Caralluma europaea (Guss.) N.E.Br.: Anti-Inflammatory, Antifungal, and Antibacterial Activities against Nosocomial Antibiotic-Resistant Microbes of Chemically Characterized Fractions
Abstract
1. Introduction
2. Results
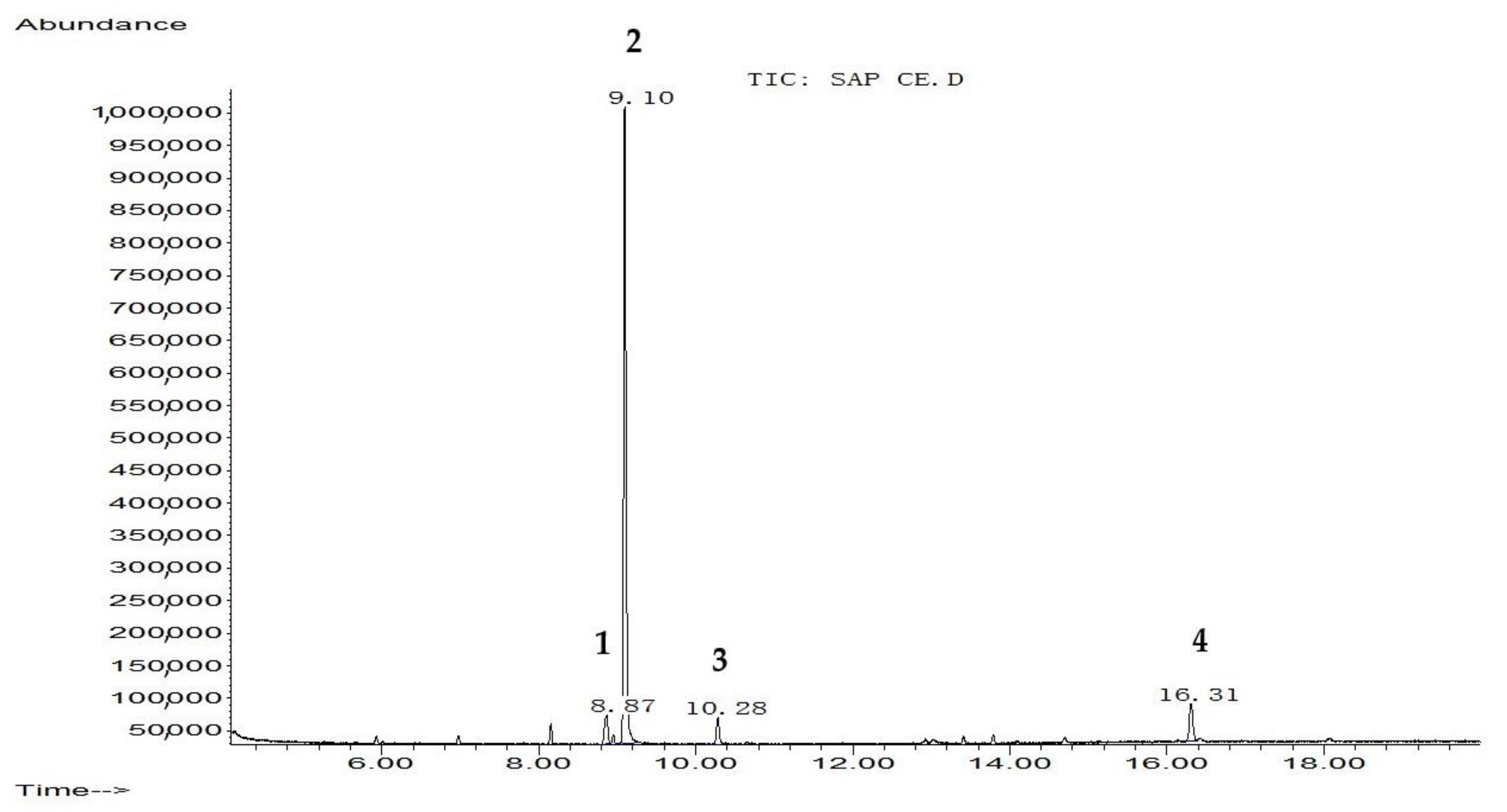
2.1. GC–MS Analysis
2.2. Anti-Inflammatory Activity
2.3. Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Solvents and Reagents
4.2. Plant Material
4.3. Animal Material
4.4. Extraction of Hydroethanol, n-Butanol, and Polyphenol-Rich Fractions from C. europaea
4.4.1. Preparation of Hydroethanol and n-Butanol Fractions
4.4.2. Preparation of the Polyphenol-Rich Fraction
4.5. GC-MS Analysis
4.6. Anti-Inflammatory Activity
- Group 1: negative control (0.9% NaCl)
- Group 2: positive control (10 mg/Kg of indomethacin)
- Group 3: hydroethanol fraction (100 mg/Kg)
- Group 4: polyphenol-rich fraction (50 mg/Kg)
- Group 5: n-butanol fraction (100 mg/Kg)
4.7. Antibacterial Activity
4.7.1. Growing Media
4.7.2. Bacterial and Yeast Strains
4.7.3. Inoculum Standardization
4.7.4. Disc Diffusion Method
4.7.5. Determination of the Minimum Inhibitory Concentration (MIC)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yang, M.; Wu, Z.; Wang, Y.; Kai, G.; Njateng, G.S.; Cai, S.; Cao, J.; Cheng, G. Acute and subacute toxicity evaluation of ethanol extract from aerial parts of Epigynum auritum in mice. Food Chem. Toxicol. 2019, 131, 110534. [Google Scholar] [CrossRef] [PubMed]
- Ezeja, M.I.; Anaga, A.O.; Asuzu, I.U. Acute and sub-chronic toxicity profile of methanol leaf extract of Gouania longipetala in rats. J. Ethnopharmacol. 2014, 151, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Matu, E.N.; van Staden, J. Antibacterial and anti-inflammatory activities of some plants used for medicinal purposes in Kenya. J. Ethnopharmacol. 2003, 87, 35–41. [Google Scholar] [CrossRef]
- Parsonnet, J. Bacterial infection as a cause of cancer. Environ. Health Perspect. 1995, 103 (Suppl. 8), 263–268. [Google Scholar]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Manouze, H.; Bouchatta, O.; Gadhi, A.C.; Bennis, M.; Sokar, Z.; Ba-M’hamed, S. Anti-inflammatory, Antinociceptive, and Antioxidant Activities of Methanol and Aqueous Extracts of Anacyclus pyrethrum Roots. Front Pharmacol. 2017, 8, 598. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules [Internet]. 2016. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6273146/ (accessed on 12 November 2020).
- Yu, Z.; Tang, J.; Khare, T.; Kumar, V. The alarming antimicrobial resistance in ESKAPEE pathogens: Can essential oils come to the rescue? Fitoterapia 2020, 140, 104433. [Google Scholar] [CrossRef]
- Mapara, N.; Sharma, M.; Shriram, V.; Bharadwaj, R.; Mohite, K.C.; Kumar, V. Antimicrobial potentials of Helicteres isora silver nanoparticles against extensively drug-resistant (XDR) clinical isolates of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2015, 99, 10655–10667. [Google Scholar] [CrossRef]
- Aboualigalehdari, E.; Ghafourian, S.; Sadeghifard, N.; Sekawi, Z. Is Candida albicans a cause of nosocomial infection in Iran? Rev. Med Microbiol. 2013, 24, 85–88. [Google Scholar] [CrossRef]
- Maenza, J.R.; Merz, W.G.; Romagnoli, M.J.; Keruly, J.C.; Moore, R.D.; Gallant, J.E. Infection due to fluconazole-resistant Candida in patients with AIDS: Prevalence and microbiology. Clin. Infect. Dis. 1997, 24, 28–34. [Google Scholar] [CrossRef]
- Dra, L.A.; Sellami, S.; Rais, H.; Aziz, F.; Aghraz, A.; Bekkouche, K.; Markouk, M.; Larhsini, M. Antidiabetic potential of Caralluma europaea against alloxan-induced diabetes in mice. Saudi J. Biol. Sci. 2019, 26, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Jan, S.; Mussarat, S.; Tariq, A.; Begum, S.; Afroz, A.; Shinwari, Z.K. A review on ethnobotany, phytochemistry and pharmacology of plant genus Caralluma, R. Br. J. Pharm. Pharmacol. 2014, 66, 1351–1368. [Google Scholar] [CrossRef] [PubMed]
- Bellakhdar, J. La Pharmacopée Marocaine Traditionnelle: Médecine Arabe Ancienne Et Savoirs Populaires-Saint-Etienne [Internet]; Persée-Portail des revues scientifiques en SHS; Ibis Press: Jerusalem, Israel, 1997; Volume 35, 759p, Available online: https://www.persee.fr/doc/horma_0984-2616_1998_num_35_1_1707_t1_0319_0000_1 (accessed on 4 December 2020).
- Amrati, F.; Bourhia, M.; Slighoua, M.; Ibnemoussa, S.; Bari, A.; Ullah, R.; Amaghnouje, A.; Di Cristo, F.; El Mzibri, M.; Calarco, A.; et al. Phytochemical Study on Antioxidant and Antiproliferative Activities of Moroccan Caralluma europaea Extract and Its Bioactive Compound Classes. Evidence-Based Complementary and Alternative Medicine [Internet]. 2020. Available online: https://www.hindawi.com/journals/ecam/2020/8409718/ (accessed on 25 April 2020).
- Hassan, H.S.; Sule, M.I.; Musa, A.M.; Musa, K.Y.; Abubakar, M.S.; Hassan, A.S. Anti-Inflammatory Activity of Crude Saponin Extracts from Five Nigerian Medicinal Plants. Afr. J. Tradit. Complement. Altern. Med. 2011, 9, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bansal, D.; Bajaj, K.; Sharma, S.; Srivastava, V.K. Synthesis of some newer derivatives of 2-amino benzoic acid as potent anti-inflammatory and analgesic agents. Bioorganic Med. Chem. 2003, 11, 5281–5291. [Google Scholar] [CrossRef]
- Mizushina, Y.; Miyazaki, S.; Ohta, K.; Hirota, M.; Sakaguchi, K. Novel anti-inflammatory compounds from Myrsine seguinii, terpeno-benzoic acids, are inhibitors of mammalian DNA polymerases. Biochim. Biophys. Acta (BBA) Gen. Subj. 2000, 1475, 1–4. [Google Scholar] [CrossRef]
- Yin, Y.; Gong, F.-Y.; Wu, X.-X.; Sun, Y.; Li, Y.-H.; Chen, T.; Xu, Q. Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. J. Ethnopharmacol. 2008, 120, 1–6. [Google Scholar] [CrossRef]
- Son, N.T. A Review on the Medicinal Plant Dalbergia odorifera Species: Phytochemistry and Biological Activity. Evid. Based Complement Altern. Med. 2017, 2017, 7142370. [Google Scholar]
- Tan, Y.-X.; Wang, H.-Q.; Chen, R.-Y. Anti-inflammatory and cytotoxic 2-arylbenzofurans from Morus wittiorum. Fitoterapia 2012, 83, 750–753. [Google Scholar] [CrossRef]
- Yin, Z.N.; Wu, W.J.; Sun, C.Z.; Liu, H.F.; Chen, W.B.; Zhan, Q.P.; Lei, Z.G.; Xuan, X.I.; Juan, J.; Kun, Y.A.; et al. Antioxidant and Anti-inflammatory Capacity of Ferulic Acid Released from Wheat Bran by Solid-state Fermentation of Aspergillus niger. Biomed. Environ. Sci. 2019, 32, 11–21. [Google Scholar]
- Lee, H.N.; Shin, S.A.; Choo, G.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Cho, S.D.; Nam, J.S.; Choi, C.S.; et al. Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB-induced atopic dermatitis animal models. Int. J. Mol. Med. 2018, 41, 888–898. [Google Scholar] [CrossRef]
- Cho, B.O.; Yin, H.H.; Park, S.H.; Byun, E.B.; Ha, H.Y.; Jang, S.I. Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of NF-κB and STAT1 activation and Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated RAW264.7 macrophages. Biosci. Biotechnol. Biochem. 2016, 80, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Kroes, B.H.; van den Berg, A.J.; Quarles van Ufford, H.C.; van Dijk, H.; Labadie, R.P. Anti-inflammatory activity of gallic acid. Planta Med. 1992, 58, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.N.M.; Islam, M.W.; Radhakrishnan, R.; Chen, H.B.; Kamil, M.; Al-Gifri, A.N.; Chan, K.; Al-Attas, A. Anti-nociceptive and anti-inflammatory properties of Caralluma arabica. J. Ethnopharmacol. 2001, 76, 155–158. [Google Scholar] [CrossRef]
- Ramesh, M.; Rao, Y.; Appa, R.A.V.N.; Prabhakar, M.C.; Seshagiri, R.C.; Muralidhar, N.; Reddy, B.M. Antinociceptive and anti-inflammatory activity of a flavonoid isolated from Caralluma attenuata. J. Ethnopharmacol. 1998, 62, 63–66. [Google Scholar] [CrossRef]
- Mokale, S.N.; Shinde, S.S.; Elgire, R.D.; Sangshetti, J.N.; Shinde, D.B. Synthesis and anti-inflammatory activity of some 3-(4,6-disubtituted-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl) propanoic acid derivatives. Bioorganic Med. Chem. Lett. 2010, 20, 4424–4426. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Tanigawa, N.; Sunghwa, F.; Ninomiya, M.; Hagiwara, M.; Matsushita, K.; Koketsu, M. Morroniside cinnamic acid conjugate as an anti-inflammatory agent. Bioorganic Med. Chem. Lett. 2010, 20, 4855–4857. [Google Scholar] [CrossRef]
- Godoy, M.E.; Rotelli, A.; Pelzer, L.; Tonn, C.E. Antiinflammatory Activity of Cinnamic Acid Esters. Molecules 2000, 5, 547–548. [Google Scholar] [CrossRef]
- Deba, F.; Xuan, T.D.; Yasuda, M.; Tawata, S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control. 2008, 19, 346–352. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.-J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Tian, F.; Li, B.; Ji, B.; Yang, J.; Zhang, G.; Chen, Y.; Luo, Y. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinensis:The polarity affects the bioactivities. Food Chem. 2009, 113, 173–179. [Google Scholar] [CrossRef]
- Longbottom, C.J.; Carson, C.F.; Hammer, K.A.; Mee, B.J.; Riley, T.V. Tolerance of Pseudomonas aeruginosa to Melaleuca alternifolia (tea tree) oil is associated with the outer membrane and energy-dependent cellular processes. J. Antimicrob. Chemother. 2004, 54, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, V.N.; Rajeswari, V.D. Preservatives in Beverages: Perception and Needs. In Preservatives and Preservation Approaches in Beverages [Internet]; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–30. Available online: http://www.sciencedirect.com/science/article/pii/B978012816685700001X (accessed on 24 June 2020).
- Cao, H.; Ke, T.; Liu, R.; Yu, J.; Dong, C.; Cheng, M.; Huang, J.; Liu, S. Identification of a Novel Proline-Rich Antimicrobial Peptide from Brassica napus. PLoS ONE 2015, 10, e0137414. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Miao, M.; Yue, T.; Zhang, Y.; Cui, L.; Wang, Z.; Yuan, Y. Antibacterial activity and mechanism of cinnamic acid and chlorogenic acid against Alicyclobacillus acidoterrestris vegetative cells in apple juice. Int. J. Food Sci. Technol. 2019, 54, 1697–1705. [Google Scholar] [CrossRef]
- Kolla, N.K.J.; Peddikotla, P.; Muvva, V.; Venkateswarlu, Y.E.; Krishna, P.S. Biological activity of phenylpropionic acid isolated from a terrestrial Streptomycetes. Polish J. Microbiol. 2007, 56, 191–197. [Google Scholar]
- Preethi, R.; Devanathan, V.V. Loganathan, M. Antimicrobial and Antioxidant Efficacy of Some Medicinal Plants Against Food Borne Pathogens. Adv. Biol. Res. 2010, 4, 122–125. [Google Scholar]
- Hassan, R.; El-Kadi, S.; Sand, M. Effect of some organic acids on fungal growth and their toxins production. Int. J. Adv. Biology. 2015, 2, 1–11. [Google Scholar] [CrossRef]
- Fadli, M.; Saad, A.; Sayadi, S.; Chevalier, J.; Mezrioui, N.-E.; Pagès, J.-M.; Hassani, L. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection–Bacteria and their synergistic potential with antibiotics. Phytomedicine 2012, 19, 464–471. [Google Scholar] [CrossRef]
- Okoh, O.O.; Sadimenko, A.P.; Afolayan, A.J. Comparative evaluation of the antibacterial activities of the essential oils of Rosmarinus officinalis L. obtained by hydrodistillation and solvent free microwave extraction methods. Food Chem. 2010, 120, 308–312. [Google Scholar] [CrossRef]
- Tepe, B.; Donmez, E.; Unlu, M.; Candan, F.; Daferera, D.; Vardar-Unlu, G.; Polissiou, M.; Sokmen, A. Antimicrobial and antioxidative activities of the essential oils and methanol extracts of Salvia cryptantha (Montbret et Aucher ex Benth.) and Salvia multicaulis (Vahl). Food Chem. 2004, 84, 519–525. [Google Scholar] [CrossRef]
- Dra, L.A.; Aghraz, A.; Boualy, B.; Oubaassine, S.; Barakate, M.; Markouk, M.; Larhsini, M. Chemical Characterization and In vitro Antimicrobial Activity of Caralluma europaea Essential Oil and Its Synergistic Potential with Conventional Antibiotics. J. Adv. Med Pharm. Sci. 2018, 19, 1–11. [Google Scholar] [CrossRef][Green Version]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.; von Wright, A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals [Internet], 8th ed.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 2011. Available online: http://www.ncbi.nlm.nih.gov/books/NBK54050/ (accessed on 9 May 2020).
- Ho, C.-T.; Chen, Q.; Shi, H.; Zhang, K.-Q.; Rosen, R.T. Antioxidative effect of polyphenol extract prepared from various Chinese teas. Prev. Med. 1992, 21, 520–525. [Google Scholar] [CrossRef]
- Kabran, G.R.M.; Mamyrbékova-Békro, J.A.; Pirat, J.-L.; Bekro, Y.-A.; Sommerer, N.; Verbaere, A.; Meudec, E. Identification de composés phénoliques extraits de deux plantes de la pharmacopée ivoirienne. J. Soc. Ouest Afr. Chim. 2014, 38, 57–63. [Google Scholar]
- Ayoola, G.; Akpanika, G.; Awobajo, F.; Osunkalu, V.; Coker, H.; Odugbemi, T. Anti-Inflammatory Properties of the Fruits of Allanblanckia floribunda oliv. (Guttiferae). Bot. Res. Int. 2009, 2, 21–26. [Google Scholar]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-Induced Edema in Hind Paw of the Rat as an Assay for Antiinflammatory Drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- da Silveira, S.M.; Luciano, F.B.; Fronza, N.; Cunha, A.; Scheuermann, G.N.; Vieira, C.R.W. Chemical composition and antibacterial activity of Laurus nobilis essential oil towards foodborne pathogens and its application in fresh Tuscan sausage stored at 7 °C. LWT Food Sci. Technol. 2014, 59, 86–93. [Google Scholar] [CrossRef]
- Atki, Y.E.; Aouam, I.; El kamari, F.; Taroq, A.; Gourch, A.; Lyoussi, B.; Abdellaoui, A. Antibacterial efficacy of Thymol, Carvacrol, Eugenol and Menthol as alternative agents to control the growth of nosocomial infection-bacteria. J. Pharm. Sci. Res. 2019, 11, 4. [Google Scholar]
- Furtado, G.; Medeiros, A. Single-disk diffusion testing (Kirby-Bauer) of susceptibility of Proteus mirabilis to Chloramphenicol: Significance of the intermediate category. J. Clin. Microbiol. 1980, 12, 550–553. [Google Scholar] [CrossRef] [PubMed]

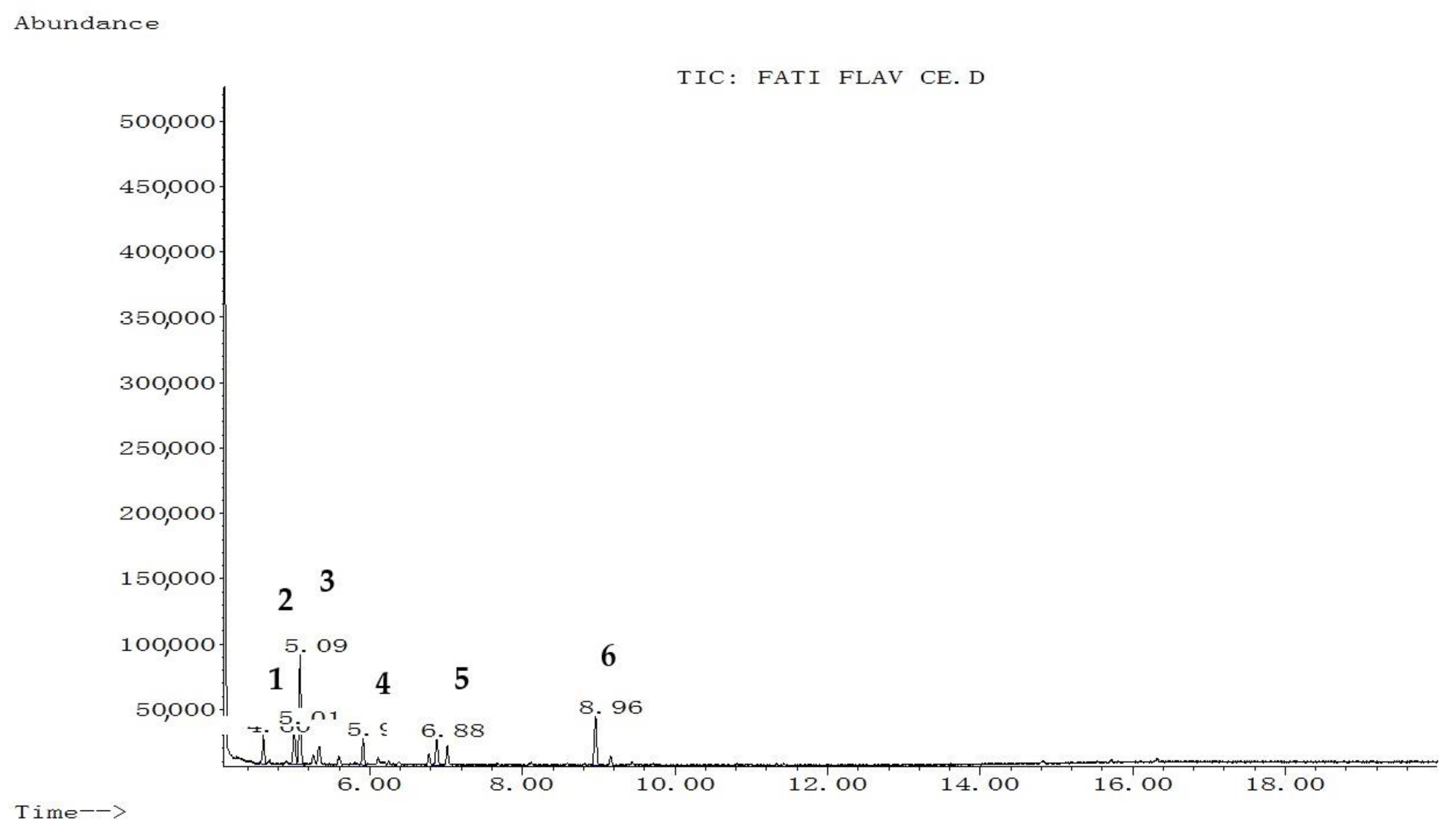
| Rank Peak No | Name of Compound | Molecular Weight (g/mol) | Structural Formula | % Area | RT (min) |
|---|---|---|---|---|---|
| 1 | Coumaran-3-one | 134.13 | C8H6O2 | 12.388 | 4.61 |
| 2 | 2-Phenylthiophene | 160 | C10H8S | 17.615 | 5.01 |
| 3 | Oxalic acid | 90.03 | C2H2O4 | 49.122 | 5.09 |
| 4 | Proline | 115.13 | C5H9NO2 | 12.718 | 5.91 |
| 5 | Propanoic acid, 2-[(trimethylsilyl)oxy]-, trimethylsilyl ester | 234 | C9H22O3Si2 | 8.157 | 7.02 |
| Rank Peak No | Name of Compound | Molecular Weight (g/mol) | Structural Formula | % Area | RT (min) |
|---|---|---|---|---|---|
| 1 | Benzoic acid, 4-methyl-2-trimethylsilyloxy-, trimethylsilyl ester | 296 | C14H24O3Si2 | 9.610 | 4.60 |
| 2 | Phenol4-(3,4-dihydro-2,2,4-trimethyl-2H-1-benzopyran-4-yl) | 268 | C18H20O2 | 14.013 | 5.01 |
| 3 | Cinnamic acid | 148.16 | C9H8O2 | 37.271 | 5.09 |
| 4 | Flavone, 2′-methoxy- | 252 | C16H12O3 | 9.297 | 5.9 |
| 5 | Butyric acid | 88.11 | C4H8O2 | 9.859 | 6.88 |
| 6 | p-coumaryl alcohol | 150.17 | C9H10O2 | 19.950 | 8.96 |
| Rank Peak No | Name of Compound | Molecular Weight (g/mol) | Structural Formula | % Area | RT (min) |
|---|---|---|---|---|---|
| 1 | Benzoic acid, 4-methyl-2-trimethylsilyloxy-, trimethylsilyl ester | 296 | C14H24O3Si2 | 5.536 | 8.87 |
| 2 | Elymoclavin | 254.33 | C16H18N2O | 83.829 | 9.10 |
| 3 | Benzenepropanoic acid, trimethylsilyl ester | 222 | C12H18O2Si | 3.758 | 10.28 |
| 4 | 5-Hydroxy-6-methoxy-2-methyl-3-phenylbenzofuran (isoparvifuran) | 254 | C16H14O3 | 6.877 | 16.31 |
| Treatment Groups | Basal Diameter (cm) | Paw Size after the Carrageenan Injection (Mean ± SEM)/Percent Inhibition of Edema | |||
|---|---|---|---|---|---|
| 3 Hour | 4 Hour | 5 Hour | 6 Hour | ||
| NaCl | 2.3 ± 0.01581 | 2.6 ± 0.04472 | 2.8 ± 0.0547 | 2.68 ± 0.0158 | 2.520 ± 0.0209 |
| Indomethacin® 10 mg/kg | 2.220 ± 0.03742 | 2.420 ± 0.03742 ** 33.33% | 2.362 ± 0.0348 * 71.60% | 2.300 ± 0.0273 78.95% | 2.266 ± 0.0331 79.09% |
| ET CE 100 mg/kg | 2.340 ± 0.02449 | 2.556 ± 0.0250 *** 28% | 2.534 ± 0.0271 *** 61.20% | 2.474 ± 0.0208 ** 64.74% | 2.398 ± 0.0217 73.64% |
| Poly CE 50 mg/kg | 2.315 ± 0.00866 | 2.523 ± 0.0062 *** 30.38% | 2.473 ± 0.0062 *** 68.5% | 2.410 ± 0.0070 *** 75% | 2.369 ± 0.0060 *** 75.68% |
| But CE 100 mg/kg | 2.350 ± 0.02887 | 2.555 ± 0.0295 *** 31.67% | 2.503 ± 0.0246 ** 69.50% | 2.440 ± 0.0291 76.32% | 2.401 ± 0.0282 76.70% |
| Fractions | Gram-Negative Bacteria | Gram-Positive Bacteria | ||
|---|---|---|---|---|
| Escherichia coli | Klebsiella pneumoniae | Pseudomonas aeruginosa | Staphylococcus aureus | |
| But CE | 6.25 | 25 | 6.25 | 6.25 |
| Poly CE | 6.25 | 12.5 | 12.5 | 3.125 |
| ET CE | 12.5 | 12.5 | 25 | 12.5 |
| STR | 0.25 | 0.003 | Resistant | 0.062 |
| AMP | Resistant | Resistant | Resistant | Resistant |
| Fractions | Gram-Negative Bacteria | Gram-Positive Bacteria | ||
|---|---|---|---|---|
| Escherichia coli | Klebsiella pneumoniae | Pseudomonas aeruginosa | Staphylococcus aureus | |
| But CE | 10 | 7 | Resistant | 9 |
| Poly CE | Resistant | Resistant | Resistant | 8 |
| ET CE | 9 | 12 | 10 | 12 |
| Streptomycine | Resistant | Resistant | Resistant | 9 |
| Ampicilline | Resistant | Resistant | Resistant | Resistant |
| Fractions | Candida albicans | Saccharomyce sereveseae |
|---|---|---|
| But CE | 25 | 25 |
| Poly CE | 50 | 50 |
| ET CE | 6.25 | 12.5 |
| Fluconazole | 0.4 | 0.2 |
| Fractions | Candida albicans | Saccharomyce sereveseae |
|---|---|---|
| But CE | Resistant | 14 |
| Poly CE | Resistant | Resistant |
| ET CE | 12 | Resistant |
| Fluconazole | 21 | 27 |
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amrati, F.E.-Z.; Bourhia, M.; Saghrouchni, H.; Slighoua, M.; Grafov, A.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Bari, A.; Ibenmoussa, S.; et al. Caralluma europaea (Guss.) N.E.Br.: Anti-Inflammatory, Antifungal, and Antibacterial Activities against Nosocomial Antibiotic-Resistant Microbes of Chemically Characterized Fractions. Molecules 2021, 26, 636. https://doi.org/10.3390/molecules26030636
Amrati FE-Z, Bourhia M, Saghrouchni H, Slighoua M, Grafov A, Ullah R, Ezzeldin E, Mostafa GA, Bari A, Ibenmoussa S, et al. Caralluma europaea (Guss.) N.E.Br.: Anti-Inflammatory, Antifungal, and Antibacterial Activities against Nosocomial Antibiotic-Resistant Microbes of Chemically Characterized Fractions. Molecules. 2021; 26(3):636. https://doi.org/10.3390/molecules26030636
Chicago/Turabian StyleAmrati, Fatima Ez-Zahra, Mohammed Bourhia, Hamza Saghrouchni, Meryem Slighoua, Andriy Grafov, Riaz Ullah, Essam Ezzeldin, Gamal A. Mostafa, Ahmed Bari, Samir Ibenmoussa, and et al. 2021. "Caralluma europaea (Guss.) N.E.Br.: Anti-Inflammatory, Antifungal, and Antibacterial Activities against Nosocomial Antibiotic-Resistant Microbes of Chemically Characterized Fractions" Molecules 26, no. 3: 636. https://doi.org/10.3390/molecules26030636
APA StyleAmrati, F. E.-Z., Bourhia, M., Saghrouchni, H., Slighoua, M., Grafov, A., Ullah, R., Ezzeldin, E., Mostafa, G. A., Bari, A., Ibenmoussa, S., & Bousta, D. (2021). Caralluma europaea (Guss.) N.E.Br.: Anti-Inflammatory, Antifungal, and Antibacterial Activities against Nosocomial Antibiotic-Resistant Microbes of Chemically Characterized Fractions. Molecules, 26(3), 636. https://doi.org/10.3390/molecules26030636






