Kinetic Modeling and Degradation Study of Liquid Polysulfide Resin-Clay Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
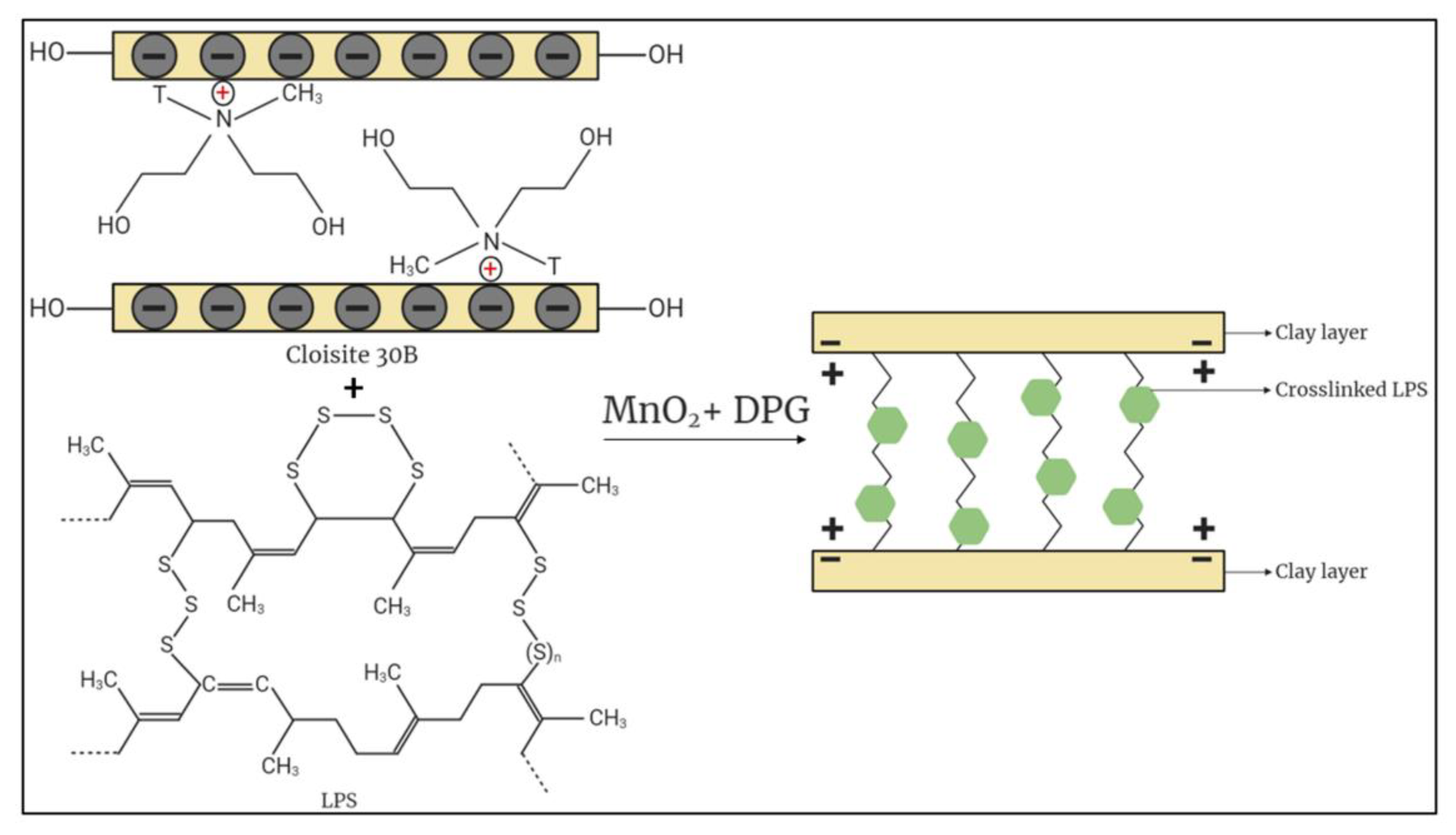
2.2. Fabrication of LPS/CNPs Nanocomposite
2.3. Characterization
2.4. Kinetic Analysis Techniques
3. Results and Discussion
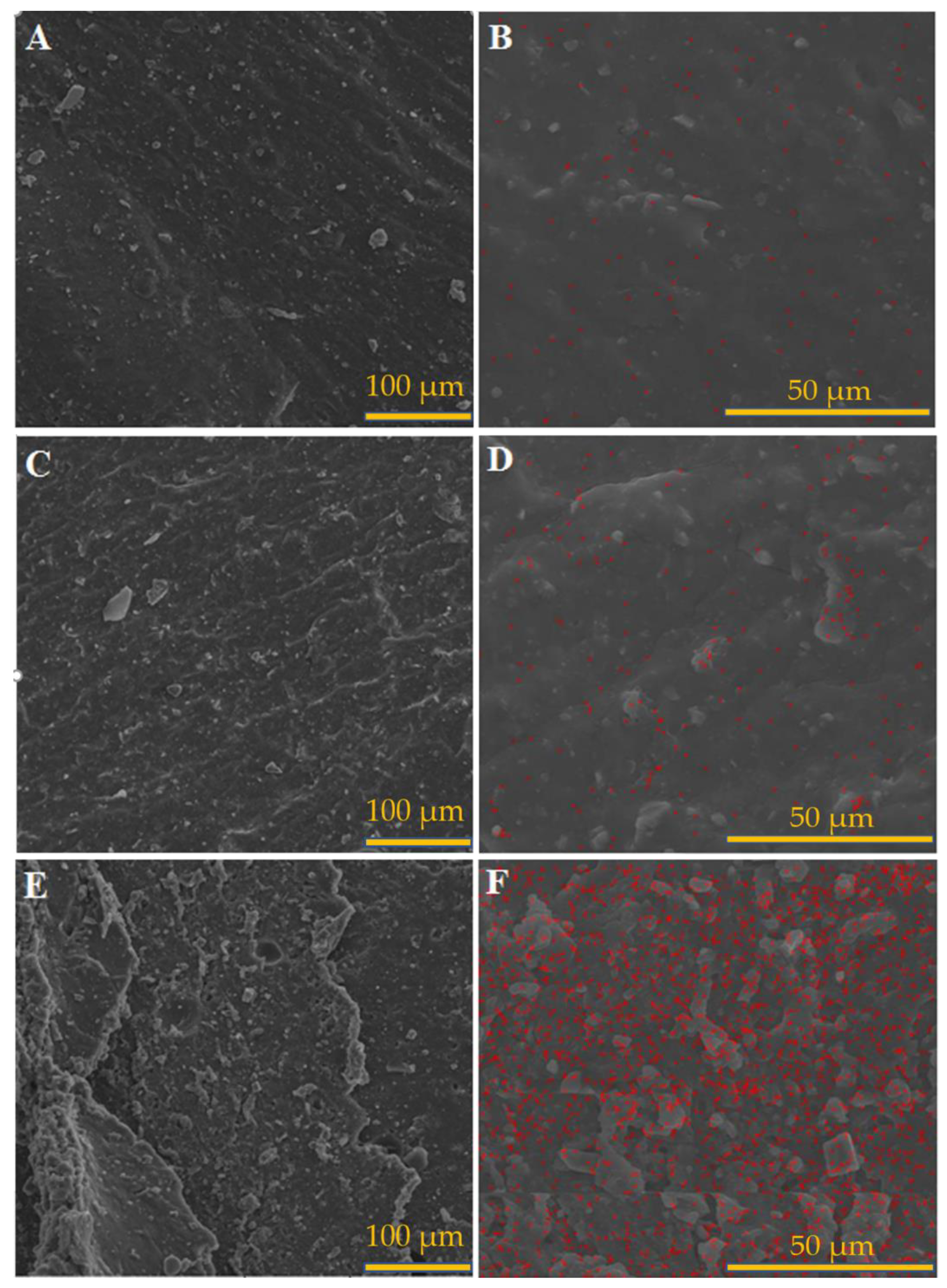
3.1. Morphological Assessment
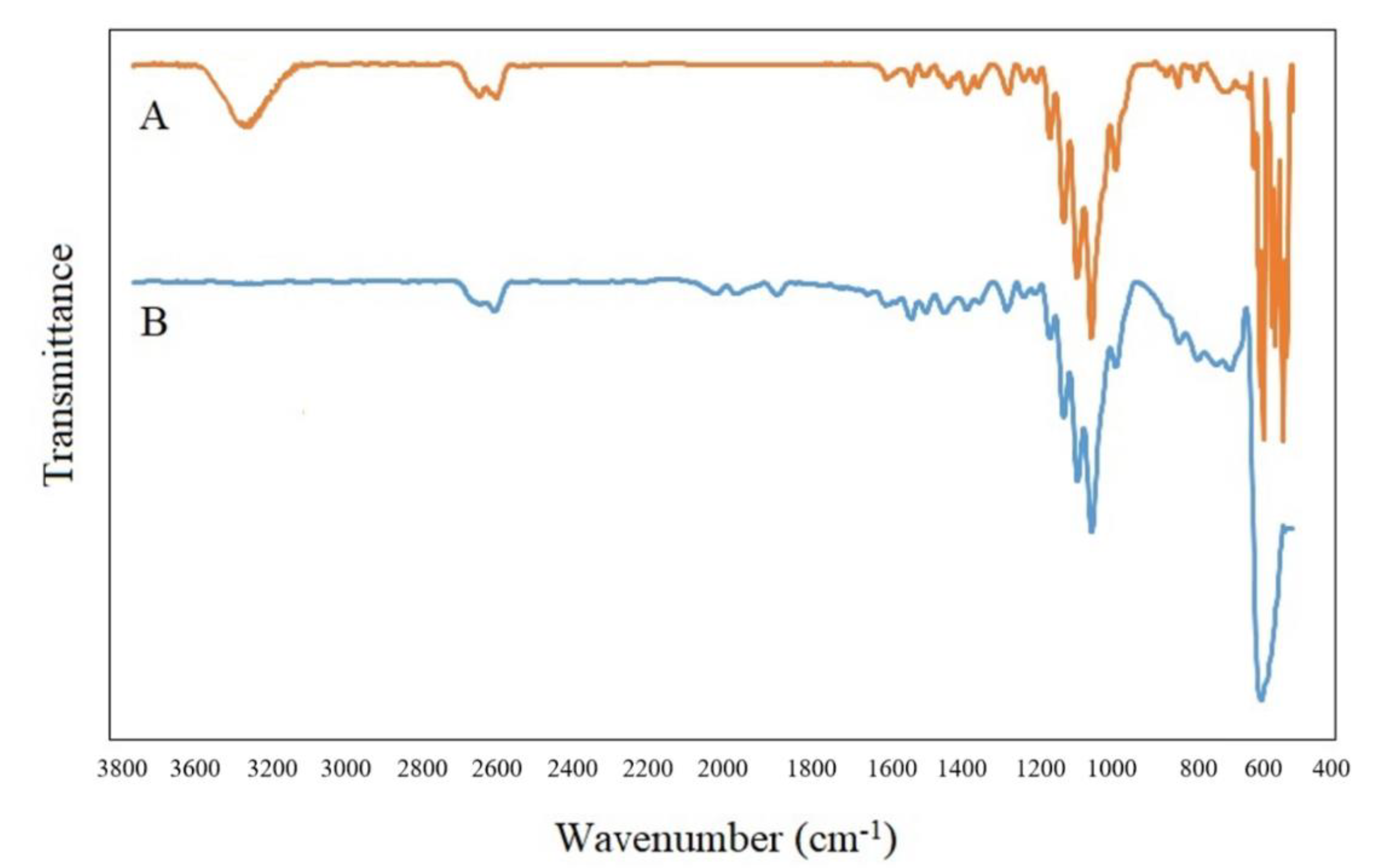
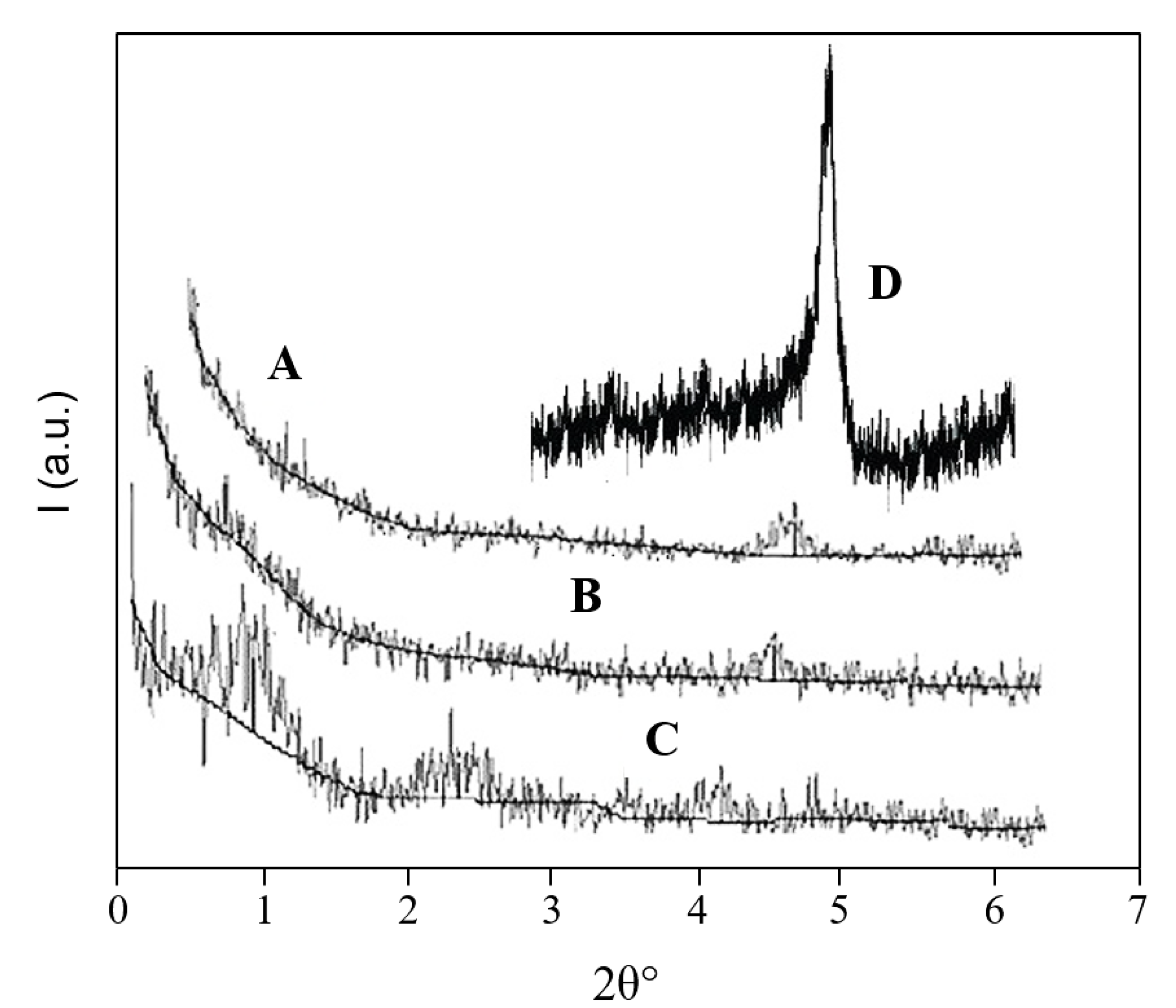
3.2. Structural Characterization
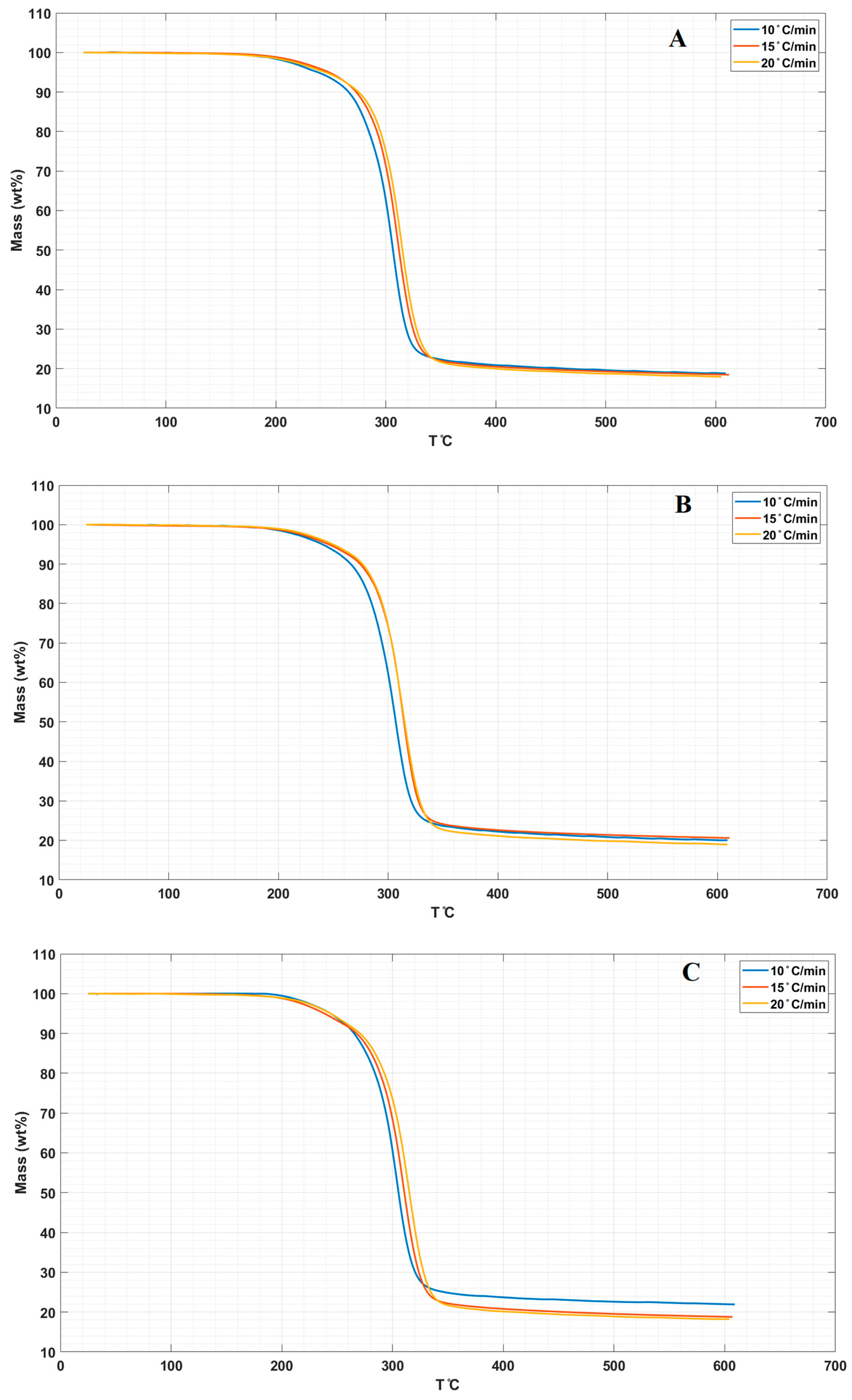
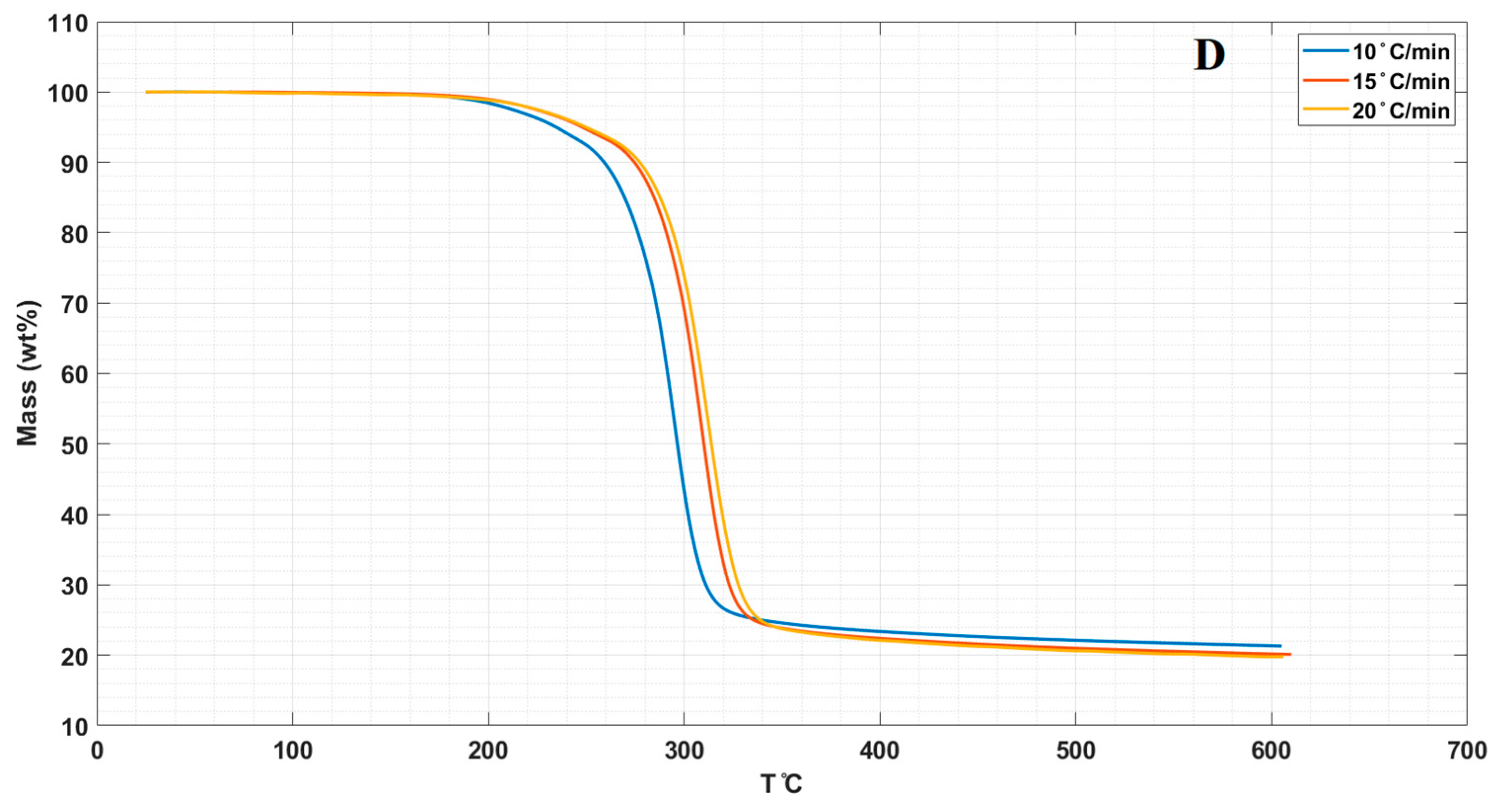
3.3. Thermal Properties
3.4. Determination of Degradation Kinetic Parameters
4. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Reid, E.E. Organic Chemistry of Bivalent Sulfur; Chemical Publishing Company: New York, NY, USA, 1958; Volume 1. [Google Scholar]
- Bertozzi, E.R. Chemistry and technology of elastomeric polysulfide polymers. Rubber Chem. Technol. 1968, 41, 114–160. [Google Scholar] [CrossRef]
- Fettes, E.; Jorczak, J. Polysulfide polymers. Ind. Eng. Chem. 1950, 42, 2217–2223. [Google Scholar] [CrossRef]
- McKeen, L.W. The Effect of UV Light and Weather on Plastics and Elastomers; William Andrew: Norwich, NY, USA, 2019. [Google Scholar]
- Kalaee, M.; Abdouss, M.; Shakiba, M. Synthesis and characterization of aliphatic polysulfides by interfacial polycondensation of sodium tetrasulfide with dichloroalkanes in the presence of quaternary onium salts. J. Sulfur Chem. 2020, 1–11. [Google Scholar] [CrossRef]
- Sheydaei, M.; Talebi, S.; Salami-Kalajahi, M. Synthesis of ethylene dichloride-based polysulfide polymers: Investigation of polymerization yield and effect of sulfur content on solubility and flexibility. J. Sulfur Chem. 2020, 1–16. [Google Scholar] [CrossRef]
- Guchhait, P.; Rahaman, M.; Nayak, L.; Chaki, T.K. Preparation and characterization of polyurethane/polysulfide miscible blend nanocomposites: Effect of nanoclay reinforcement on morphology, mechanical and thermal properties. Int. Polym. Process. 2012, 27, 461–468. [Google Scholar] [CrossRef]
- Pirayesh, A.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Najafi, F. Polysulfide polymers: Synthesis, blending, nanocomposites, and applications. Polym. Rev. 2019, 59, 124–148. [Google Scholar] [CrossRef]
- Amangah, M.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Nanoconfinement effect of graphene on thermophysical properties and crystallinity of matrix-grafted graphene/crosslinked polysulfide polymer nanocomposites. Diam. Relat. Mater. 2018, 83, 177–183. [Google Scholar] [CrossRef]
- Moqadam, S.; Kalajahi, M.S.; Mahdavian, M. Synthesis and characterization of sunflower oil-based polysulfide polymer/cloisite 30B nanocomposites. Iran. J. Chem. Chem. Eng. 2018, 37, 185–192. [Google Scholar]
- Guchhait, P.K.; Bhandari, S.; Singh, S.; Rahaman, M. Study on the effect of nanosilica particles on morphology, thermo-mechanical and electrical properties of liquid polysulfide modified epoxy hybrid nanocomposites. Int. J. Plast. Technol. 2011, 15, 150–162. [Google Scholar] [CrossRef]
- Syao, O.; Malysheva, G. Properties and application of rubber-based sealants. Polym. Sci. Ser. D 2014, 7, 222–227. [Google Scholar] [CrossRef]
- Pirayesh, A.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Dehghani, E. Investigation of thermophysical and adhesion/mechanical properties of amine-cured epoxidized polysulfide polymer/epoxidized graphene nanocomposites. Prog. Org. Coat. 2019, 131, 211–218. [Google Scholar] [CrossRef]
- Kariminejad, B.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Thermophysical behaviour of matrix-grafted graphene/poly(ethylene tetrasulphide) nanocomposites. RSC Adv. 2015, 5, 100369–100377. [Google Scholar] [CrossRef]
- Sheydaei, M.; Alinia-Ahandani, E. Synthesis and characterization of methylene-xylene-based polysulfide block-copolymer/carbon nanotube nanocomposites via in situ polymerization method. J. Sulfur Chem. 2020, 41, 421–434. [Google Scholar] [CrossRef]
- Sheydaei, M.; Edraki, M.; Alinia-Ahandani, E.; Rufchahi, E.O.M.; Ghiasvandnia, P. Poly (p-xylene disulfide) and poly(p-xylene tetrasulfide): Synthesis, cure and investigation of mechanical and thermophysical properties. J. Macromol. Sci. Part A 2020, 1–7. [Google Scholar] [CrossRef]
- Pradhan, S.; Guchhait, P.K.; Kumar, K.D.; Bhowmick, A.K. Influence of nanoclay on the adhesive and physico-mechanical properties of liquid polysulfide elastomer. J. Adhes. Sci. Technol. 2009, 23, 2013–2029. [Google Scholar] [CrossRef]
- Thanki, J.D.; Parsania, P.H. Dynamic DSC curing kinetics and thermogravimetric study of epoxy resin of 9, 9′-bis (4-hydroxyphenyl) anthrone-10. J. Therm. Anal. Calorim. 2017, 130, 2145–2156. [Google Scholar] [CrossRef]
- Gu, A.; Liang, G. Thermal degradation behaviour and kinetic analysis of epoxy/montmorillonite nanocomposites. Polym. Degrad. Stab. 2003, 80, 383–391. [Google Scholar] [CrossRef]
- Bhowmick, A.; Bhattacharya, M.; Mitra, S.; Kumar, K.D.; Maji, P.K.; Choudhury, A.; George, J.J.; Basak, G.C. Morphology–Property Relationship in Rubber-Based Nanocomposites: Some Recent Developments, in Advanced Rubber Composites; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–83. [Google Scholar]
- Guo, T.; Gu, L.; Zhang, Y.; Chen, H.; Jiang, B.; Zhao, H.; Jin, Y.; Xiao, H. Bioinspired self-assembled films of carboxymethyl cellulose–dopamine/montmorillonite. J. Mater. Chem. A 2019, 7, 14033–14041. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, X.; Chu, J.; Li, Z.; Zhang, Q.; Wang, R.; Wu, S. Synthesize ordered macroporous MnO2 and their application as curing agent for polysulfide polymers. J. Nanoparticle Res. 2019, 21, 58. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Neisiany, R.E.; Khorasani, S.N.; Dinari, M.; Ataei, S.; Koochaki, M.S.; Ramakrishna, S. Development of an epoxy self-healing coating through the incorporation of acrylic acid-co-acrylamide copolymeric gel. Prog. Org. Coat. 2020, 149, 105948. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Khorasani, S.N.; Kichi, M.K.; Dinari, M.; Ataei, S.; Enayati, M.H.; Koochaki, M.S.; Neisiany, R.E. Synthesis and characterization of TiO2/acrylic acid-co-2-acrylamido-2-methyl propane sulfonic acid nanogel composite and investigation its self-healing performance in the epoxy coatings. Colloid Polym. Sci. 2020, 298, 213–223. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kissinger method in kinetics of materials: Things to beware and be aware of. Molecules 2020, 25, 2813. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Dowdy, D. Meaningful activation energies for complex systems II: Evaluation of the Friedman method when applied to multiple reactions, and comparison with the Ozawa-Flynn-Wall method. J. Therm. Anal. Calorim. 1987, 32, 1177–1187. [Google Scholar] [CrossRef]
- Alvarado Flores, J.J.; Rutiaga-Quiñones, J.G.; Rodríguez, M.L.Á.; Alcaraz-Vera, J.V.; Espino, J.; Guevara-Martínez, S.J.; Montesino, F.M.; Rosas, A.A. Thermal degradation kinetics and FT-IR analysis on the pyrolysis of pinus pseudostrobus, pinus leiophylla and pinus montezumae as forest waste in Western Mexico. Energies 2020, 13, 969. [Google Scholar] [CrossRef]
- Khosravi, F.; Khorasani, S.N.; Ghomi, E.R.; Kichi, M.K.; Zilouei, H.; Farhadian, M.; Neisiany, R.E. A bilayer GO/nanofibrous biocomposite coating to enhance 316L stainless steel corrosion performance. Mater. Res. Express 2019, 6, 086470. [Google Scholar] [CrossRef]
- Owonubi, S.J.; Malima, N.M.; Revaprasadu, N. Metal Oxide–Based Nanocomposites as Antimicrobial and Biomedical Agents. In Antibiotic Materials in Healthcare; Elsevier: Amsterdam, The Netherlands, 2020; pp. 287–323. [Google Scholar]
- Rezvani Ghomi, E.; Khosravi, F.; Mossayebi, Z.; Ardahaei, A.S.; Dehaghi, F.M.; Khorasani, M.; Neisiany, R.E.; Das, O.; Marani, A.; Mensah, R.A.; et al. The flame retardancy of polyethylene composites: From fundamental concepts to nanocomposites. Molecules 2020, 25, 5157. [Google Scholar] [CrossRef]
- Hatami, L.; Haddadi-Asl, V.; Roghani-Mamaqani, H.; Ahmadian-Alam, L.; Salami-Kalajahi, M. Synthesis and characterization of poly (styrene-co-butyl acrylate)/clay nanocomposite latexes in miniemulsion by AGET ATRP. Polym. Compos. 2011, 32, 967–975. [Google Scholar] [CrossRef]
- Khezri, K.; Haddadi-Asl, V.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Synthesis and characterization of exfoliated poly (styrene-co-methyl methacrylate) nanocomposite via miniemulsion atom transfer radical polymerization: an activators generated by electron transfer approach. Polym. Compos. 2011, 32, 1979–1987. [Google Scholar] [CrossRef]
- Ali, A.; Hakim, S.; Nekoomanesh, M. Effect of NH3/methylaluminoxane/dodecylamine modifiednanoclay on morphology and properties of polyethylene/clay nanocomposites prepared by in-situ polymerization. Polyolefins J. 2018, 5, 125–139. [Google Scholar]



| Appearance | Content SH (%) | Viscosity (pa.s) | Average Molecular Weight (g/mol) | Sulfur Content (%) |
|---|---|---|---|---|
| Brown liquid | 5–7 | 1.3 | 1100 | 37–38 |
| Samples | LPS (g) | DPG (g) | MnO2 (g) | CNP (g) |
|---|---|---|---|---|
| LPS | 20 | 0.2 | 3 | 0 |
| LPS/CNPs 1% | 20 | 0.2 | 3 | 0.2 |
| LPS/CNPs 3% | 20 | 0.2 | 3 | 0.6 |
| LPS/CNPs 5% | 20 | 0.2 | 3 | 1 |
| 10 °C/min | |||
|---|---|---|---|
| Samples | T0.1(°C) a | T0.5(°C) b | Tm (°C) c |
| LPS | 252 | 290 | 280 |
| LPS/CNPs 1% | 256.7 | 294.5 | 281 |
| LPS/CNPs 3% | 258.7 | 297.9 | 292 |
| LPS/CNPs 5% | 257 | 295 | 290 |
| 15 °C/min | |||
| Samples | T0.1(°C) a | T0.5(°C) b | Tm (°C) c |
| LPS | 258.5 | 300.5 | 300 |
| LPS/CNPs 1% | 264.9 | 302.4 | 302 |
| LPS/CNPs 3% | 267.9 | 301.3 | 300 |
| LPS/CNPs 5% | 265 | 300.5 | 297 |
| 20 °C/min | |||
| Samples | T0.1(°C) a | T0.5(°C) b | Tm (°C) c |
| LPS | 264.5 | 304.7 | 306 |
| LPS/CNPs 1% | 268.8 | 306.5 | 303 |
| LPS/CNPs 3% | 269.5 | 306.9 | 302 |
| LPS/CNPs 5% | 268.6 | 308.6 | 304 |
| R2 | Ea (Kj/mol) | Samples |
|---|---|---|
| 0.9712 | 119 | LPS |
| 0.9451 | 136 | LPS/CNPs 1% |
| 0.8888 | 206 | LPS/CNPs 3% |
| 0.9542 | 181 | LPS/CNPs 5% |
| R2 | Ea (Kj/mol) | Samples |
|---|---|---|
| 0.9859 | 105 | LPS |
| 0.9198 | 126 | LPS/CNPs 1% |
| 0.9859 | 186 | LPS/CNPs 3% |
| 0.9728 | 170 | LPS/CNPs 5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakiba, M.; Kakoei, A.; Jafari, I.; Rezvani Ghomi, E.; Kalaee, M.; Zarei, D.; Abdouss, M.; Shafiei-Navid, S.; Khosravi, F.; Ramakrishna, S. Kinetic Modeling and Degradation Study of Liquid Polysulfide Resin-Clay Nanocomposite. Molecules 2021, 26, 635. https://doi.org/10.3390/molecules26030635
Shakiba M, Kakoei A, Jafari I, Rezvani Ghomi E, Kalaee M, Zarei D, Abdouss M, Shafiei-Navid S, Khosravi F, Ramakrishna S. Kinetic Modeling and Degradation Study of Liquid Polysulfide Resin-Clay Nanocomposite. Molecules. 2021; 26(3):635. https://doi.org/10.3390/molecules26030635
Chicago/Turabian StyleShakiba, Mohamadreza, Arash Kakoei, Iman Jafari, Erfan Rezvani Ghomi, Mohammadreza Kalaee, Davood Zarei, Majid Abdouss, Saeid Shafiei-Navid, Fatemeh Khosravi, and Seeram Ramakrishna. 2021. "Kinetic Modeling and Degradation Study of Liquid Polysulfide Resin-Clay Nanocomposite" Molecules 26, no. 3: 635. https://doi.org/10.3390/molecules26030635
APA StyleShakiba, M., Kakoei, A., Jafari, I., Rezvani Ghomi, E., Kalaee, M., Zarei, D., Abdouss, M., Shafiei-Navid, S., Khosravi, F., & Ramakrishna, S. (2021). Kinetic Modeling and Degradation Study of Liquid Polysulfide Resin-Clay Nanocomposite. Molecules, 26(3), 635. https://doi.org/10.3390/molecules26030635









