability, and Safety Issues for Food Application. Essent. Oils Bioact. Compd. New Perspect. Appl. 2020. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Peter, J.G.; Trubiano, J.A.; Phillips, E.J. Antibiotic Allergy. Lancet Lond. Engl. 2019, 393, 183–198. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of Essential Oils to Enhance Their Antimicrobial Activity in Foods. LWT Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Oroojalian, F.; Kasra-Kermanshahi, R.; Azizi, M.; Bassami, M.R. Phytochemical Composition of the Essential Oils from Three Apiaceae Species and Their Antibacterial Effects on Food-Borne Pathogens. Food Chem. 2010, 120, 765–770. [Google Scholar] [CrossRef]
- Yu, Z.; Tang, J.; Khare, T.; Kumar, V. The Alarming Antimicrobial Resistance in ESKAPEE Pathogens: Can Essential Oils Come to the Rescue?—ScienceDirect. ELSEVIER 2020, 140. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Nutrition, Well-Being and Health; BoD—Books on Demand: Norderstedt, Germany, 2012; ISBN 978-953-51-0125-3. [Google Scholar]
- Manion, C.R.; Widder, R.M. Essentials of Essential Oils. Am. J. Health. Syst. Pharm. 2017, 74, e153–e162. [Google Scholar] [CrossRef] [PubMed]
- Dagli, N.; Dagli, R.; Mahmoud, R.S.; Baroudi, K. Essential Oils, Their Therapeutic Properties, and Implication in Dentistry: A Review. J. Int. Soc. Prev. Community Dent. 2015, 5, 335–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupler, D.; Odle, T.G.; Newton, D.E. Essential oils. In The Gale Encyclopedia of Alternative Medicine; Fundukian, L.J., Ed.; Gale: Farmington Hills, MI, USA, 2014; Volume 2, pp. 859–862. [Google Scholar]
- Samie, A. Escherichia Coli: Recent Advances on Physiology, Pathogenesis and Biotechnological Applications; BoD—Books on Demand: Norderstedt, Germany, 2017; ISBN 978-953-51-3329-2. [Google Scholar]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential Oils, a New Horizon in Combating Bacterial Antibiotic Resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Krishnan, T.; Chan, K.-G.; Lim, S.H.E. Antibacterial Mode of Action of Cinnamomum Verum Bark Essential Oil, Alone and in Combination with Piperacillin, Against a Multi-Drug-Resistant Escherichia Coli Strain. J. Microbiol. Biotechnol. 2015, 25, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The Natural Plant Compound Carvacrol as an Antimicrobial and Anti-Biofilm Agent: Mechanisms, Synergies and Bio-Inspired Anti-Infective Materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef]
- Do, T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of Essential Oils. TrAC Trends Anal. Chem. 2014, 66, 146–157. [Google Scholar] [CrossRef]
- Muturi, E.J.; Ramirez, J.L.; Doll, K.M.; Bowman, M.J. Combined Toxicity of Three Essential Oils against Aedes Aegypti (Diptera: Culicidae) Larvae. J. Med. Entomol. 2017, 54, 1684–1691. [Google Scholar] [CrossRef]


| Antimicrobial Activity | EO | Main Compound | Structure | Microorganism | MIC/IC50 | MBC/MFC | Reference |
|---|---|---|---|---|---|---|---|
| a,bAntibacterial activity | Peppermint and mint oils | Menthol |  | Methicillin-resistant Staphylococcus aureus—ATCC 33591 Escherichia coli—ATCC 10798 Streptococcus mutans—ATCC 25175 Aggregatibacter actinomycetemcomitans—ATCC 33384 | 1000 μg/mL >2500 μg/mL 1000 μg/mL 500 μg/mL | 1000 μg/mL >2500 μg/mL 1000 μg/mL 1000 μg/mL | [45,46] |
| Lemon and cinnamon oil | Linalool | 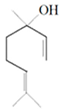 | S. aureus—ATCC 25923 E. coli—ATCC O157:H7 | 5.0 μg/mL 6.0 μg/mL | 5.5 μg/mL 6.0 μg/mL | [45,47,48] | |
| Clove oil | Eugenol |  | Methicillin-resistant S. aureus E. coli—S17 strain E. coli—ATCC 8739 S. aureus—ATCC 25923 Bacillus cereus—ATCC 14579 Salmonella typhimurium—ATCC 14028 | 1300 μg/mL 400 μg/mL 30 μg/mL 3 μg/mL 70 μg/mL 70 μg/mL | 1.5 mg/mL 0.5 mg/mL Bacterial growth Bacterial growth Bacterial growth 0.06 mg/mL | [45,49,50,51,52,53] | |
| Ginger oil | Gingerols | 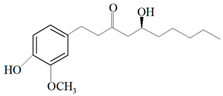 6-Gingerol 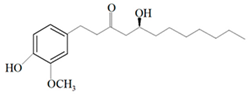 8-Gingerol  10-Gingerol | Porphyromonas gingivalis—ATCC 53978 Porphyromonas endodontalis—ATCC 35406 Prevotella intermedis—ATCC 25611 | 6–30 µg/mL 6–30 µg/mL 6–30 µg/mL | 4–20 µg/mL 4–20 µg/mL 4–20 µg/mL | [54] | |
| Mustard oil | AITC |  | S. aureus—ATCC 29413 Listeria monocytogenes—(Scott A) Salmonella enteritidis—(PT 30) | 500–1000 μg/mL 500–1000 μg/mL 500–1000 μg/mL | Not Available | [55] | |
| a,c Antifungal activity | Clove oil | Compound not specified in article | Cladosporium cladosporioides—air-borne Chaetomium globosum—air-borne Aspergillus fumigatus—air-borne | 500 μg/mL 250 μg/mL 250 μg/mL | 0.075% (w/v) 0.05% (w/v) 0.075% (w/v) | [56,57] | |
| Tea tree oil | Compound not specified in article | Epidermophyton floccosum Microsporum canis Trichophyton rubrum Aspergillus niger Penicillium spp. Alternaria spp. Fluconazole-Resistant Candida albicans—ATCC 10231 | 80–300 μg/mL 40–300 μg/mL 80–300 μg/mL 600–1200 μg/mL 300–600 μg/mL 160–1200 μg/mL 1250 μg/mL | 0.12–0.25% (v/v) 0.06–0.25% (v/v) <0.03–0.25% (v/v) 2–8% (v/v) 0.5–2% (v/v) 0.06–2% (v/v) 0.25% (v/v) | [58] | ||
| Arborvitae | Compound not specified in article | C. globosum—air-borne | 100 μg/mL | 0.025% (w/v) | [57] | ||
| Oregano | Compound not specified in article | A. fumigatus—air-borne C. cladosporioides—air-borne Alternaria alternata—air-borne | 250 μg/mL 100 μg/mL 100 μg/mL | 0.075% (w/v) 0.075% (w/v) 0.05% (w/v) | [57] |
| Antimicrobial activity | Main Compound | Structure | Microorganism | MIC/IC50 | MBC/MFC | Reference |
|---|---|---|---|---|---|---|
| a,bAntibacterial | Cinnamaldehyde | 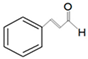 | Escherichia coli—S17 strain Methicillin-resistant S. aureus | 200 μg/mL 400 μg/mL | 0.3 mg/mL 0.5 mg/mL | [50,53] |
| Carvacrol | 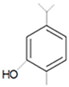 | Methicillin-resistant S. aureus E. coli—S17 strain S. mutans—ATCC 25175 A. actinomycetemcomitans—ATCC 33384 | 200 μg/mL 200 μg/mL 400 μg/mL 200 μg/mL | 0.3 mg/mL 0.4 mg/mL 600 μg/mL 200 μg/mL | [46,51,53,59] | |
| Thymol | 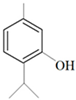 | Methicillin-resistant S. aureus E. coli—S17 strain Methicillin-resistant S. aureus—ATCC 33591 E. coli—ATCC 10798 S. mutans ATCC 25175 A.actinomycetemcomitans—ATCC 33384 E. coli—ATCC 8739 S. aureus—ATCC 25923 B. cereus—ATCC 14579 S. typhimurium—ATCC 14028 | 200 μg/mL 200 μg/mL 200 μg/mL 200 μg/mL 200 μg/mL 100 μg/mL 7 μg/mL 7 μg/mL 7 μg/mL 3 μg/mL | 0.3 mg/mL 0.3 mg/mL 200 μg/mL 400 μg/mL 400 μg/mL 200 μg/mL 0.12 mg/mL 0.12 mg/mL Bacterial growth 0.12 mg/mL | [46,51,52,53,60] | |
| β-caryophyllene and Squalene |  β-caryophyllene  Squalene | Methicillin-resistant S. aureus E. coli—S17 strain | >4000 μg/mL >4000 μg/mL | >4.0 mg/mL >4.0 mg/mL | [53] | |
| Terpineol |  | E. coli—ATCC 8739 S. aureus—ATCC 25923 B. cereus—ATCC 14579 S. typhimurium—ATCC 14028 | 60 μg/mL 30 μg/mL 120 μg/mL 120 μg/mL | Bacterial growth 0.12 mg/mL Bacterial growth 0.25 | [52,54] | |
| Benzoquinone - embelin | 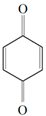 Benzoquinone 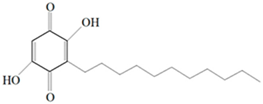 Embelin Embelin | S. aureus—ATCC 6538 B. cereus—ATCC 10876 E. coli—ATCC 4157 Pseudomonas aeruginosa—ATCC 9027 | 20 μg/mL 20 μg/mL 45 μg/mL 25 μg/mL | 20 μg/mL 75 μg/mL 325 μg/mL 125 μg/mL | [54,61] | |
| Carveol | 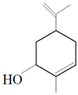 | E. coli S. aureus | 200 µg/mL 2000 µg/mL | 1500 µg/mL 2500 µg/mL | [51] | |
| Citronellol | 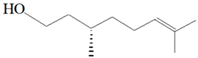 | E. coli S. aureus | 5 µg/mL 375 µg/mL | 15 µg/mL 400 µg/mL | [51] | |
| Citronellal |  | E. coli S. aureus | 300 µg/mL 400 µg/mL | 500 µg/mL 800 µg/mL | [51] | |
| a,c Antifungal | Nerol |  | A. niger Aspergillus ochraceus Aspergillus flavus | 300 µg/mL 300 µg/mL 200 µg/mL | 300 µg/mL 500 µg/mL 200 µg/mL | [62] |
| Thyme | 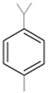 p-cymene  Thymol 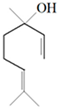 Linalool 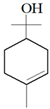 α-terpineol 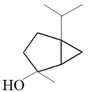 Sabinene hydrate | A. alternata—air-borne C. globosum—air-borne | 250 μg/mL 250 μg/mL | 0.05% (w/v) 0.05% (w/v) | [57] | |
| d Antiviral | Thymol | 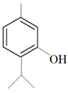 | Herpes simplex virus type 1 | 7 µM | [63] | |
| Carvacrol | 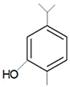 | HSV-1 | 7 µM | [63] | ||
| Farnesol |  | HSV-1 | 3.5 µg/mL | [64] | ||
| Eugenol | 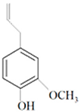 | HSV-1 | 35 µg/mL | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules 2021, 26, 628. https://doi.org/10.3390/molecules26030628
Aljaafari MN, AlAli AO, Baqais L, Alqubaisy M, AlAli M, Molouki A, Ong-Abdullah J, Abushelaibi A, Lai K-S, Lim S-HE. An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules. 2021; 26(3):628. https://doi.org/10.3390/molecules26030628
Chicago/Turabian StyleAljaafari, Mariam Nasser, Asma Obaid AlAli, Laila Baqais, Maream Alqubaisy, Mudhi AlAli, Aidin Molouki, Janna Ong-Abdullah, Aisha Abushelaibi, Kok-Song Lai, and Swee-Hua Erin Lim. 2021. "An Overview of the Potential Therapeutic Applications of Essential Oils" Molecules 26, no. 3: 628. https://doi.org/10.3390/molecules26030628
APA StyleAljaafari, M. N., AlAli, A. O., Baqais, L., Alqubaisy, M., AlAli, M., Molouki, A., Ong-Abdullah, J., Abushelaibi, A., Lai, K.-S., & Lim, S.-H. E. (2021). An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules, 26(3), 628. https://doi.org/10.3390/molecules26030628






