Abstract
Reaction of 2,2′-bipyridine (2,2′-bipy) or 1,10-phenantroline (phen) with [Mn(Piv)2(EtOH)]n led to the formation of binuclear complexes [Mn2(Piv)4L2] (L = 2,2′-bipy (1), phen (2); Piv− is the anion of pivalic acid). Oxidation of 1 or 2 by air oxygen resulted in the formation of tetranuclear MnII/III complexes [Mn4O2(Piv)6L2] (L = 2,2′-bipy (3), phen (4)). The hexanuclear complex [Mn6(OH)2(Piv)10(pym)4] (5) was formed in the reaction of [Mn(Piv)2(EtOH)]n with pyrimidine (pym), while oxidation of 5 produced the coordination polymer [Mn6O2(Piv)10(pym)2]n (6). Use of pyrazine (pz) instead of pyrimidine led to the 2D-coordination polymer [Mn4(OH)(Piv)7(µ2-pz)2]n (7). Interaction of [Mn(Piv)2(EtOH)]n with FeCl3 resulted in the formation of the hexanuclear complex [MnII4FeIII2O2(Piv)10(MeCN)2(HPiv)2] (8). The reactions of [MnFe2O(OAc)6(H2O)3] with 4,4′-bipyridine (4,4′-bipy) or trans-1,2-(4-pyridyl)ethylene (bpe) led to the formation of 1D-polymers [MnFe2O(OAc)6L2]n·2nDMF, where L = 4,4′-bipy (9·2DMF), bpe (10·2DMF) and [MnFe2O(OAc)6(bpe)(DMF)]n·3.5nDMF (11·3.5DMF). All complexes were characterized by single-crystal X-ray diffraction. Desolvation of 11·3.5DMF led to a collapse of the porous crystal lattice that was confirmed by PXRD and N2 sorption measurements, while alcohol adsorption led to porous structure restoration. Weak antiferromagnetic exchange was found in the case of binuclear MnII complexes (JMn-Mn = −1.03 cm−1 for 1 and 2). According to magnetic data analysis (JMn-Mn = −(2.69 ÷ 0.42) cm−1) and DFT calculations (JMn-Mn = −(6.9 ÷ 0.9) cm−1) weak antiferromagnetic coupling between MnII ions also occurred in the tetranuclear {Mn4(OH)(Piv)7} unit of the 2D polymer 7. In contrast, strong antiferromagnetic coupling was found in oxo-bridged trinuclear fragment {MnFe2O(OAc)6} in 11·3.5DMF (JFe-Fe = −57.8 cm−1, JFe-Mn = −20.12 cm−1).
1. Introduction
Polynuclear coordination compounds of transition metals are widely used as catalysts in various reactions [1,2,3,4,5], as starting materials for the preparation of nanosized oxides or metals [6,7,8], as well as the basis for the creation of new magnetic materials [9,10,11,12,13] and coordination polymers with remarkable properties [14,15,16,17,18,19,20]. In the majority of these applications the stability or reactivity of the polynuclear core play an important role. In many cases polynuclear complexes undergo rearrangement or dissociation in solution because of instability in solvent or upon interaction with the “additional” ligands [21,22,23,24,25,26,27], and the resulting compounds may contain a metal core different from the one existing in the starting complex. Such rearrangement reactions lead to the formation of certain polynuclear cores, which are most stable under the reaction conditions. For example, binuclear cores M2(O2CR)4 (MII = Cu, Zn, Co, Ni) [28], trinuclear cores FeIII3 or FeIII2MII (M = Ni, Co, Mn) [29,30,31,32], MII2LnIII (MII = Zn, Co, Ni; Ln is lanthanide) [33,34,35] often form due to their exceptional stability in various media. However, the result of such rearrangements is not always predictable, since many factors may influence the reaction pathway.
In this work manganese complexes were chosen as the objects of research due to some specific features of this ion, which are manifested in the reactivity of its polynuclear complexes [25,26]. The reasons for such differences include the wide range of stable oxidation states of manganese with low energy barriers for redox-transformations [36,37], as well as, in the case of MnII, the relatively high kinetic lability of this ion [36]. The additional reasons for interest to homo- and heterometallic manganese complexes is that such species have found applications as building blocks for the synthesis of single-molecule magnets [9,15,38] or various coordination polymers capable of absorbing guest molecules [39,40]. It is also known that molecular manganese compounds can oligomerize upon crystallization during solvent changes, giving rise to complexes with higher nuclearity [9,26,26].
The aim of this study was to reveal the stability limits of MnII homometallic (pivalate) or heterometallic (Fe2Mn acetate) complexes in reactions with N-donor ligands (Scheme 1) and to compare the reactivity of MnII species with the reactivity of NiII and CoII analogues. An additional aim of the study was to see the influence of the structure of new compounds on their ability to uptake guest molecules or on the magnetic exchange interactions in the polymetallic cores.
Scheme 1.
Formulae of pivatale (Piv−) and N-donor ligands used in the study.
In this study we used pivalate manganese(II) polymer [Mn(Piv)2(EtOH)]n and trinuclear manganese(II)-iron(III) oxoacetate [MnFe2O(OAc)6(H2O)3]n (Piv− is the anion of pivalic acid) as starting compounds. Reactions of [Mn(Piv)2(EtOH)]n with both chelating (2,2′-bipyridine (2,2′-bipy) and 1,10-phenantroline (phen)) and non-chelating bridging (pyrimidine (pym), pyrazine (pz)) N-donor ligands in the absence/presence of oxygen as oxidant were investigated. In contrast to NiII and CoII, the reaction of MnII pivalate with FeCl3 led to formation of a compound with a Mn4Fe2O2(Piv)10 core. Reaction of the same MnII pivalate with a N-donor ligand led to the assembly of various polynuclear cores, while reaction of FeIII2MnII acetate with N-donors of the pyridine type led to the formation of new complexes where the trinuclear FeIII2MnII core was preserved. The crystal and molecular structures of eleven new compounds were determined—[Mn2(Piv)4L2] (L = 2,2′-bipy (1), phen (2)), [Mn4O2(Piv)6L2]·MeCN (L = 2,2′-bipy (3·MeCN), phen (4·0.5MeCN)), [Mn6(OH)2(Piv)10(pym)4] (5), [Mn6O2(Piv)10(pym)2]n (6), [Mn4(OH)(Piv)7(pz)2]n∙2nMeCN (7∙2MeCN), [Mn4Fe2O2(Piv)10(MeCN)2(HPiv)2]·2MeCN (8·2MeCN), [MnFe2O(OAc)6L2]n·2nDMF (L = 4,4′-bipy (9·2DMF), bpe (10·2DMF), [MnFe2O(OAc)6(bpe)(DMF)]n·4nDMF (11·3.5DMF). Sorption of alcohols by porous coordination polymers, as well as the magnetic properties of several polynuclear Mn-containing complexes were studied.
2. Results and Discussion
2.1. Synthesis
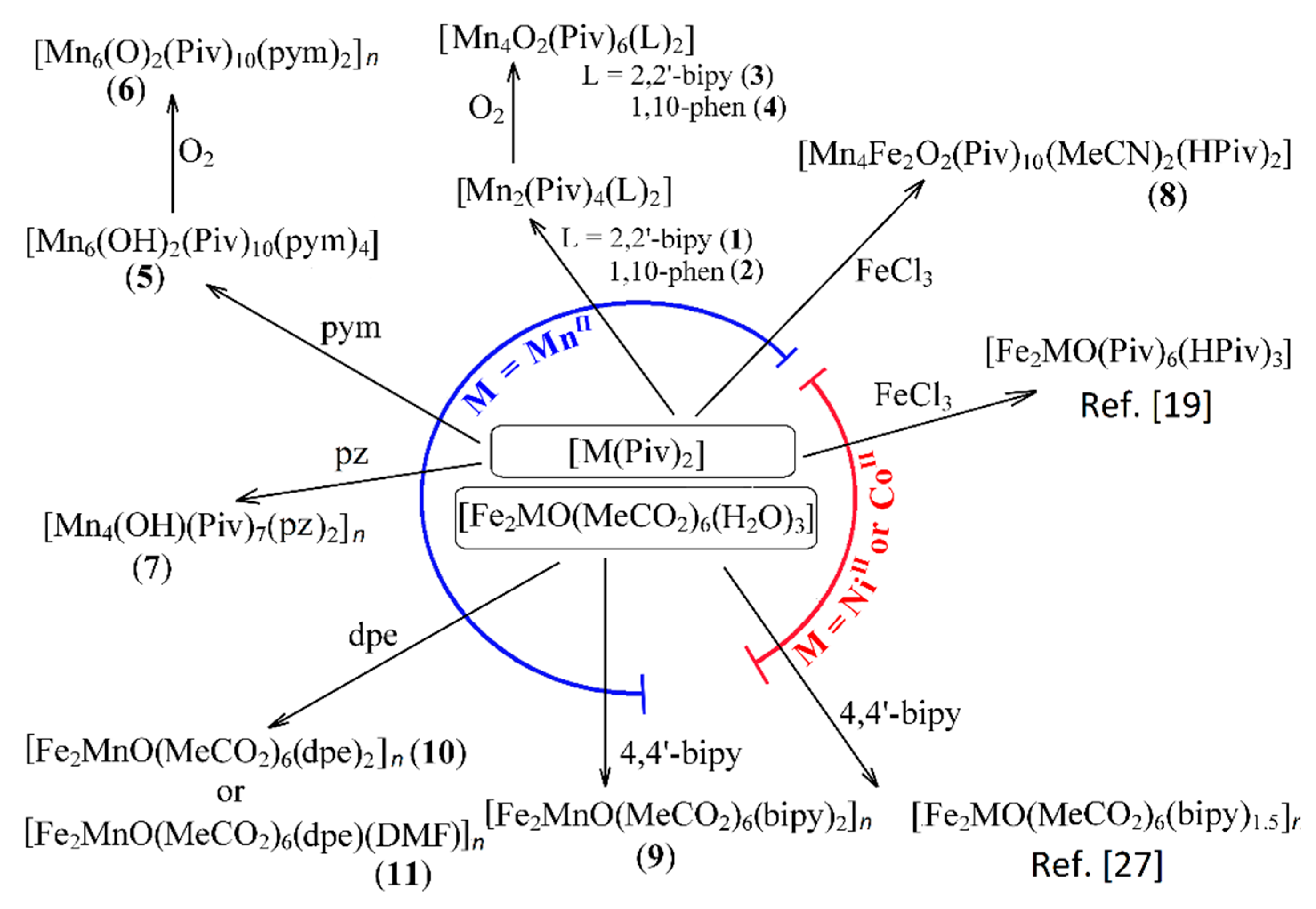
The reaction of [Mn(Piv)2(EtOH)]n and 2,2′-bipyridine (2,2′-bipy) or 1,10-phenantroline (phen) in MeCN under an argon atmosphere led to formation of molecular complexes [Mn2II(Piv)4L2] (L = 2,2′-bipy (1), phen (2), Figure 1). Oxidation of these complexes or the initial reaction system resulted in rearrangement of the dinuclear molecules to tetranuclear complexes [Mn2IIMn2IIIO2(Piv)6L2] (L = 2,2′-bipy (3), phen (4)).
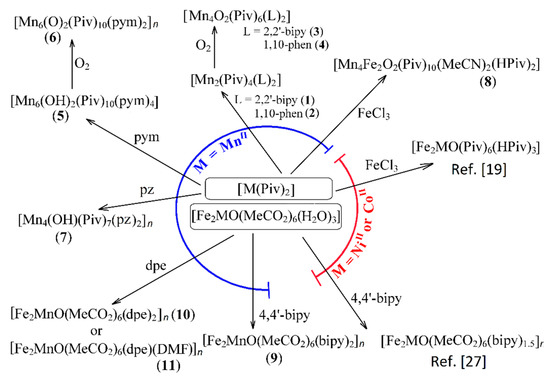
Figure 1.
Scheme illustrating the formation of complexes 1–11. 2,2′-bipy is 2,2′-bipyridine, 4,4′-bipy is 4,4′-bipyridine, bpe is 1,2-bis-trans-(4-pyridyl)ethylene, pym = pyrimidine, pz = pyrazine. M(Piv)2 in the center of the scheme means [Mn(Piv)2(EtOH)]n or Ni(Piv)2(H2O)2 or [Co(Piv)2]n.
Binuclear complexes 1 and 2 are similar to reported binuclear complexes of other d-metals with +2 charge [41,42,43,44,45,46,47,48]. The complex 3 is known and was obtained earlier by reaction of [Mn(Piv)2(EtOH)]n and 2,2′-bipy in THF in air [49].
Reaction of [Mn(Piv)2(EtOH)]n and pyrimidine (pym) in MeCN under an argon atmosphere led to the molecular hexanuclear complex [Mn6(OH)2(Piv)10(pym)4] (5). On exposure to air in MeCN compound 5 was oxidized to give the 1D polymer [MnII4MnIII2(µ4-O)2(Piv)10(µ2-pym)(pym)]n (6).
Previously reported carboxylate complexes of transition metals ions formed polymeric compounds with pyrimidine [50], so the formation of the molecular complex 5 is uncommon. At the same time, the formation of the mixed valence hexanuclear fragment {MnII4MnIII2(µ4-O)2(Piv)10} is similar to that in 5 and quite typical, for example, oxidation of [Mn(Piv)2(EtOH)]n (the same starting compound as in formation of 5) by air led to [MnII4MnIII2(µ4-O)2(Piv)10(HPiv)(EtOH)3] [51]; a similar hexanuclear Mn6 core was found in polymeric compounds [40,52]. Introduction of pyrimidine ligands resulted in formation of new coordination polymers.
The use of pyrazine (pz) instead of pyrimidine in the reaction with [Mn(Piv)2(EtOH)]n in MeCN under an argon atmosphere led to the formation of the 2D-coordination polymer [Mn4(OH)(Piv)7(µ2-pz)2]n (7). This complex was stable in air and was not soluble in MeCN, and this reason probable precluding oxidation of MnII. Pyrazine is a quite typical bridging ligand in the chemistry of manganese, forming polymers with MnII carboxylates [53,54], as well as with the mixed-valence hexanuclear fragment {MnII4MnIII2(µ4-O)2(O2CR)10} [54,55,56]. From an analysis of literature, as well as from the results of this study it can be noted that the tetranuclear unit {MnII2MnIII2O2(O2CR)6} usually forms in the presence of chelating ligands, such as 2,2′-bipy and phen, while the hexanuclear unit {MnII4MnIII2O2(O2CR)10} is produced in reactions with monodentate N-donor ligands or in the absence of such ligands.
It is known that the MII ions (such as MnII, CoII or NiII) and FeIII ions quite typically form trinuclear acetates [Fe2MO(OAc)6(H2O)3] [30]. Earlier we reported that CoII and NiII pivalates with FeCl3·6H2O in acetonitrile gave similar trinuclear complexes [Fe2MO(Piv)6(HPiv)3] (M = Co, Ni) [19]. Unexpectedly, [Mn(Piv)2(EtOH)]n reacted with FeCl3·6H2O in MeCN under an argon atmosphere with formation of the hexanuclear complex [MnII4FeIII2O2(Piv)10(MeCN)3] (8). On the other hand, with an excess of pivalic acid in the reaction of FeCl3·6H2O, Mn(NO3)2·6H2O and KOH [MnIIFeIII2O(Piv)6(HPiv)3] is formed [57]. The trinuclear fragments {Fe2MnO(Piv)6} were also generated in situ in the synthesis of coordination polymers [58].
The synthesis of compounds 9–11 was based on substitution of coordinated water molecules in [Fe2MnO(OAc)6(H2O)3] by 4,4′-bipyridine (4,4′-bipy) or 1,2-trans-(4-pyridyl)ethene (bpe). In compounds 9 and 10 all vacancies in the coordination spheres of the metal ions are filled by the nitrogen atoms of 4,4′-bipy or bpe ligands, while 4,4′-bipy or bpe molecules act both as bridging and non-bridging (capping) ligands, as will be described in details in the X-ray structures description (vide infra). In compound 11 all bpe molecules link trinuclear blocks but only two of three possible “vacant” positions in the coordination spheres of metal ions are occupied by a pyridine group of bpe; the third position is filled by DMF.
Formation of coordination polymers 9–11 can be formally described as the generation of a 1D-chain of [MnFe2O(OAc)6(L)]n (L = 4,4′-bipy or bpe) and filling of the third positions by terminal 4,4′-bipy and [MnFe2O(OAc)6(4,4′-bipy)3]n (for 9), by bpe or DMF (for 10 and 11, respectively). Synthesis of compounds [M3O(RCO2)6(4,4′-bipy)3]0/+ which can potentially bind metal ions was reported earlier [59]. In contrast to the reaction of [Fe2MO(OAc)6(H2O)3] (M = Co, Ni) with 4,4′-bipy which led to destruction of the trinuclear blocks or to the formation of porous [MFe2O(OAc)6(4,4′-bipy)1.5]n coordination polymers [27], compound [Fe2MO(OAc)6(H2O)3] was stable under the same conditions and formed coordination polymers with a ratio of trinuclear block to bridging ligand equal to 1:1 or 1:2.
2.2. Crystal and Molecular Structures
The crystal structures of molecular complexes 1–5, 8 and coordination polymers 6, 7, 9–11 were determined by single crystal X-ray analysis.
2.2.1. Compounds 1 and 2
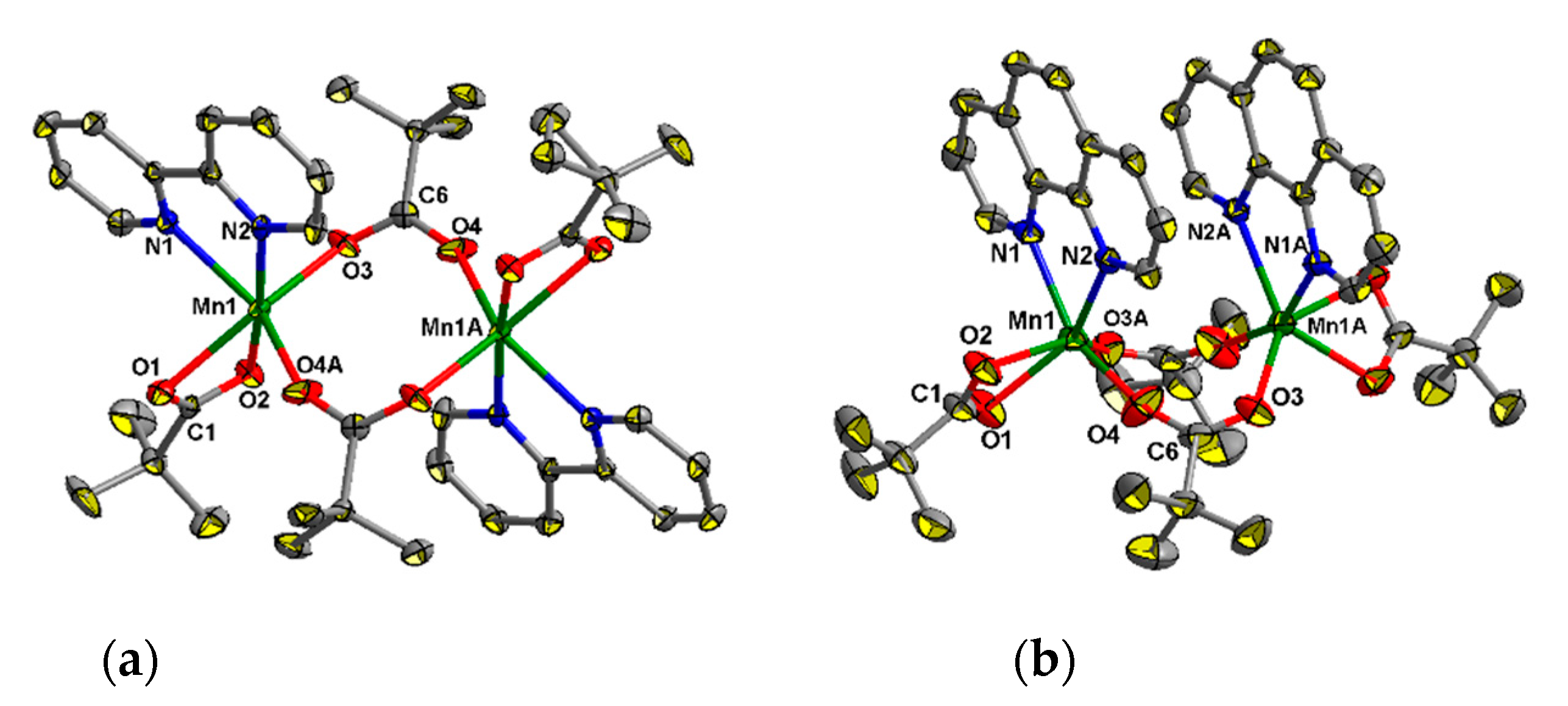
Complexes 1 and 2 have dinuclear cores (Mn…Mn 4.448(2) and 4.015(3) Å, respectively) with a common metal-carboxylate fragment {Mn2(µ-Piv)2(η-Piv)2}. Despite their similar composition, the structures of these fragments are different (Figure 2a,b). The binuclear complex in 1 is centrosymmetric (the inversion center lies between the metal atoms), while compound 2 possesses axial symmetry (a 21 axis passes between the metal atoms). In both 1 and 2 oxygen donors occupy four positions in the coordination sphere of each of MnII ion, and two N atoms from 2,2′-bipy or phen complete the coordination spheres of these ions to form distorted octahedra (Figure 2a,b). The Mn–O and Mn–N bond lengths in 1 and 2 fall in range 2.039(4)–2.607(6) Å and 2.263(4)–2.342(4) Å respectively, which is typical for complexes of MnII with carboxylates and 2,2′-bipy or phen [44,45,60,61,62].
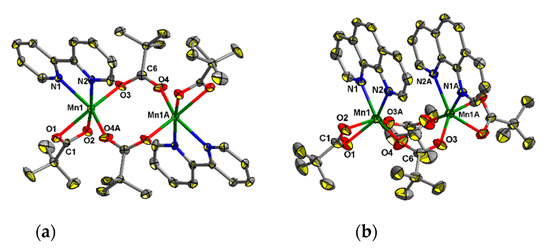
Figure 2.
The molecular structures of 1 ((a), atoms with an additional character in the atom labels are at (1 − x, 1 − y, 2 − z)) and 2 ((b), atoms with an additional character in the atom labels are at (1 − x, y, 1/2 − z))H atoms at carbon atoms are omitted for clarity, the displacement ellipsoids are drawn at the 30% probability level.
To the best of our knowledge, the dinuclear core in 1 is the first example of a Mn2 structural unit block possessing a syn,syn-binding µ-COO-group and chelating 2,2′-bipy ligands. A compound of similar composition, Mn2(ad(O2C)2)2(2,2′-bipy)2·0.5H2O (where ad(O2C)22− is 1,3-adamantanedicarboxylate) [60] contained a dinuclear Mn2(µ2-O2C)2(η-O2C)2 unit with syn,anti-coordination mode of the µ-O2C-groups.
The aromatic rings of 2,2′-bipy ligands of the neighboring molecules in 1 are not parallel, and the angle between the mean planes of these molecules is 7.6(3)°. The closest distance between centroids of pairs rings of different 2,2′-bipy ligands is 3.591(4) Å (the slippage is 0.707 Å). This leads to the formation of a supramolecular chain along the c axis (Figure 3b), probably due to π-stacking interactions.
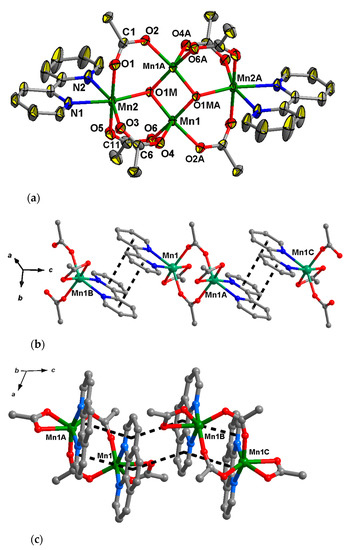
Figure 3.
, The molecular structure of 3 ((a), atoms with an additional character in the atom labels are at (−x, 1 − y, 1 − z)), intra- (only for 2) and intermolecular (for 1 and 2) π-stacking interaction and formation of supramolecular chain structure in crystal lattices of 1 (a) and 2 (b) (H atoms at carbon atoms and methyl groups of pivalate ions are omitted for clarity, (c) the displacement ellipsoids are drawn at the 30% probability level).
The aromatic rings of two phen ligands in one molecule of 2 are not parallel, and the angle between the mean planes of these molecules is 9.8(2)°. The closest distance between centroids of pairs rings (N2C18-C22, C14-C19), belonging to these different phen ligands, is 3.695(4) Å (the slippage is 1.055 Å). The mean planes of phen ligands from the neighboring different molecules of 2 are parallel and the centroids of these pairs of phen rings are located in 3.730(4) Å (the slippage is 1.561 Å). Such an arrangement of aromatic phen molecules can allow for π-stacking interactions between them and as a result formation of a supramolecular pile structure along the c axis (Figure 3c). However, it cannot be excluded that intramolecular π-stacking between phen molecules in 2 may be the reason for the difference between structures of this compound and 1: the 2,2′-bipy molecules in 1 are located on different sides in respect to the inversion center, located between the MnII ions.
2.2.2. Compounds 3 and 4
The molecules of compounds 3 and 4 are centrosymmetric (an inversion center lies between the central metal ions) and possess a similar tetranuclear Mn4O2(Piv)6 core (Figure 3a) of a “butterfly” type, which is quite typical for Mn carboxylates [63,64,65,66]. Each MnIII ion in the center of the butterfly is located in a square pyramidal coordination polyhedron (MnO5 chromofore, τ = 0.15 [63,64,65,66]), while MnII ions on the wings of the butterfly are hexacoordinate and donor atoms in their coordination environment form highly distorted octahedra (MnO4N2 chromophores, where N atoms belong to 2,2′-bipy or phen in 3 and 4, respectively). MnIII–(µ3–O) and MnII–(µ3–O) bond lengths in 3 and 4 fall in range 1.843(2)–1.851(2) Å and 2.073(3)–2.099(2) Å, respectively, which is typical for Mn-O distances within trinuclear units MnII2MnIII2O2 [63,64,65,66]. MnIII–O and MnII–O (O atoms from pivalate) bond lengths fall in range 1.957(2)–2.097(2) and 2.116(2)–2.213(3) respectively for 3, and 1.955(3)–2.105(3) Å and 2.105(3)–2.196(3) Å respectively for 4, making these bonds longer than the corresponding bonds of Mn ions with µ3-O atoms. Terminal MnII atoms fill up their coordination sphere by coordination of 2,2′–bipy or phen with Mn–N bond lengths 2.271(3), 2.284(3) Å for 3, and 2.256(4), 2.284(4) Å for 4.
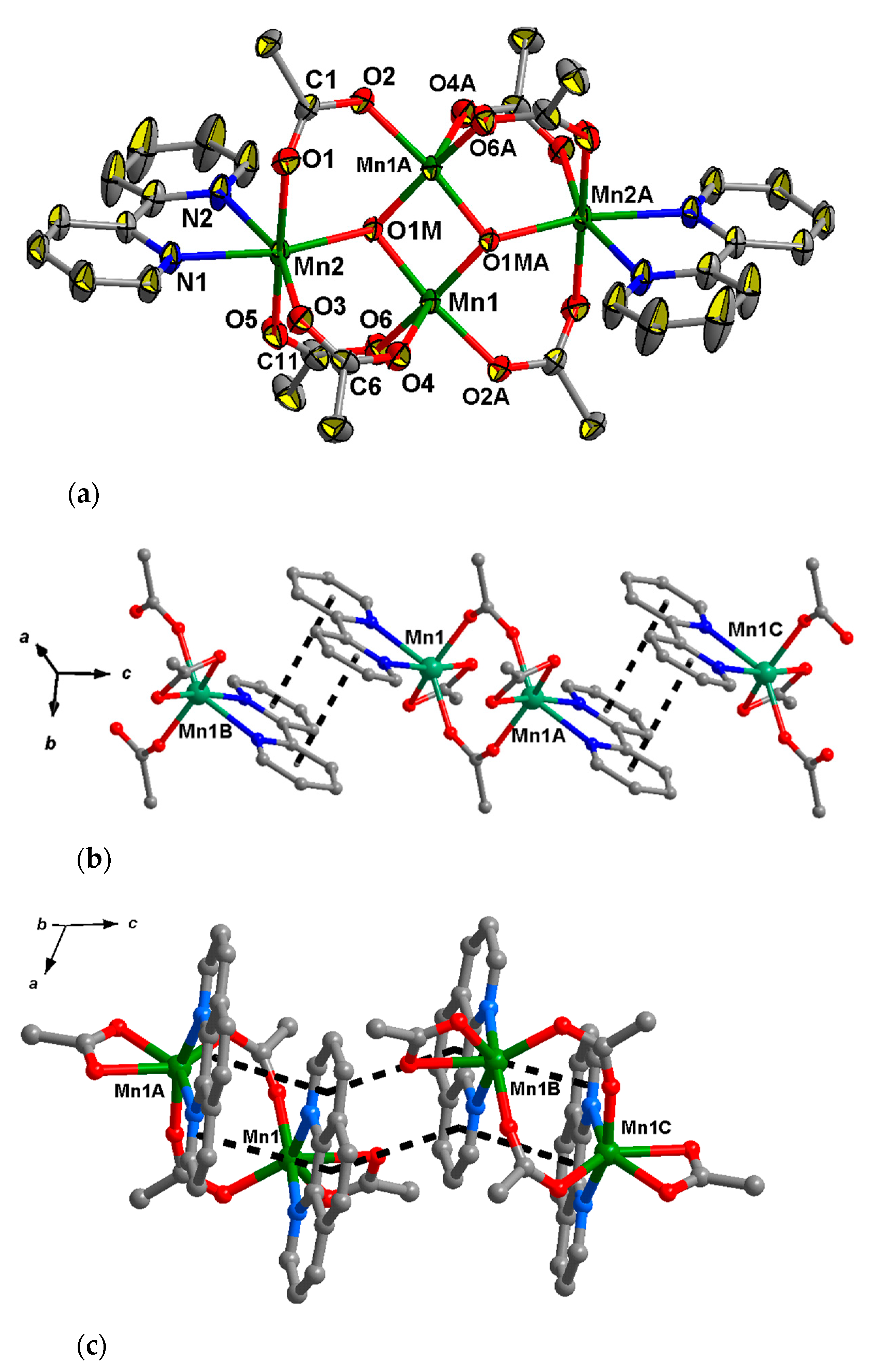
2.2.3. Compound 5
Compound 5 crystallizes in the monoclinic space group P21/n as a discrete centrosymmetric hexanuclear complex (the inversion center lies between the central metal ions Mn2, Mn3, Mn2A and Mn3A). The hexanuclear core of 5 can be considered as two identical triangular fragments {Mn3(OH)(Piv)3(pym)2} linked by four carboxylic acid groups (two µ2-Piv and two µ3-Piv) (Figure 4a). In each trinuclear fragment MnII ions are linked by µ3-OH (bond lengths Mn–O are equal to 2.049(2)–2.184(2) Å), and the O atom is located on 0.66(2) Å above the Mn1Mn2Mn3 plane, which can be an additional proof that the central oxygen atom belongs to a µ3-hydroxo group rather than a µ3-oxo (Mn3(µ3-O) unit that is expected to be planar [67,68,69,70]). One µ-O2C bridging group links Mn1 and Mn2, and two µ2-O2C groups link Mn1 with Mn3 (bond lengths Mn–O(Piv) and lie in the 2.095(3)–2.142(3) Å) range. In addition to the oxygen atoms of carboxylate groups, the nitrogen atoms of two pyrimidine molecules are coordinated to Mn1, completing its coordination polyhedron to form a distorted octahedron (Mn1–N bond lengths are 2.293(3) and 2.322(3) Å). Mn2 is located in the distorted tetrahedral donor set O4 (Mn2–O(Piv) bond lengths are in range 2.067(3)–2.097(3) Å)), and Mn3 is in a distorted octahedral donor set O6 (Mn3–O(Piv) bond lengths are in range 2.094(3)–2.262(2) Å)).
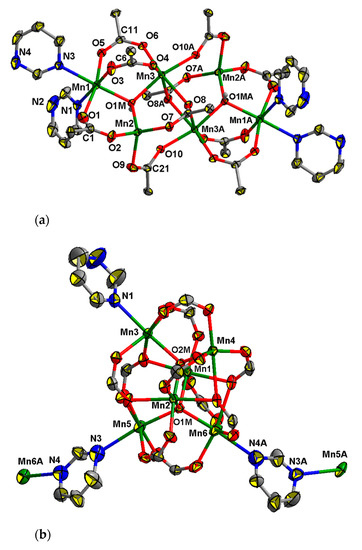
Figure 4.
Structures of polynuclear units in 5 (a) and 6 (b) respectively. H atoms at carbon atoms, and methyl (in 5) and tert-butyl (in 6) groups of pivalate ions are omitted for clarity, the displacement ellipsoids are drawn at the 30% probability level).
2.2.4. Compound 6
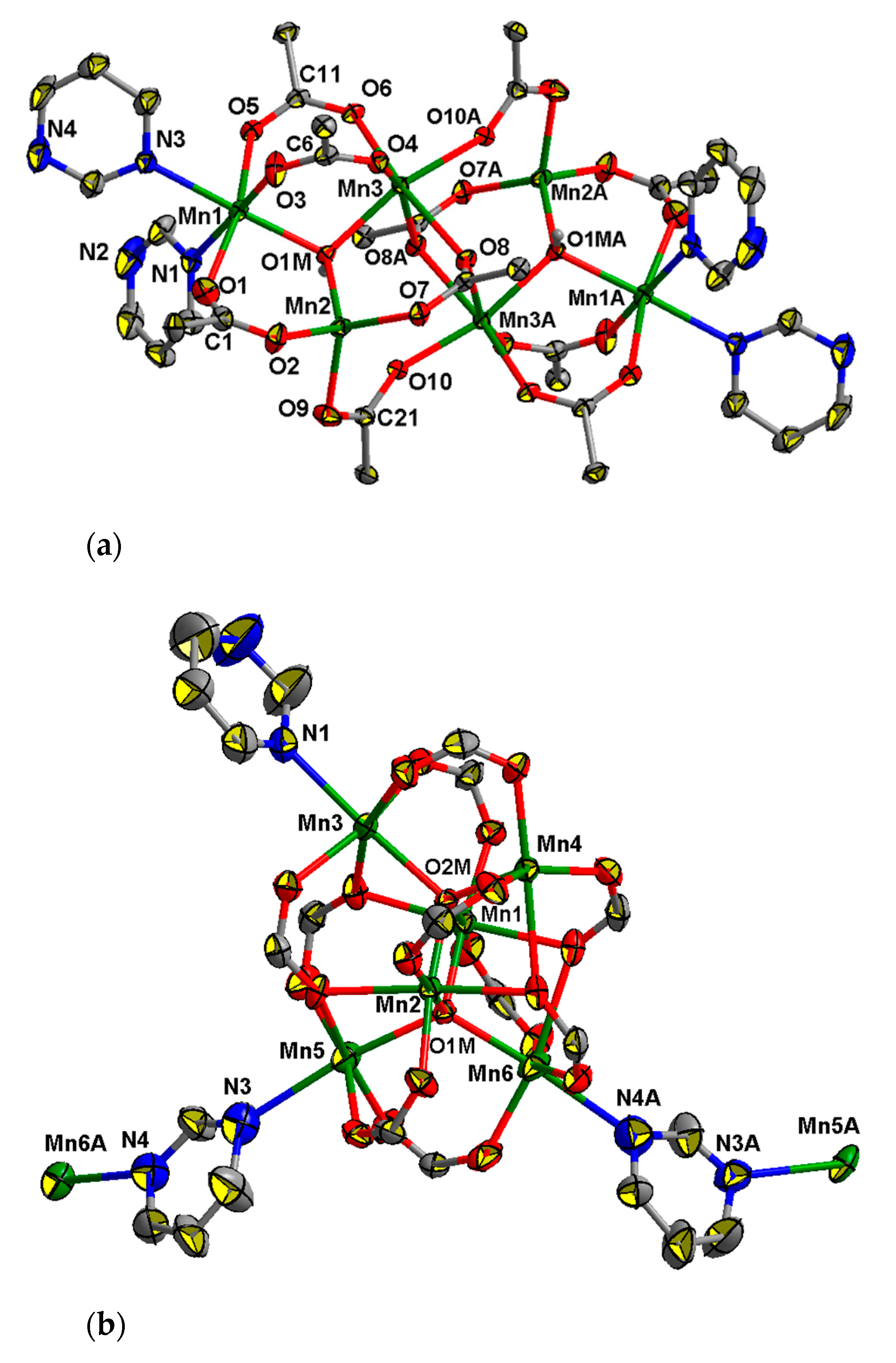
This compound crystallizes as a 1D polymer in the space group Pn, in which hexanuclear units {MnII4MnIII2O2(Piv)10} are linked by pyrimidine bridges (Figure 4b). Generally, the structure of the Mn6 core in 6 is similar to that of the hexanuclear units observed in [MII4MIII2(O)2(O2CR)10(L)4] complexes, where L is a neutral N- or O-donor ligands [71,72,73,74] with the difference that one Mn4 atom in 6 possesses coordination number four and is located in a coordination polyhedron, close to a distorted square-pyramid (τ = 0.12) [75]. Central MnIII ions (Mn1…Mn2 2.835(3) Å) possess O6 donor sets (Mn–O(µ4–O) 1.878(7)–1.914(8) Å, Mn–O 1.933(9)–2.267(9) Å), three of four terminal MnII ions are located in O5N donor sets (Mn–O(µ4–O) 2.019(8)–2.200(8) Å, Mn–O 1.970(10)–2.457(10) Å, Mn3–N1 2.255(12), Mn5–N3 2.511(16), Mn6–N4 2.471(18) Å), where N is an atom of a bridging (N3 and N4) or non-bridging (N1) pyrimidine molecule. In the crystal lattice 1D chains of 6 are arranged parallel to the a axis. The crystal lattice of 6 is retained upon sample storage in air, as confirmed by powder XRD (Figure S1, Supporting information).
2.2.5. Compound 7
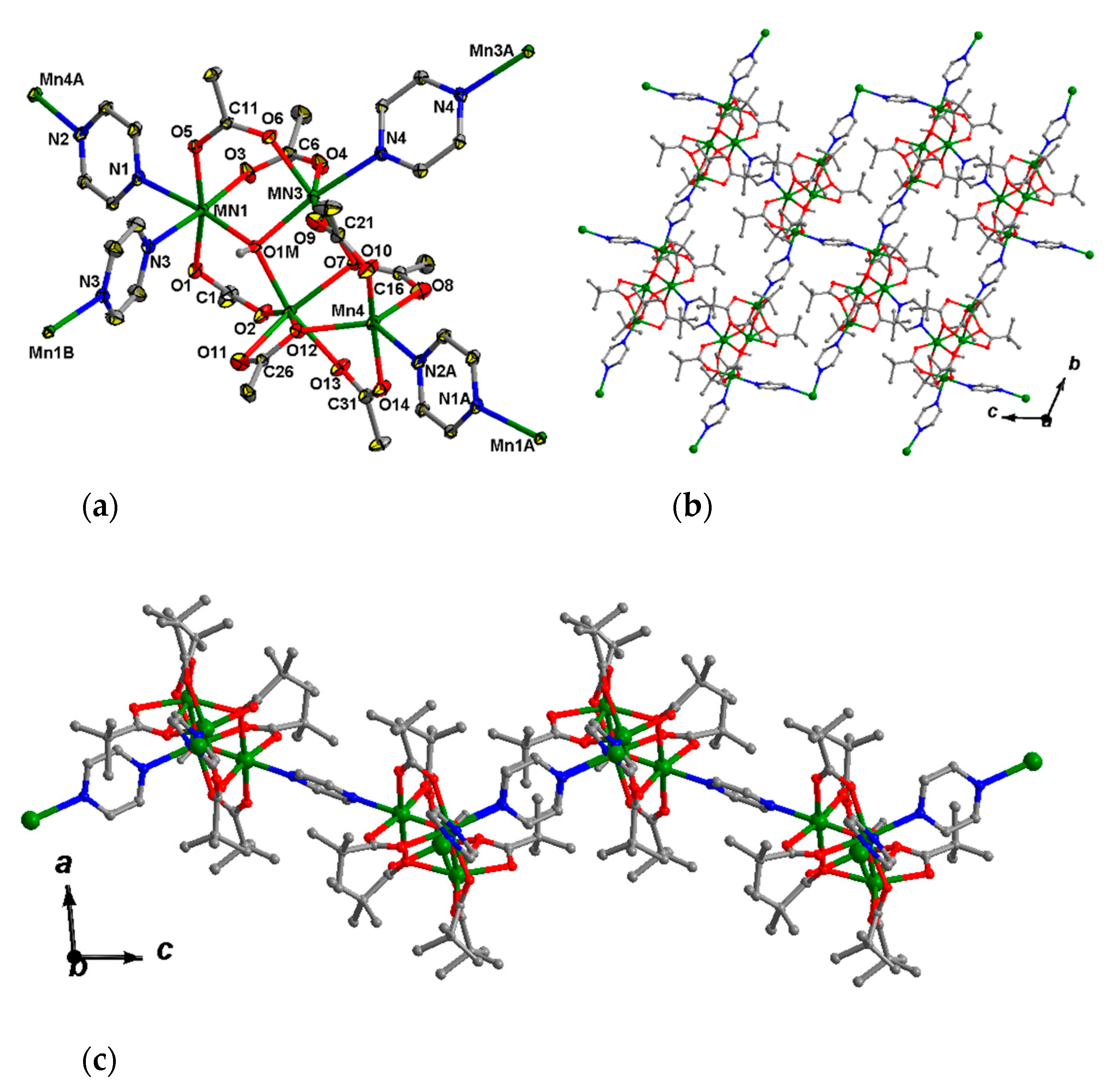
This complex crystallizes in the triclinic space group P-1 as a solvate with two molecules of MeCN. The tetranuclear core {MnII4(OH)(Piv)7} (Figure 5a) in 7 can be described as a trinuclear µ3-hydroxo-centered unit Mn3(OH)(Piv)4 (Mn…Mn 3.371(2)–3.735(2) Å), linked with the fourth Mn4 ion (Mn4…Mn 3.451(2), 4.157(2) Å) by four pivalate anions. Such a Mn4 unit is not symmetric. The Mn3(OH)(Piv)4 bond lengths of Mn–O1M fall in the 2.131(2)–2.134(2) Å range, with atom O1M located above Mn1Mn2Mn3 plane at 0.64(3) Å, d(Mn–O(Piv) = 2.111(3)–2.238(3) Å. The Mn1 ion is bound to two pyrazine ligands (Mn1–N = 2.346(4), 2.389(4) Å), while the Mn3 and Mn4 ions are bound to one molecule of pyrazine (Mn3–N 2.277(4) Å, Mn4–N 2.315(4) Å). Thus, the Mn1, Mn2 and Mn3 ions possess distorted octahedral donor sets O4N2, O6 and O5N, respectively, and the Mn4 ion is located in a distorted square-pyramidal coordination environment (O4N donor set; τ = 0.08) [75]. All pyrazine molecules in 7 are bridging. Local symmetry centers of the unit cell are located in the centers of the pyrazine rings which link Mn1-Mn1′ and Mn3-Mn3′ ions.
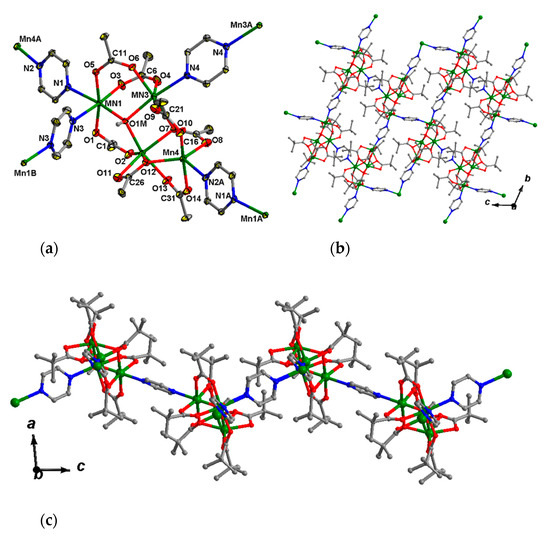
Figure 5.
Fragment of crystal lattice (a) and 2D-grid (b,c) of 7. H atoms at carbon atoms and methyl groups of pivalate ions (in a) are omitted for clarity, the displacement ellipsoids are drawn at the 30% probability level (in a).
Each Mn4 unit is connected with other four Mn4 units by four pyrazine bridges, while each pyrazine links two tetranuclear units. Such an arrangement results in the formation of a 2D polymer. The 2D-layers are parallel to the bc plane (Figure 5b,c).
2.2.6. Compound 8
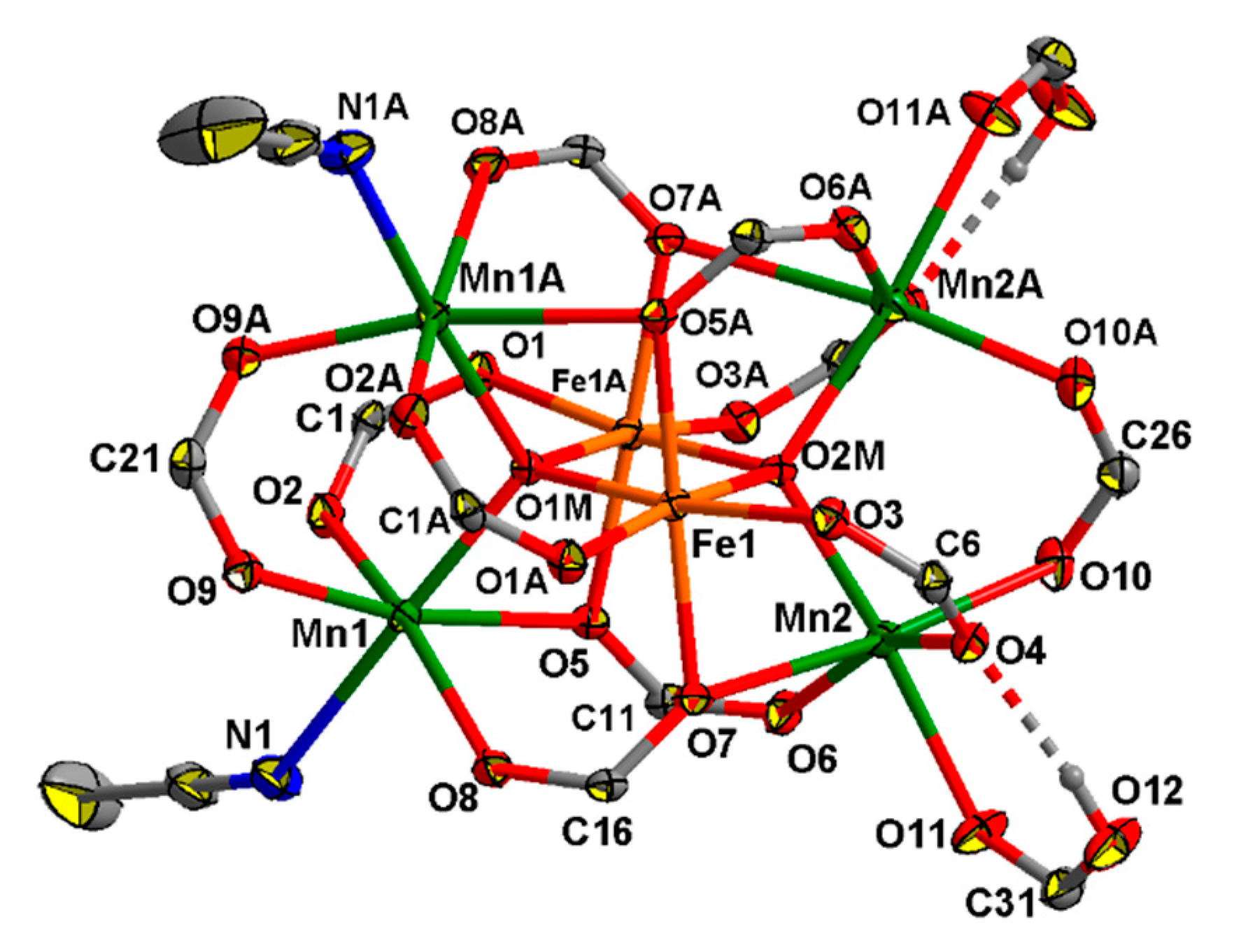
The complex 8 crystallizes in the monoclinic space group C2/c as a solvate with four molecules of MeCN. The molecule of 8 has axial symmetry, with axis 2 passing between the Fe1 and Fe1A atoms through the O1M, O2M, C21, C22, C26 and C27 atoms. The structure of the complex is similar to that of the known hexanuclear complexes [MnII4MnIII2(O)2(O2CR)10(L)4], where L is a neutral N- or O-donor ligans [71,72,73] but where the central atoms are FeIII instead of MnIII. The central FeIII ions (Fe1…Fe1A 2.8824(10) Å) possess O6 donor sets (Fe–O(µ4-O) 1.953(2), 1.962(2) Å, Fe–O(Piv) 2.020(2)–2.070(2) Å), two terminal MnII ions (Mn…Fe 3.1806(8)–3.5075(8) Å, Mn…Mn 3.4831(11), 3.4841(11) Å) are located in O5N donor sets (Mn–O(µ4-O) 2.085(2) Å, Mn–O(Piv) 2.101(3)–2.492(2) Å, Mn-N 2.343(4), where N is an atom of a MeCN molecule, and two terminal MnII ions are located in O6 donor sets (Mn–O(µ4-O) 2.086(2) Å, Mn–O(Piv) 2.104(3)–2.522(2) Å, Mn–O(HPiv) 2.240(3) (Figure 6). H-bonds are formed between of a coordinated molecule of acid and the O atom of a bridging carboxylate group (O12…O4 2.603(4) Å, O12–H 0.84 Å, O4…H 1.77 Å, angle O12–H–O4 169°).
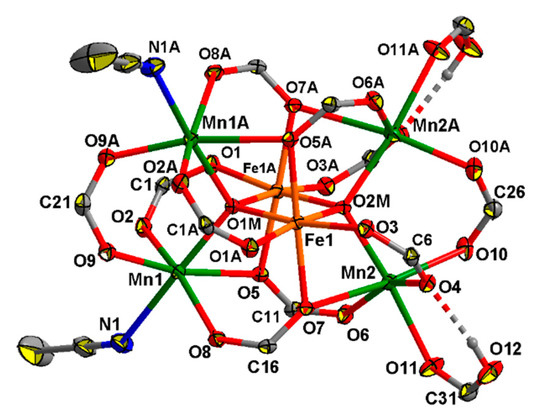
Figure 6.
The structure of [Mn4Fe2O2(Piv)10(MeCN)2(HPiv)2] in 8 (atoms with an additional character in the atom labels are at (1 − x, y, 1/2 − z)). H atoms at carbon atoms and methyl groups of pivalate ions are omitted for clarity, the displacement ellipsoids are drawn at the 30% probability level.
2.2.7. Compounds 9–11
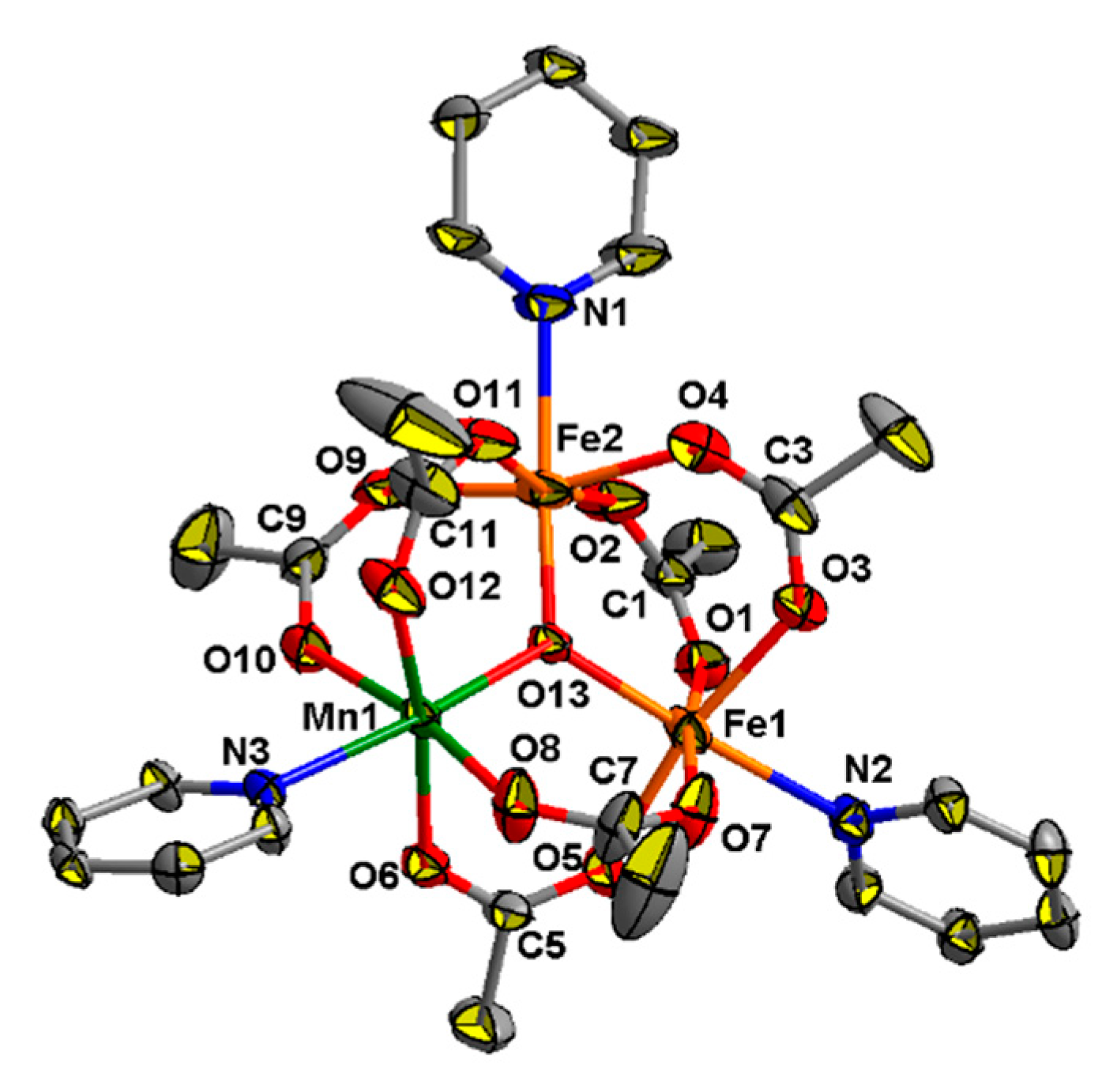
Coordination polymers 9–11 are built by linking a neutral trinuclear block, {Fe2MnO(OAc)6}, with neutral pyridine-containing bridges. The structure of the {Fe2MnO(OAc)6} unit in all these complexes is almost the same (such unit in compound 9 is shown on Figure 7 as example). In this block three metal ions (two FeIII and MnII) are located in the corners of an irregular triangle and generally cannot be distinguished by X-ray crystallography, so the assignment of metal ions was arbitrary. These metal ions are linked by μ3-O atoms and six bridging acetates, so oxygen donors occupy five positions in the coordination sphere of each metal ion. The sixth positions are taken up by a donor atom (N or O) from other ligands (4,4′-bipy, bpe or DMF), so that each metal ion is located in a distorted octahedral donor set.
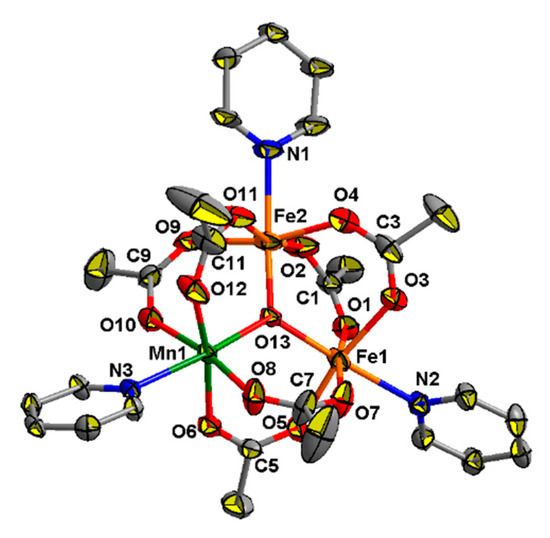
Figure 7.
The structure of {MnFe2O(OAc)6(C5H4N)3} fragment in 10. All hydrogen atoms are omitted for clarity. Only one pyridine ring from each 4,4′-bipy ligand is shown.
M---M separations within trinuclear acetates in compounds 9–11 fall in range 3.240(2)–3.353(2) Å. M–(μ3-O) bond lengths lie in the range from 1.837(6) to 2.056(7) Å, M–O(carboxylate) bonds vary between 2.011(6) to 2.155(8) Å, which is typical for μ3-oxocentered carboxylates [30].
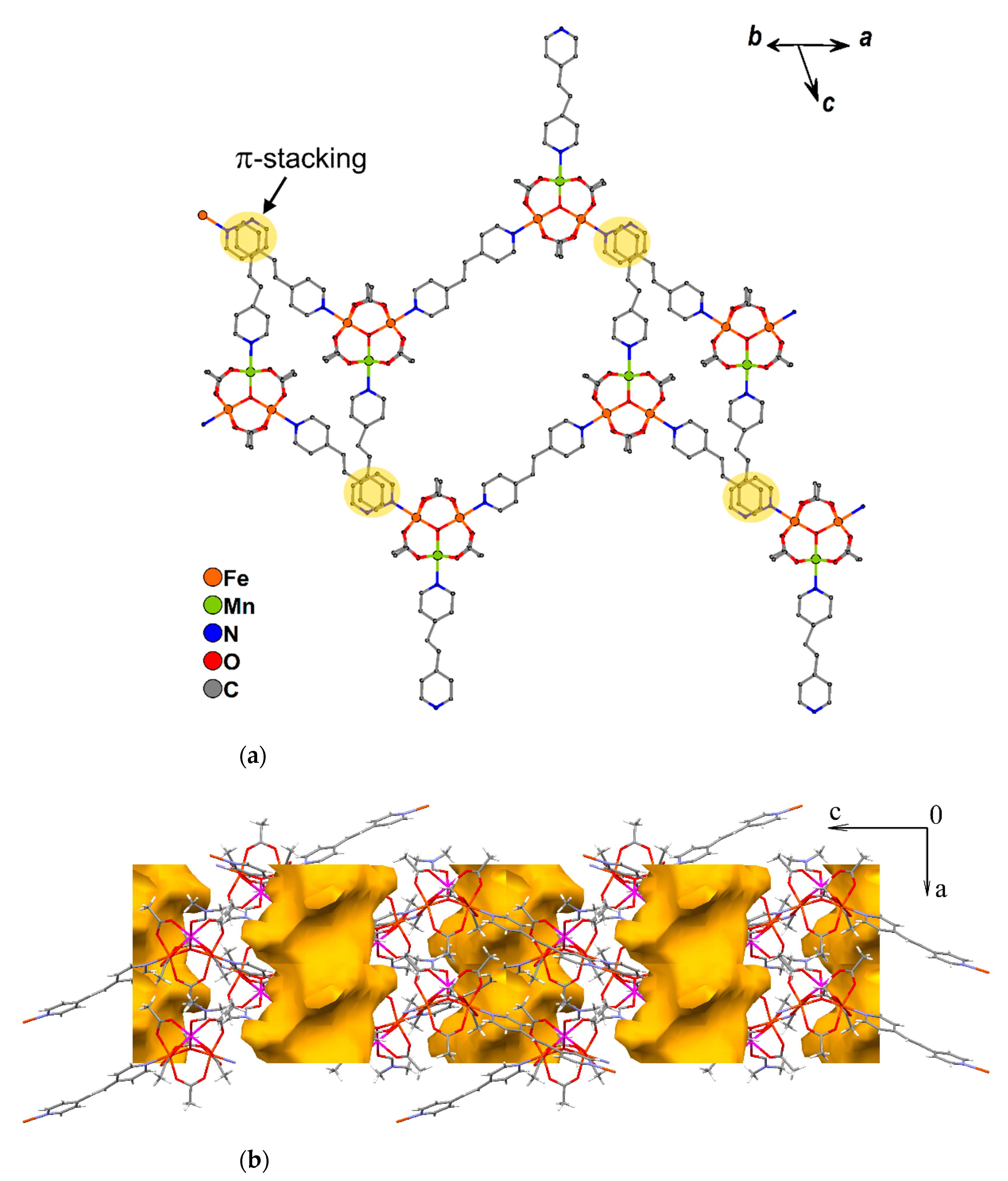
In the crystal lattice of 9 trinuclear μ3-oxocentered units {MnFe2O(OAc)6} are connected by 4,4′-bipy molecules. 3/4 of trinuclear blocks are linked by 4,4′-bipy with formation of a 1D zig-zag chain. Two metal ions from each Fe2Mn unit take part in such chain formation, while the third metal ion is bound to a non-bridging 4,4′-bipy molecule or a “terminal block” {MnFe2O(OAc)6(4,4′-bipy)3}. Thus, there are three types of trinuclear MnFe2 blocks in 9 (Figure 8):
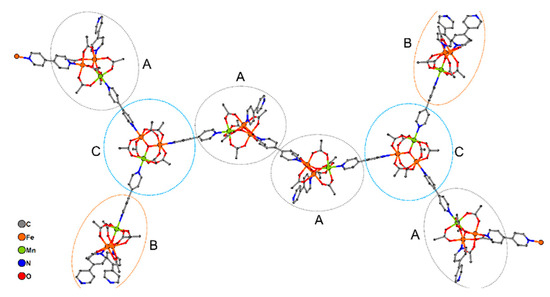
Figure 8.
A fragment of 1D chain of 9. Hydrogen atoms and solvent are omitted for clarity. Note that one bipy molecule in Fragment A is non-bridging; no coordinated metal ions was deleted for fragment A on the figure.
- (1)
- MnFe2 units in 1D chains, bound to two bridging 4,4′-bipy and one additional non-bridging 4,4′-bipy (type A);
- (2)
- MnFe2 units in {MnFe2O(OAc)6(4,4′-bipy)3} residues (type B);
- (3)
- MnFe2 unit in 1D chains, bound to two bridging 4,4′-bipy and one type B unit (type C).
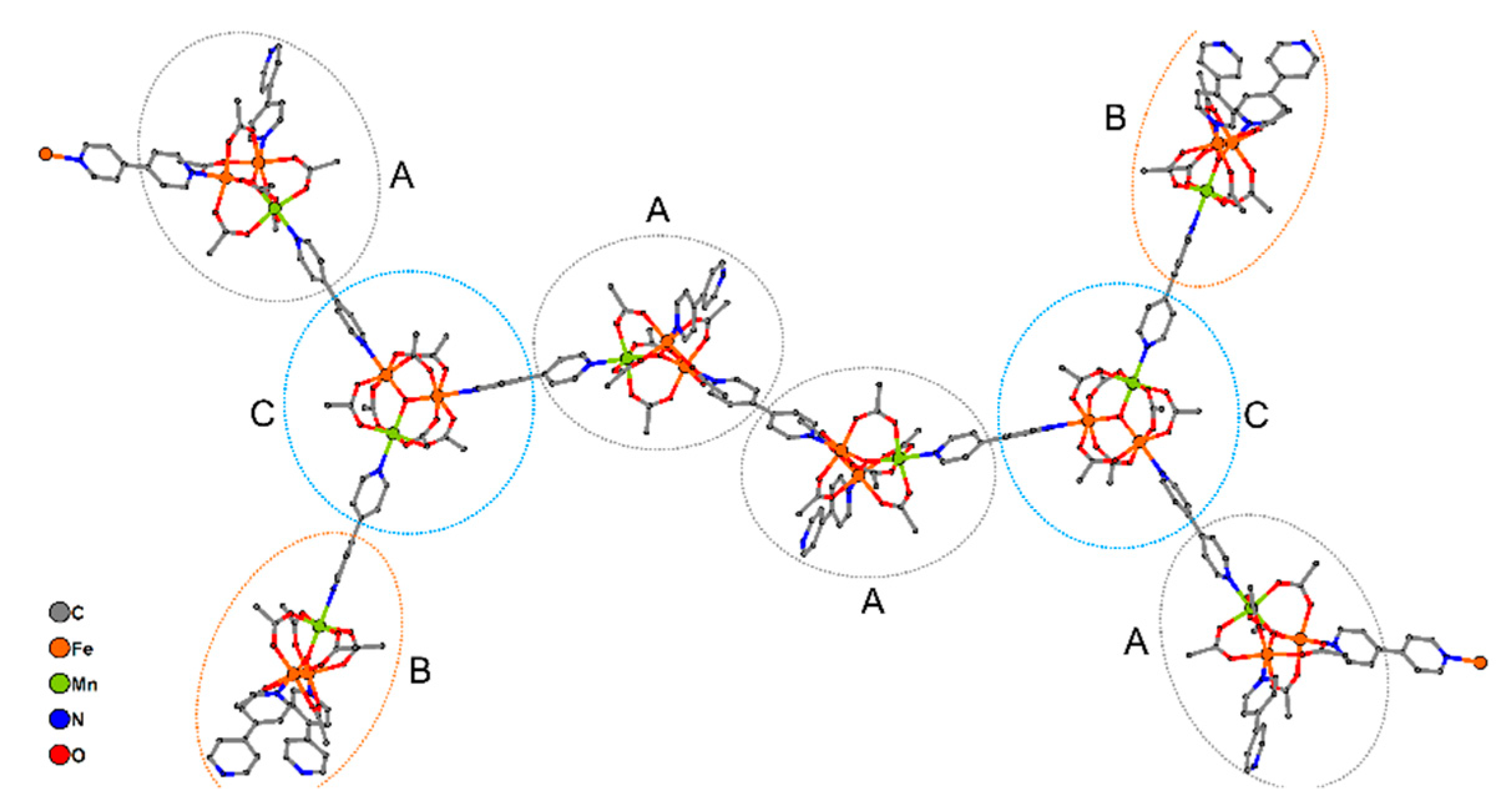
The distance between µ3-O atoms of neighboring Fe2Mn blocks in one 1D chain in 9 is equal to 15.222(8) Å in the case of AA blocks, 15.323 Å for AC blocks and 15.49(1) Å for BC blocks, while the angles between lines connecting the µ3-O atoms of the neighboring trinuclear blocks, are close to 120° (from 116.83(4)° to 120.99(2)°). Torsion angles between lines connecting four µ3-O atoms of adjacent Fe2Mn blocks, are equal to 180 ° for a CAAC fragment (i.e., all µ3-O atoms for this fragment belong to one plane) and ±119.64(5)° for ACAA and AACA fragments. In other words, one 1D chain of 9 turns clockwise twice by 119.64(5)°, as implied by the torsion angles for ACAA or AACA, then all µ3-O atoms lie in one plane in the CAAC fragment and finally the chain turns twice counterclockwise by 119.64(5)° in the ACAA and AACA fragments. The main axes of chains in compound 9 are directed along the (c-½a) vector (Figure 9).
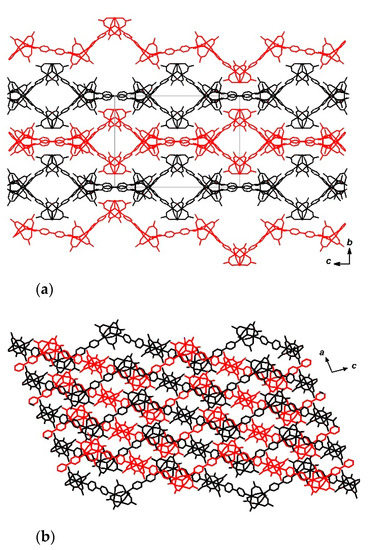
Figure 9.
Fragment of crystal structure of 9. View along crystallographic axes a (a) and b (b). All non-bridging bipy molecules, terminal Fe2MnO(OAc)6(4,4′-bipy)3 blocks, hydrogen atoms and non-coordinated DMF molecules are omitted for clarity.
The twofold axis passes through µ3-O atoms of trinuclear blocks B and C. Also the local inversion centers are located between the pyridine groups of 4,4′-bipy molecules which bind two A type trinuclear blocks. The crystal lattice of 9 does not contain continuous channels (see Figure S2, Supporting Information).
2.2.8. Complexes 10 and 11
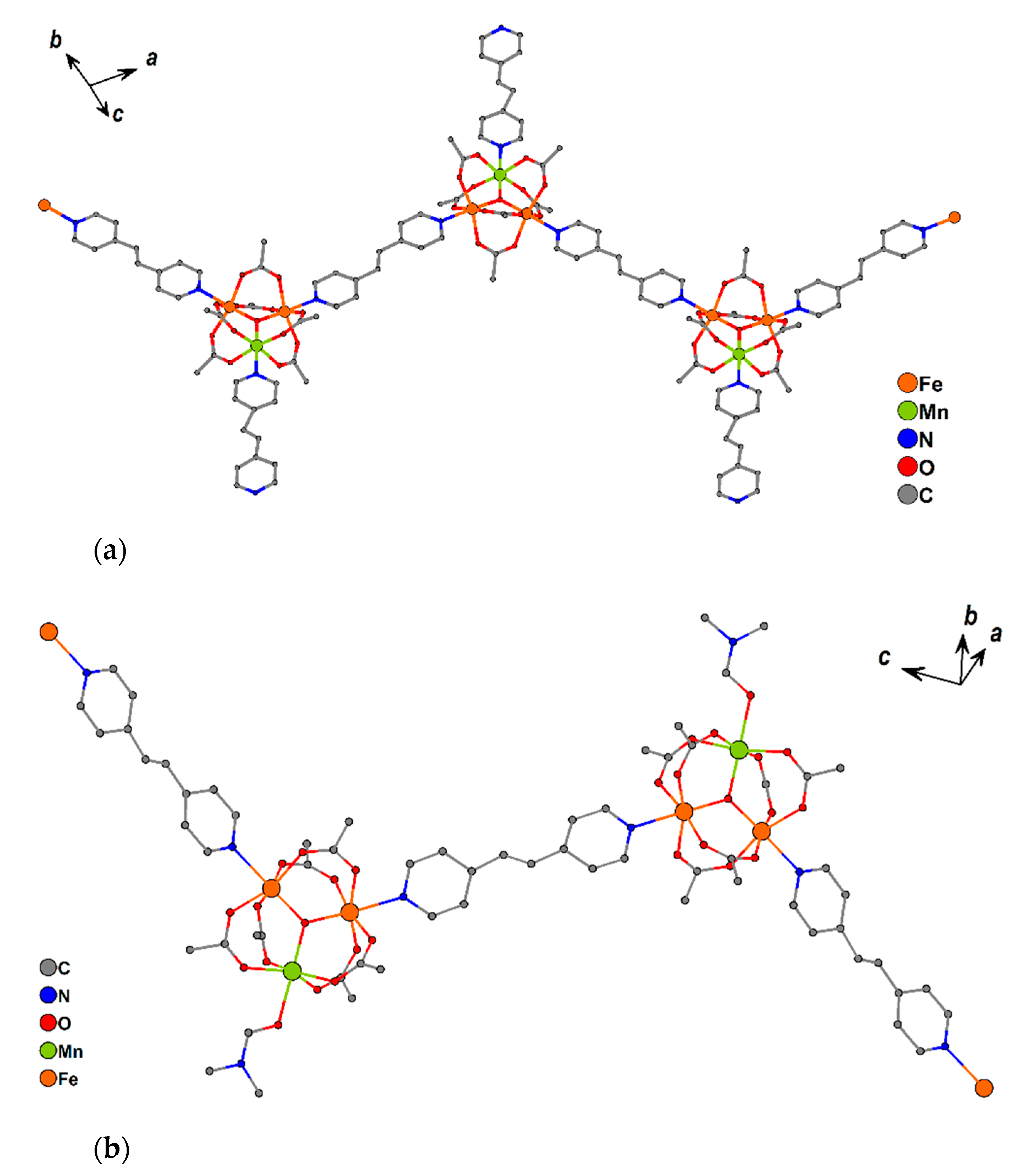
These are built from trinuclear units {Fe2MnO(OAc)6} bound by bpe molecules (Figure 10a). Two metal ions in each trinuclear block coordinate with pyridine rings from bridging bpe, leading to 1D-chain formation. The third metal ion is bound to a nitrogen atom of a terminal (non-bridging) bpe in 10 or oxygen atom of coordinated DMF in 11 (Figure 10b).
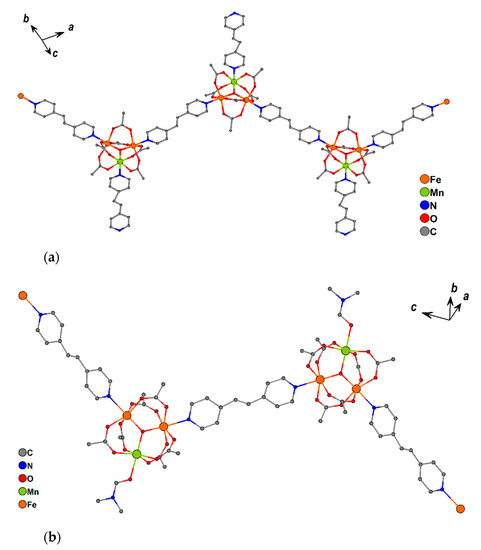
Figure 10.
Fragments of 1D chains of 10 (a) and 11 (b). Hydrogen atoms and non-coordinated solvent molecules are omitted for clarity.
Chains of compound 10 are parallel and directed along the a-½b vector. The non-coordinated pyridine ring of bpe in one chain and the pyridine ring of a bridging bpe ligand from the neighboring chain are almost parallel (the angle between mean planes of these rings is 4.1(5)°), and the closest distance between these rings is 3.38(2) Å (the distance between centroids of the rings is 3.634(6) Å), the slippage is 1.179 Å, which can allow for π-interactions (Figure 11a).
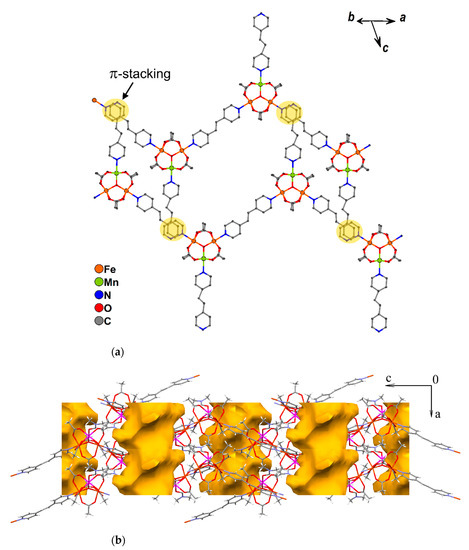
Figure 11.
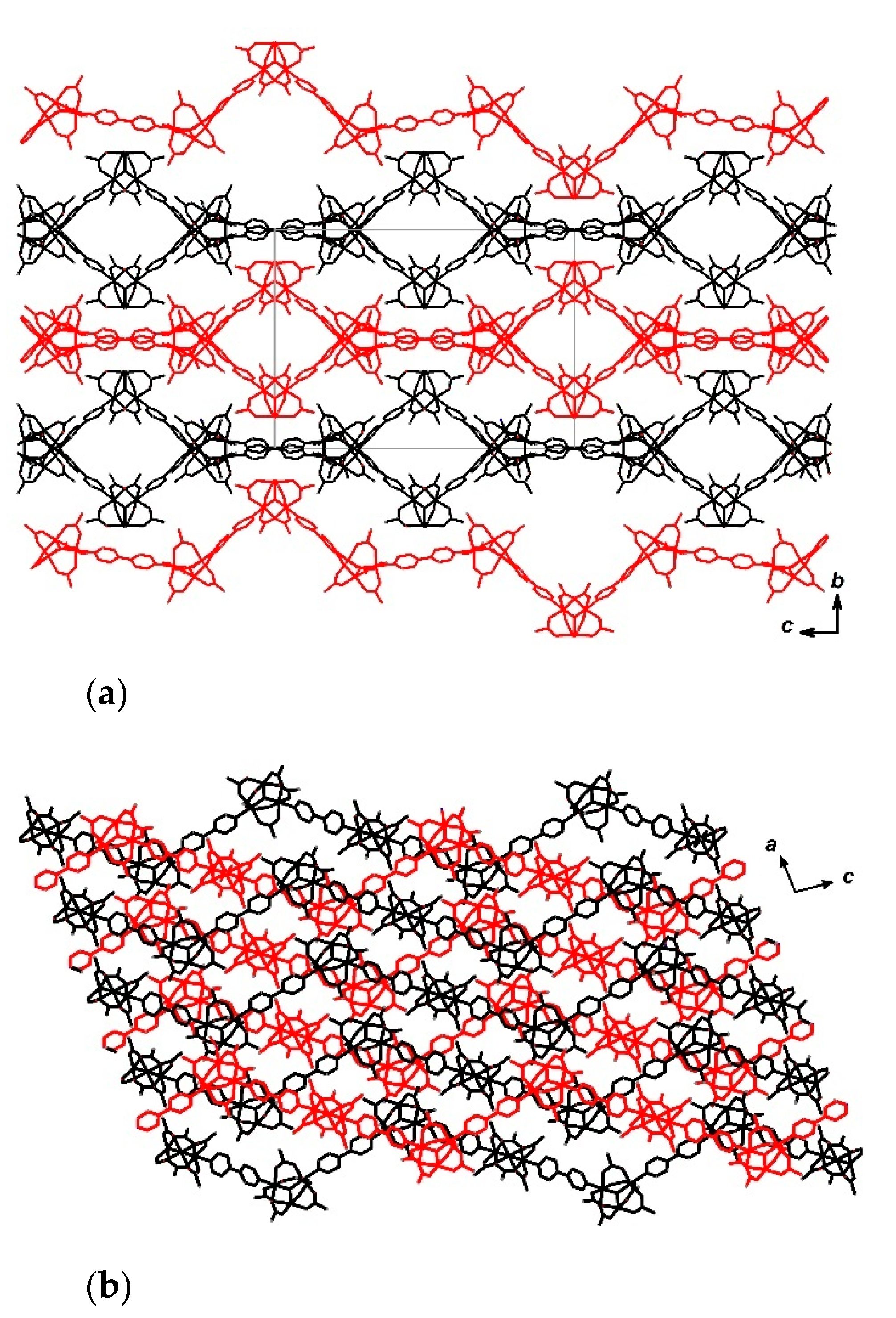
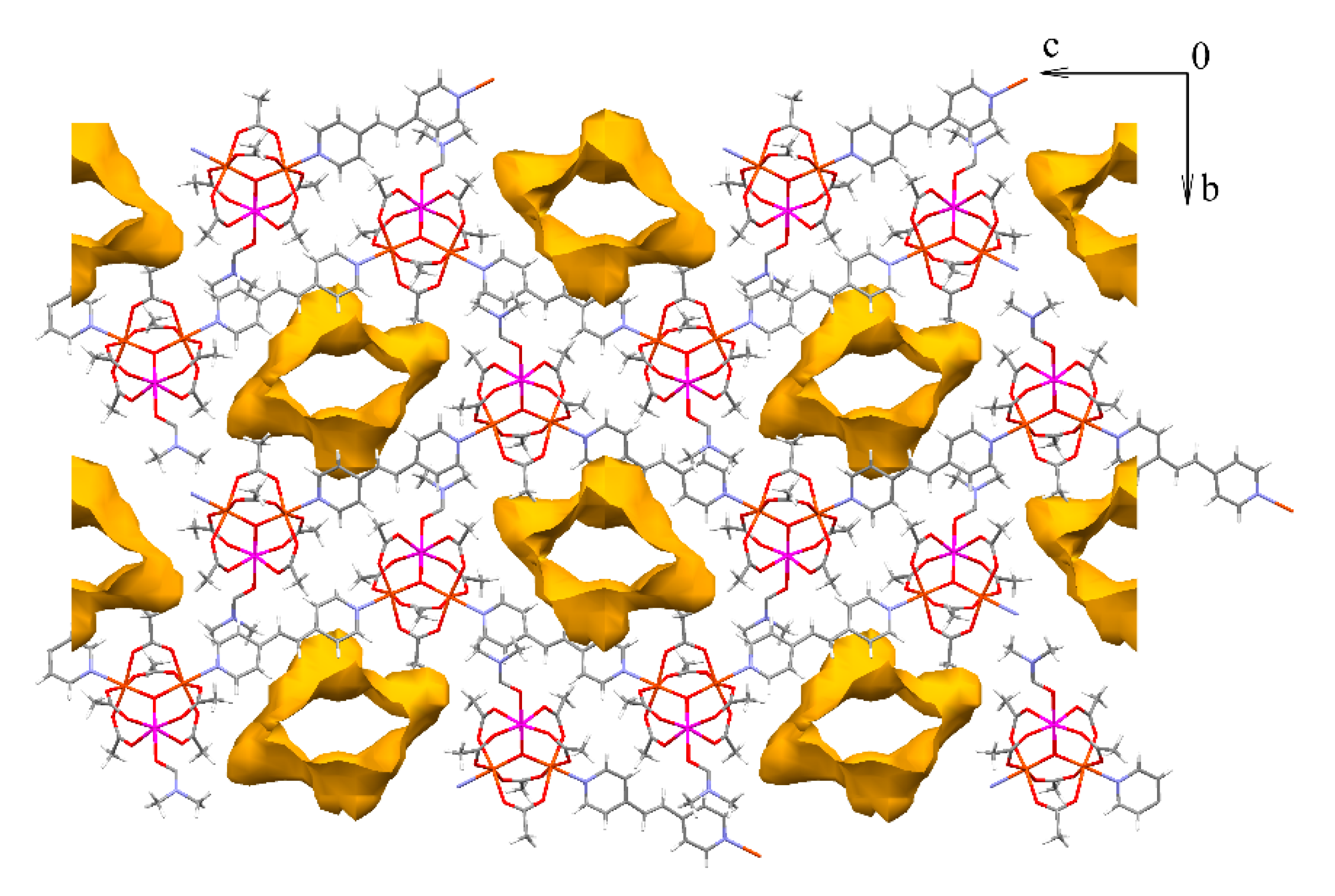
π-stacking interactions between the neighboring chains of 10 (a) and 1D-channels in crystal lattice of 11(-DMF) (b). Hydrogen atoms and solvent molecules are omitted for clarity.
Chains of compound 11 are also parallel and directed along the c vector (Figure 11). No specific interactions between different chains are found. Due to this peculiarities of the chain packing channels of dimensions 5 × 12 Å directed along the a axis form in the crystal lattice (Figure 12). Estimation of solvent-accessible volume, performed by PLATON software [76], gives a value of 36% for 11, containing DMF molecules coordinated to metal ions, or 45% for a structure, if coordinated DMF is removed assuming that such removal does not lead to crystal lattice collapse (calculated for a probe molecule with r = 1.4 Å). These values correspond to ca. 0.32 cm3 g−1 pore volume, occupied by solvent in 11, assuming that the volume occupied by coordinated DMF is not included in this value, or 0.40 cm3 g−1, if the volume of coordinated DMF is included.
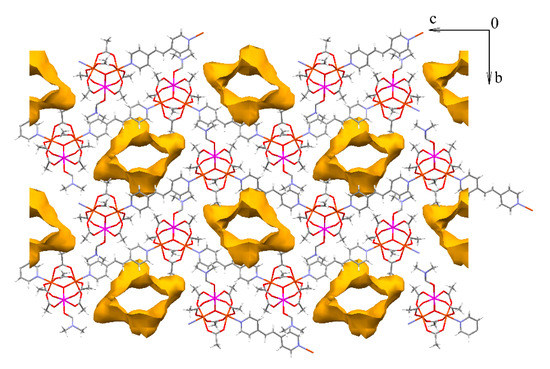
Figure 12.
Channels in crystal lattice of 11·3DMF (projection along a axis). Solvent molecules (including coordinated DMF) and hydrogen atoms are omitted for clarity.
2.3. Thermal Stability and Sorption Properties of 11·3.5DMF
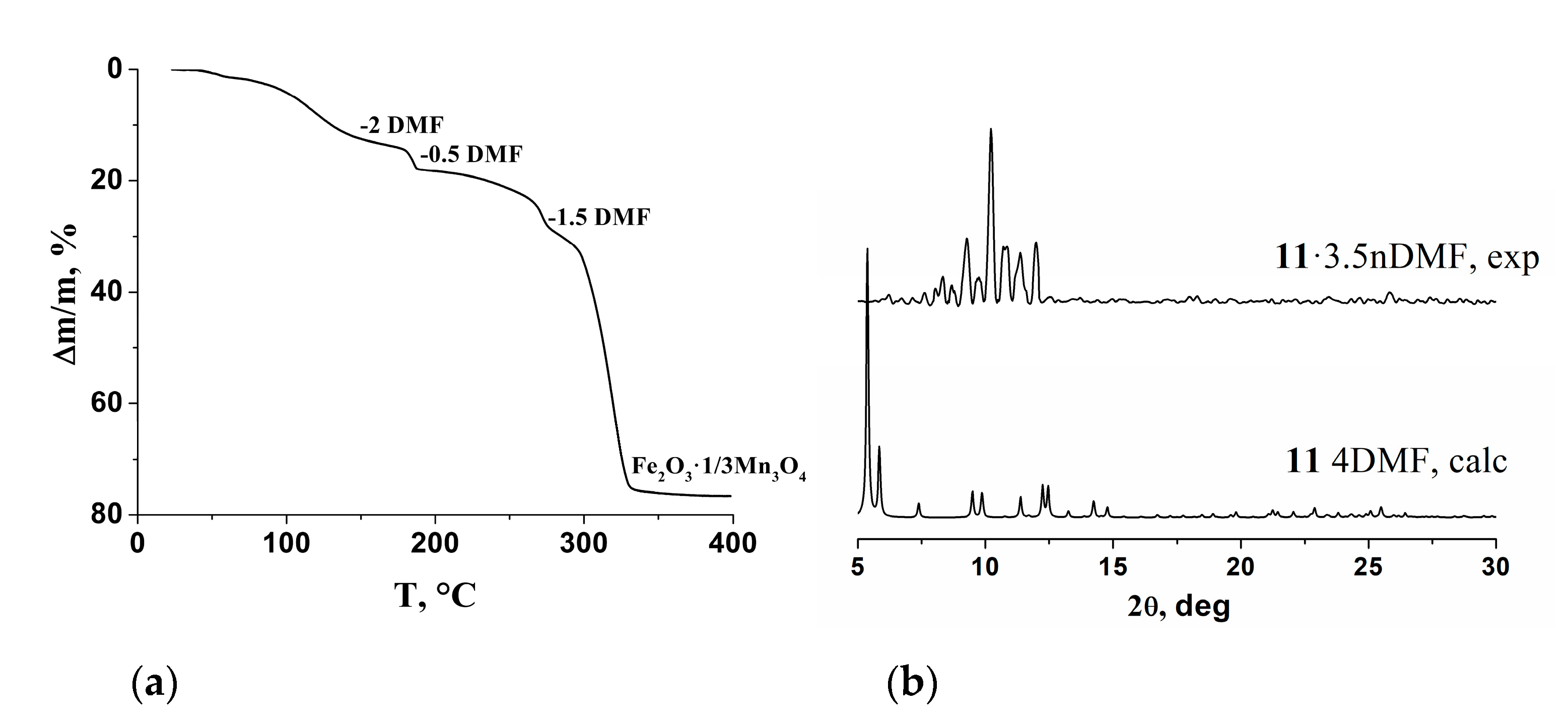
The thermal stability of 11·3.5DMF was studied by thermogravimetry. Upon heating to 275 °C, compound 11·3.5DMF lost 26% of its weight, which corresponds to the release of both non-coordinated and coordinated solvent (Figure 13). An abrupt weight loss began at 275 °C, which was completed at 400 °C and could be associated with decomposition of the compound. The total weight loss was equal to 76.6% and corresponded to the formation of Fe2O3·1/3Mn3O4 (Figure 13a). Loss of solvent and coordinated DMF led to significant lattice disorder, as it can be concluded by comparison of the powder XRD pattern of vacuum-dried product at 145 °C and the powder XRD pattern, calculated from the single-crystal structure (Figure 13b).
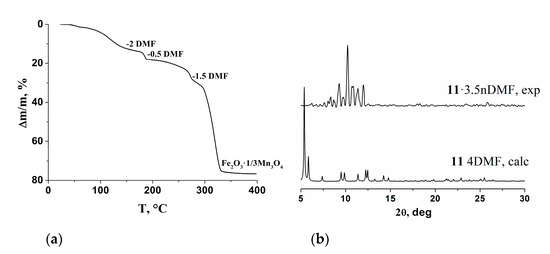
Figure 13.
TG curve for compound 11·3.5DMF (a) and powder XRD patterns for vacuum-dried at 145 °C sample of 11·3.5DMF and calculated from single-crystal X-ray data for 11 (b).
For sorption experiments compound 11·3.5DMF was heated in vacuum at 153 °C during 6 h, which led to removal of non-coordinated and coordinated DMF. The desolvated sample is hereinafter referred to as 11′.
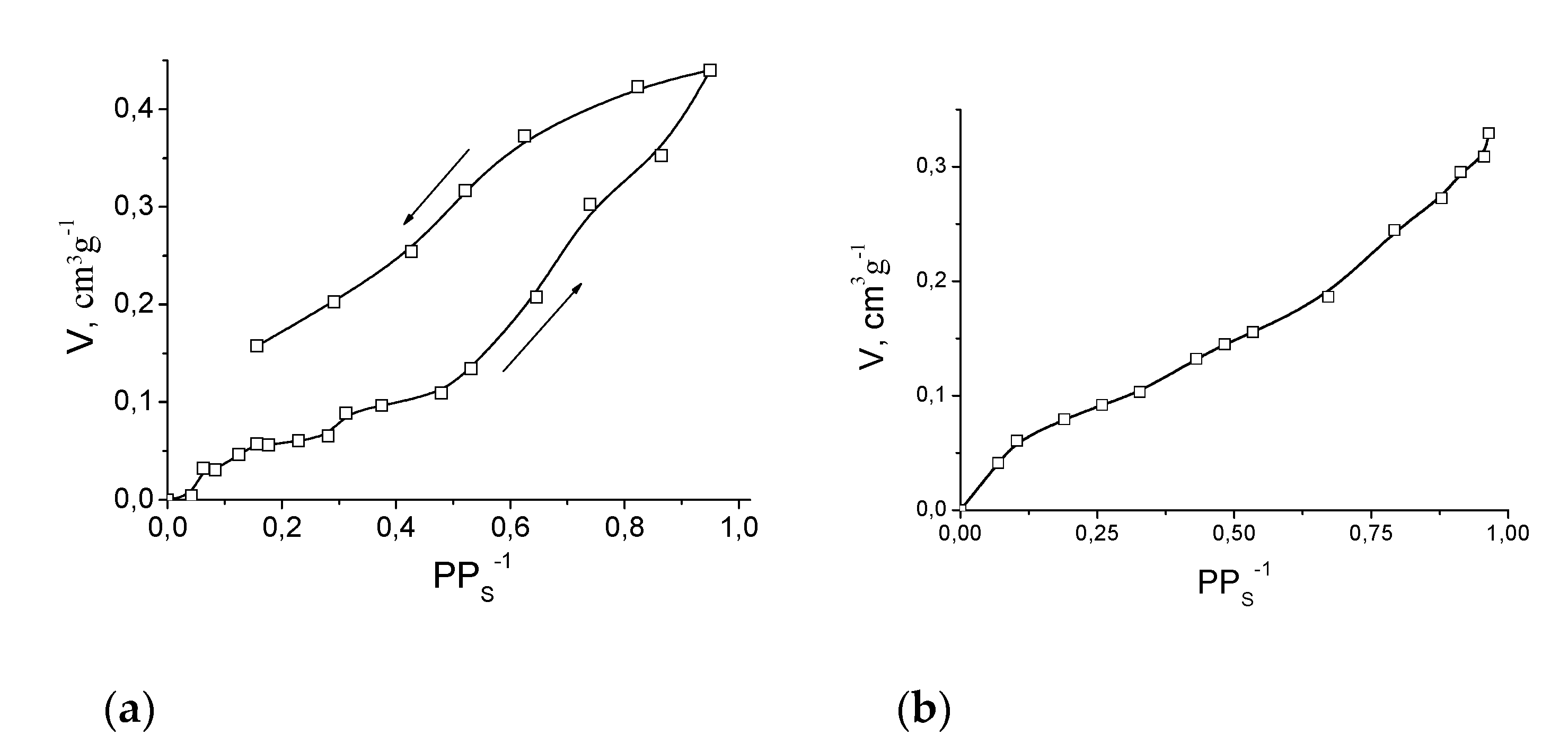
Compound 11′ showed only surface sorption of N2 or H2 at 78 K, which is evidence of crystal lattice collapse and is consistent with the powder XRD data. In contrast, 11′ absorbed significant quantities of methanol and ethanol at 298 K (Figure 14). Such a difference between absorption of gases and alcohols can be caused by expansion of crystal lattice of 11′ upon interaction with methanol and ethanol, similarly to reported gate-opening phenomena [77] and previously reported cases of alcohol absorption by coordination polymers [18]. Both in the cases of methanol and ethanol, the sorption capacity gradually increased to ca. 0.42 cm3·g−1 (methanol) or 0.35 cm3·g−1 (ethanol), which is in good agreement with the value of solvent-accessible volume estimated from the crystallographic data (vide supra).
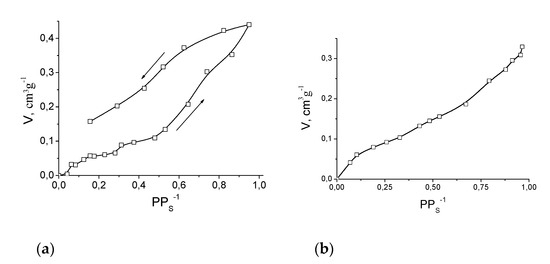
Figure 14.
Sorption isotherms of methanol (a) and ethanol (b) by 11′ at 293 K. Arrows on Figure (a) indicate directions of absorption and desorption.
The plateau in the methanol absorption isotherm at PPS−1 ca. 0.07–0.3 (Vabs. about 0.05 cm3 g−1) corresponds to a methanol to Fe2Mn molar ratio 1:1 and can be associated with methanol coordination to the metal ion (in a position which was occupied by coordinated DMF in 11). It can be concluded from the presence of such a plateau that there is a noticeable difference between the energy of methanol coordination to a metal ion in 11′ and the energy of further methanol interaction with 11′·CH3OH. In contrast, a similar plateau was not found in the ethanol absorption isotherm: ethanol binding by 11′ after filling of unsaturated metal sites seems to be as efficient as ethanol binding due to its coordination (which most probably does occur). Anyhow, the maximal achieved sorption capacity of 11′ corresponds to ca. 10.5 moles of methanol or ca. 5 moles of ethanol per 1 mole of Fe2Mn, which is significantly higher than the sorption capacity associated with coordination.
2.4. EPR Spectroscopy
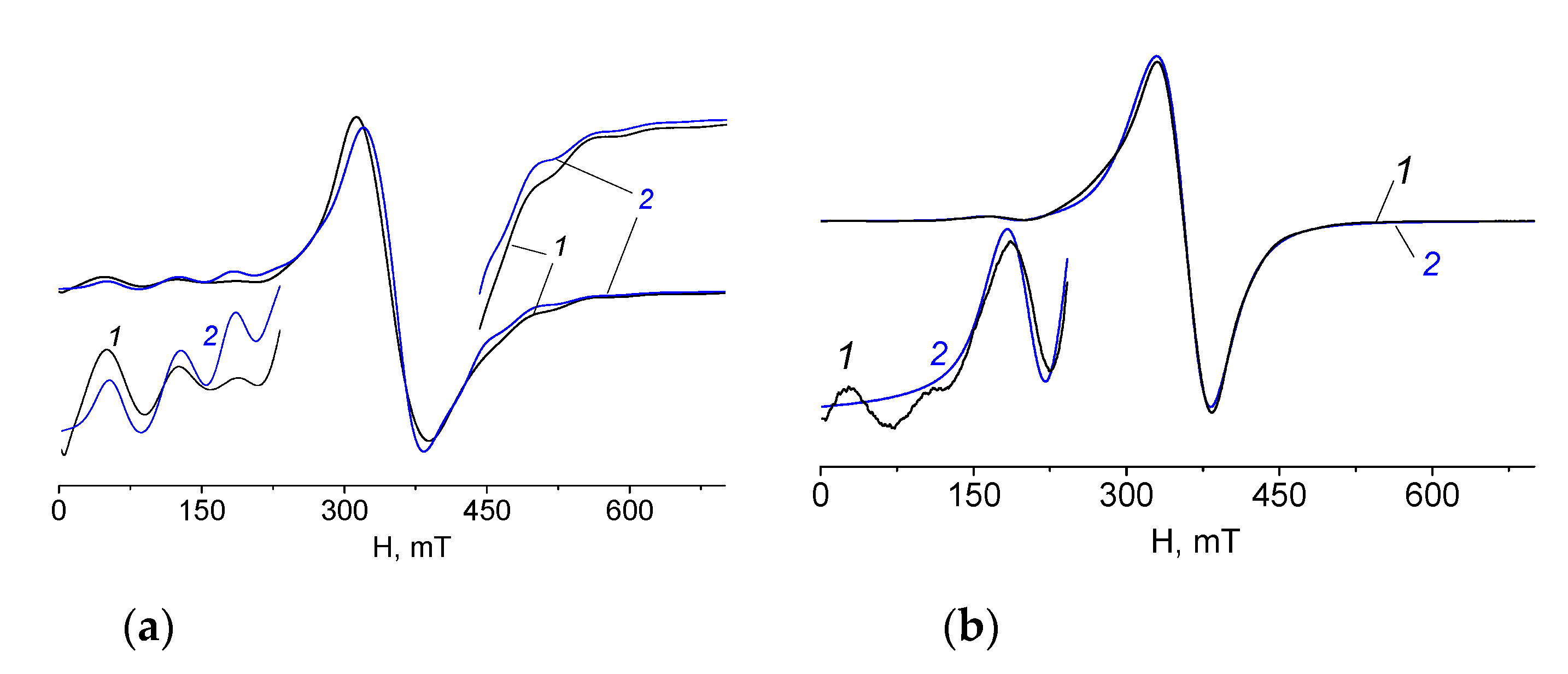
X-band EPR experiments for polycrystalline samples 1 and 2 were performed at 293 K. The spectra of 1 and 2 show an intense singlet without hyperfine structure with g ≈ 2.00. In the low magnetic field lines of low intensity are observed; their origin can be explained by the exchange interactions between paramagnetic manganese ions (Figure 15).
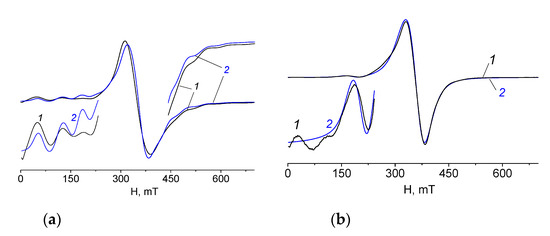
Figure 15.
EPR spectra of polycrystalline samples of 1 (a) and 2 (b) at 293 K (1—experimental, 2—calculated).
Since antiferromagnetic interactions with J = −1.03 cm−1 were found in 1 and 2 by magnetic data analysis (see below), all possible magnetic states of dimer of two Mn2+ ions with spins S1,2 = 5/2, notably, S = 0, 1, 2, 3, 4, 5 (S = S1 + S2) were equally populated. Furthermore, since |J| > hν ≈ 0.3 cm−1, transitions between states with different total spin S can be neglected. Thus, the spin Hamiltonian for 1 and 2 is the sum of spin Hamiltonians of five dimers with different total spins. The spin Hamiltonian (1) for single ion in 1 or 2 has a rhombic symmetry:
where giz, gix, giy—z, x, y—g-tensor components of monomer i, where i = 1, 2; Siz, Six, Siy–projections of spin operator of monomer on coordinate axes, Si = 5/2; di, ei—component of fine interaction tensor (so-called, single-ion). Mn2+ ion has half-filled d5 shell and S-state, so g-tensor is isotropic and close to spin-only value, so, giz = gix = giy = g = 2.0023.
Spin Hamiltonian (2) of dimer is the sum of two Hamiltonians for interactions within mononuclear fragments of molecule and the part of their interaction:
where d12, e12 are the components of fine interaction tensor, caused by dipole interaction of manganese ions.
For the total spin of dimer and neglecting of transitions between multiplets with different total spin S = S1 + S2, spin Hamiltonian (3) can be used:
where D and E are the components of fine interaction tensor, associated with parameters d1,2, e1,2, d12 и e12 by the following formula [78]:
The spin Hamiltonian (3) was diagonalized numerically. Calculations of resonance fields of spin Hamiltonian (3) required to build a theoretical spectrum were carried out by the Belford method [79], which involves finding the values of magnetic field H, for which two eigenvalues of spin Hamiltonian (3) matrix, corresponding to two different eigenvectors, would differ on the hν.
The spin Hamiltonian parameters for compounds 1 and 2 are given in Table 1. Thus, while isotropic exchange of two dimers is same, fine interaction tensor causes a noticeable difference in the EPR spectra.

Table 1.
Spin Hamiltonian parameters for 1 and 2.
2.5. Magnetic Properties of Complexes 1, 2, 7·2MeCN and 11·3.5DMF
Magnetic properties of the representative complexes from the prepared series—compounds 1, 2, 7·2MeCN and 11 3.5DMF—were characterized by their temperature dependence of the molar magnetic susceptibility, χM.
2.5.1. Magnetic Properties of Complexes 1 and 2
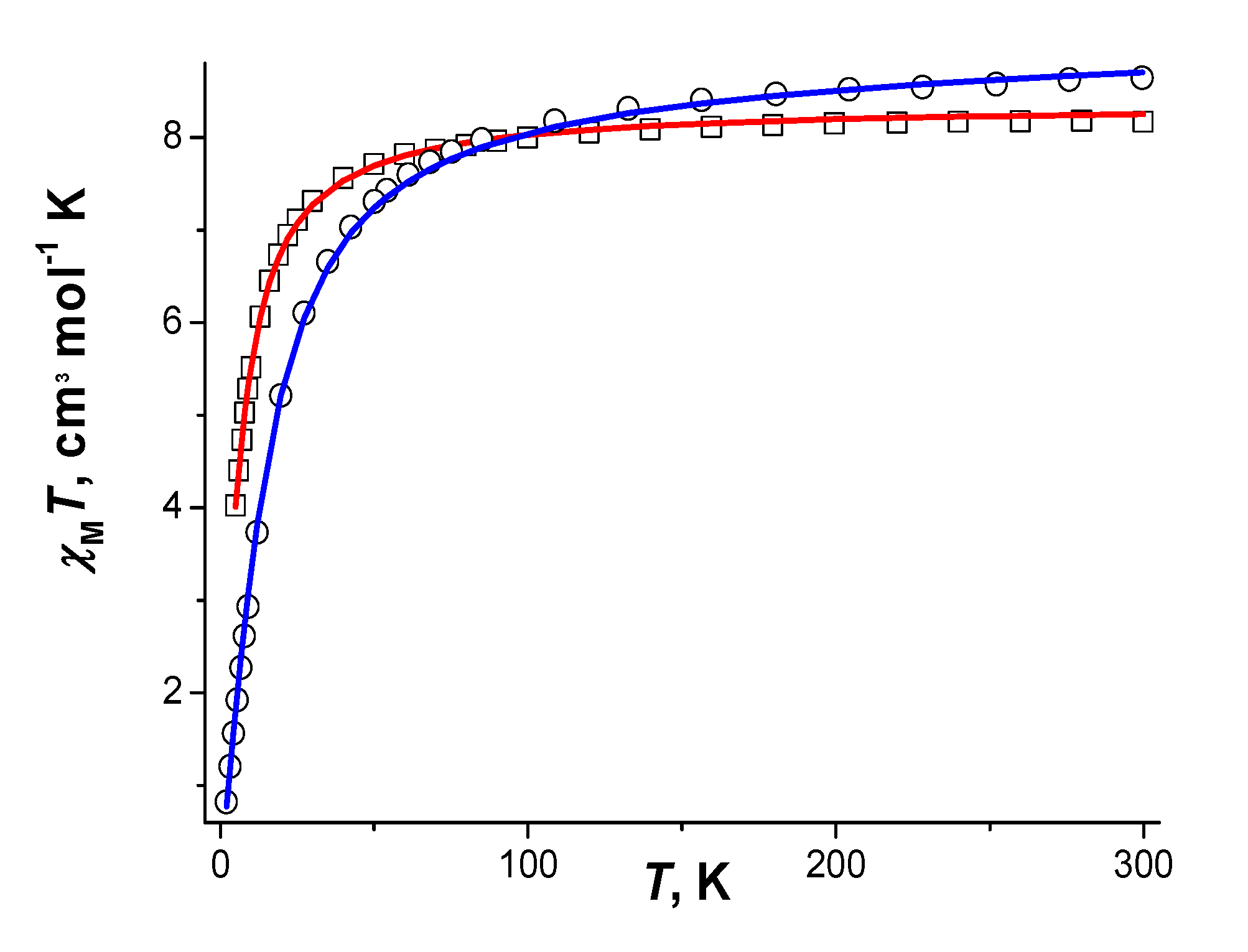
For both compounds χMT values (here and below χM is the magnetic susceptibility per formula unit and T is the temperature in K) monotonously decrease upon lowering the temperature from 8.17 (for 1) or 8.64 (for 2) cm3·K·mole−1 at 300 K to 7.81 (for 1 at 60 K) or 7.74 (for 2 at 68 K) cm3·K·mole−1, after which it falls sharply to 4.01 (for 1 at 5 K) or 0.82 (for 2 at 2 K) cm3·K·mole−1. Room-temperature values of χMT are close to the expected spin-only value (8.75 cm3·K·mole−1 for a system with two non interacting magnetic centers with S = 5/2).
The spin Hamiltonian for dinuclear blocks Mn2 in 1 and 2 is shown as Equation (5).
where the first summand corresponds to the superexchange interactions between Heisenberg spins localized at metal sites (JMn-Mn), and the second summand corresponds to the isotropic interactions between local spins and the external field through Zeeman interactions [80].
It should be noted that the spin-Hamiltonian proposed for interpretation of the magnetic properties differed from the one employed for interpretation of the EPR spectra of the same compounds. There was no contradiction between these Hamiltonians, as both of them were “partial” variations of the complete spin-Hamiltonian describing the system of the unpaired electron within the species of the compounds. This complete spin-Hamiltonian had to include all the terms: (i) Zeeman interactions with the external magnetic field; (ii) exchange interactions between the ions; (iii) zero-field splitting. However, the variations of exchange interaction parameters could not notably influence the studied EPR spectra (vide supra), while the influence of zero-field splitting on the magnetization curves was negligible compared to the influence of the exchange interactions. Thus, introduction of the corresponding terms into the spin-Hamiltonians and efforts to extract the corresponding parameters from the data simulations would not produce any reliable values. Regarding Zeeman interactions, their principal parameters—g-factors—were consistent (within accuracy of the methods) for the EPR and magnetochemical data.
Temperature-independent paramagnetism (tip) term was also introduced. Intermolecular interactions were taken into account within molecular field model (zJ’ term).
Analytical expression for the χMT values for the Mn2 unit [80] is the following:
where x = −J/kT.
The best agreement between experimental and calculated χMT curves (Figure 16) was achieved at parameters JMn-Mn = −1.03(2) cm−1, gMn = 2.0, zJ’ = −0.19(1) cm−1 (R2 = 2.44·10−5) for compound 1 and JMn-Mn = −1.03(2) cm−1; gMn = 2.0, tip = 7·10−4 (R2 = 9.87·10−5) for compound 2 (where R2 = ∑[(χMT)obs. − (χMT)calc.]2/(∑(χMT)obs.2)).
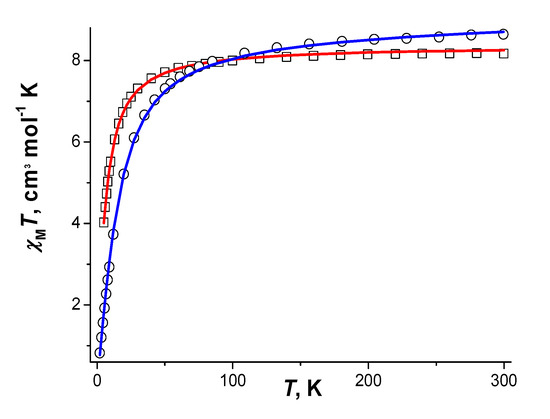
Figure 16.
χMT vs. T dependencies and the calculated curves (─) for 1 (Υ), 2 (⊄).
Absolute values of JMn-Mn for 1 and 2 are higher than those reported for benzoato- and phtalato-bridged dinuclear blocks Mn2(µ-O2C)2(η-O2C)2 [81,82], which is consistent with the higher electron-donating ability of the tert-butyl groups in pivalates compared to phenyl group.
2.5.2. Magnetic Properties of Complex 7 ·2MeCN
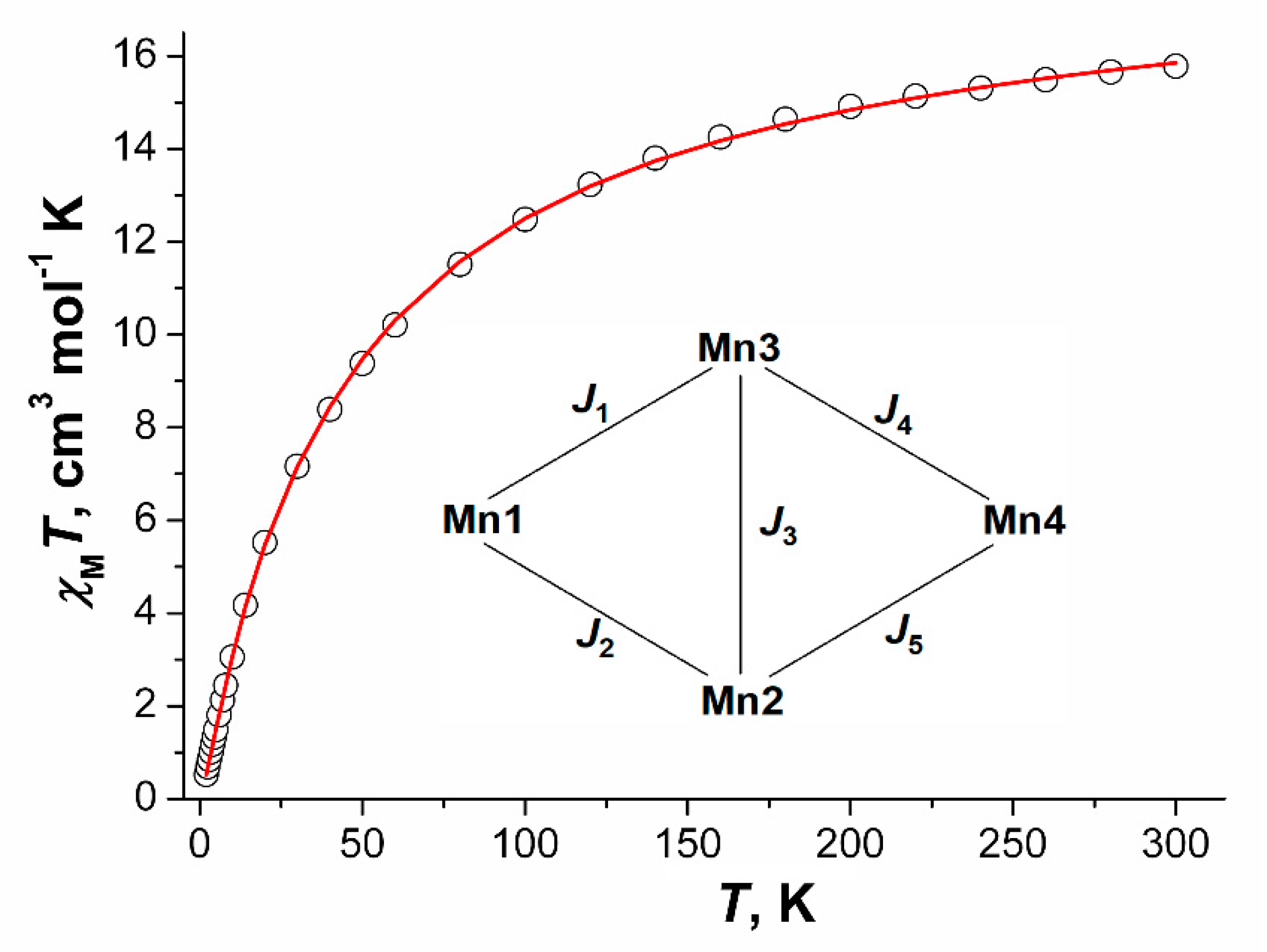
For the compound 7 2MeCN the χMT value monotonically decreases upon lowering the temperature from 15.78 cm3·K·mole−1 at 300 K to 0.53 cm3·K·mole−1 at 3 K. The room-temperature value of χMT is lower than the expected spin-only value (17.5 cm3·K·mole−1 for a system with four non-interacting magnetic centers with S = 5/2). The coupling scheme within a tetranuclear unit is presented in Figure 17. The spin Hamiltonian for tetranuclear block Mn4 takes the form:
where the first five summands correspond to the superexchange interactions between Heisenberg spins localized at metal sites (J1–J5), and the last summand corresponds to the isotropic interactions between local spins and the external field through Zeeman interactions (gMn), respectively [80]. A temperature-independent paramagnetism (tip) term was also introduced.
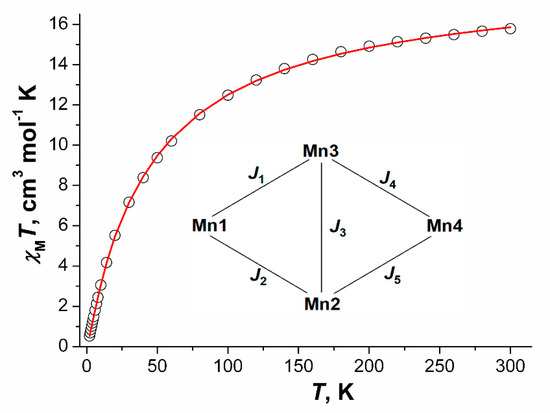
Figure 17.
χMT vs. T dependence, the calculated curve (─) and coupling scheme within a tetranuclear unit Mn4 for compound 7 2MeCN.
Calculation of the exchange coupling parameters were performed by full-matrix diagonalization using the Mjöllnir software [16,83]. Uncertainty values of simulation parameters were estimated as described previously [33]. Briefly, digits in brackets indicated deviation of the value, which caused 10% increase of R2.
The best correspondence between experimental and calculated χMT values for compound 7 was achieved for the parameters J1 = −2.69(2) cm−1, J2 = −2.38(2) cm−1, J3 = −0.8(1) cm−1, J4 = −0.42(2) cm−1, J5 = −0.8(2) cm−1, gMn = 2.0023 (fixed), zJ’ = −0.5(1) cm−1, tip = 0.00129 (R2 = 2.3·10−5).
Exchange coupling parameters have larger values for magnetic interactions of Mn1 ion with Mn2 and Mn3 which agree with the structural data: the Mn1 ion has in its coordination sphere two N atoms from two pyrazine molecules which increase its electron density and amplify the antiferromagnetic interactions. Additionally, the most effective way of magnetic interactions transfer through the OH group in the trinuclear Mn1Mn2Mn3 unit which also agrees with the received data.
The Mn4 units can be selected in the 2D-coordination polymer [Mn4(μ3-OH)(Piv)7(μ-pz)2]n, and exchange coupling within these units can be presented by five integrals J1–J5 (Figure 17). From the experimental χMT vs. T curve the following values could be calculated by full-matrix diagonalization (performed using the Mjöllnir software [16,83]) (cm−1): J1 = −2.7, J2 = −2.4, J3 = −0.8, J4 = −0.4, J5 = −0.8. Signs and magnitudes of these J were estimated using broken-symmetry DFT calculations with TPSSh functional and LANLTZ ECPs (3d ions)/def2-SVP basis set. For calculation of each J value all Mn2+ ions in the Mn4 core except the two ions taking part in the coupling were “substituted” by diamagnetic Zn2+ ions, and then calculations of the high-spin (HS) and the broken symmetry (BS) states’ energies (see Experimental part for details of their construction) were performed. The results evidence that two exchange integrals, J1 and J2, have the same order of magnitude (−6.9 and −6.4 cm−1, respectively); while J3, J4, J5 fall in the range from −0.9 to −1.2 cm−1. The exchange through pz bridge is estimated as −0.2 cm−1. These results correlate with J values, found from χMT vs. T curve simulation (|J1|, |J2| the highest and close to each other, |J3|–|J5|—lower and close to each other).
It should be noted that fitting of the χMT vs. T curve for 7 2MeCN could be fitted with simpler Hamiltonian (8):
with J1 = −2.08 ± 0.02 cm−1, J2 = −3.92 ± 0.07 cm−1, g = 2 (fixed), however there are no reasons to neglect interactions between other MnII ions, since structural features of the bridges between them are similar. The χMT vs. T curve calculated with these parameters is visually the same as shown on Figure 17.
2.5.3. Magnetic Properties of Complex 11·3.5DMF
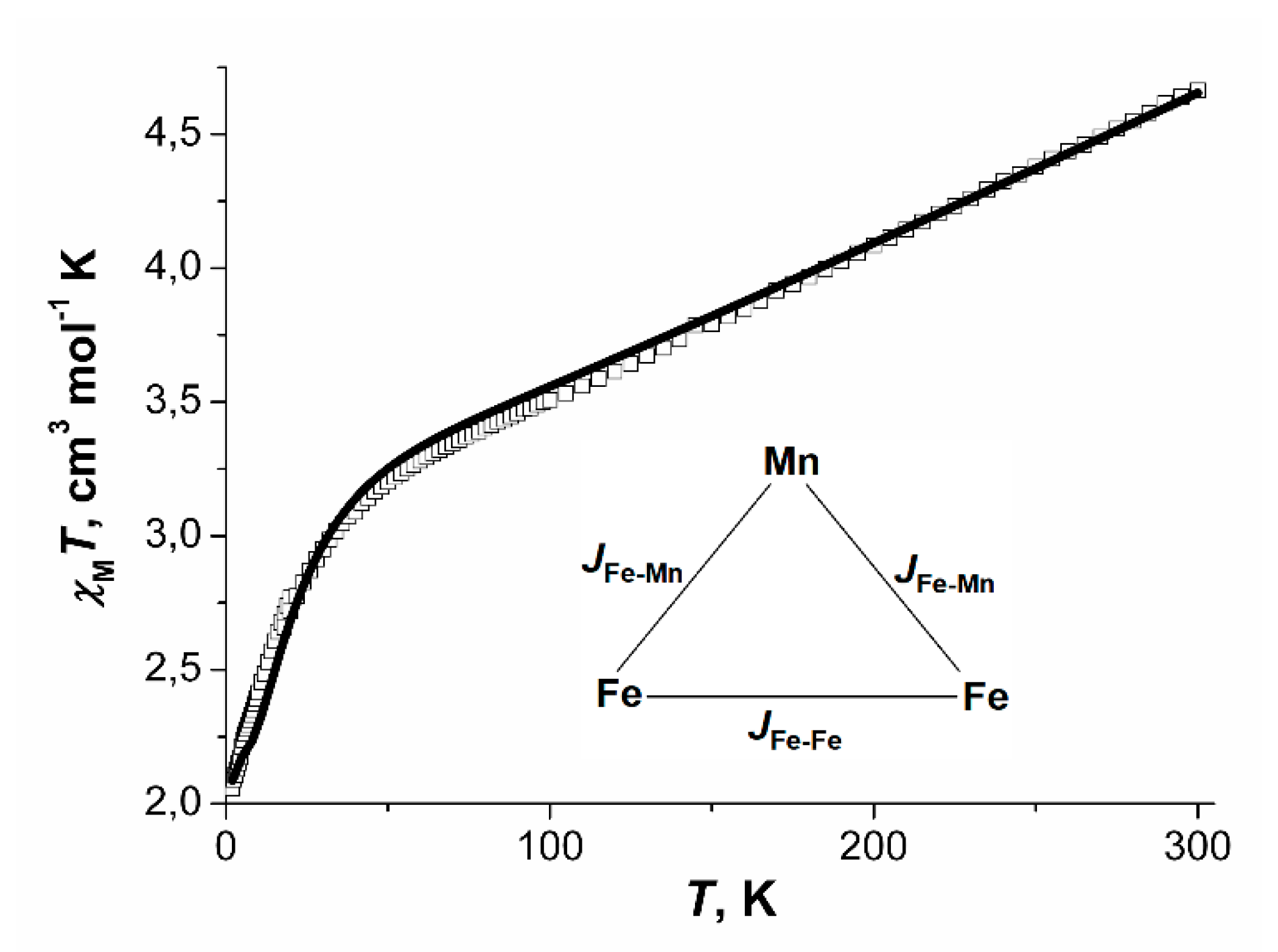
For compound 11·3.5DMF, the value of χMT at 300 K was 4.66 cm3·K·mol−1, which is significantly lower than the expected spin-only value for three non-interacting spins 5/2 (13.125 cm3·K·mol−1). On cooling, the χMT vs. T curve decreased monotonically to 3.20 cm3·K/mol at 50 K, after which it sharply fell to 2.08 cm3·K·mol−1 at 2 K.
The coupling scheme within a trinuclear unit is represented on Figure 18. The spin Hamiltonian for trinuclear blocks Fe2Mn takes the form:
where first line corresponds to the superexchange interactions between Heisenberg spins localized at metal sites (JFe-Fe and JFe-Mn), the second line corresponds to the isotropic interactions between local spins and the external field through Zeeman interactions (gFe and gMn), respectively [80]. Intermolecular interactions were taken into account within molecular field model.
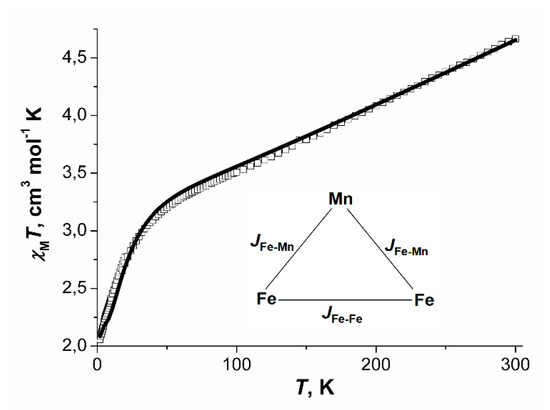
Figure 18.
χMT vs. T dependence, the calculated curve (─) and coupling scheme within a trinuclear unit Fe2Mn for compounds 11·3.5DMF.
The best correspondence between experimental and calculated χMT values for the compound was achieved at parameters JFe-Fe = −57.8(2) cm−1, JFe-Mn = −20.12(7) cm−1, gFe = gMn = 2.0023 (fixed), zJ’ = −0.10(1) cm−1, χimpurities = 3.2% (Simpurities = 5/2).
Absolute values of JFe-Mn and JFe-Mn for 11 3.5DMF are higher than those reported for the trifluoroacetate complex [Fe2MnO(O2CCF3)6(H2O)3] (JFe-Fe = −56.50(7) cm−1, JFe-Mn = −16.23(4) cm−1 [31], which is consistent with the higher electron-donating ability of the methyl group in acetate relative to CF3-group. However, for {Fe2MnO(Piv)6} blocks exchange coupling parameters [58] have close values to exchange coupling parameters for 11·3.5DMF. It can be explained by different nitrogen ligands: pyridine group in 11·3.5DMF in comparison with hexamethylenetetramine [58] compensates lower electron donor efficiency of acetate group in comparison with pivalate group [84].
3. Experimental
3.1. Materials and Methods
Reagents and solvents were commercially available (Sigma-Aldrich, Aldrich, St. Louis, MO, USA) and were used without further purification. Manganese pivalate [Mn(Piv)2(EtOH)]n and trinuclear acetate [MnFe2O(OAc)6(H2O)3], used as starting compound, were prepared as previously reported [30,51]. C,H,N-analyses were performed using a 1106 instrument (Carlo Erba, Instruments, Egelsbach, Germany). IR-spectra were measured in KBr pellets on a Spectrum BX FT-IR spectrometer (Perkin Elmer, Waltham, MA, USA) in 400–4000 cm−1 range. The X-ray powder diffraction analysis of 6 was carried out on a G670 (HUBER, Offenburg, Germany) Guinier camera using CuKα1 radiation on air. The X-ray powder diffraction analysis of 11 was performed on a D8 Advance instrument (Bruker, Billerica, MA, USA) in air.
Thermogravimetric analyses (TGA) were performed in air on Q1500 instrument, (Paulik-Paulik-Erdey, Budapest, Hungary). The heating rate was 5 °C per minute. Sorption of methanol and ethanol by [MnFe2O(OAc)6(dpe)] was studied gravimetrically, using a tungsten microbalance at 293 K. Each point on the absorption and desorption isotherms corresponds to equilibrium conditions (no change of sample weight at certain p·pS−1, where pS is the pressure of saturated vapor of the compound at 293 K). This sample was thermally activated at 150 °C in vacuum at 10−2 Torr. Volume of pores was estimated from the quantity of adsorbed alcohol using its density in liquid phase at 293 K.
Magnetic measurements were performed on a MPMS-XL (for 11), MPMS-5S (for 7) and PPMS (for 1 and 2) SQUID magnetometers (Quantum Design, San Diego, CA, USA) and intrinsic diamagnetic corrections were calculated using Pascal’s constants [80]. The X-band EPR spectra for 1 and 2 were measured on a Bruker Elexsys E680-X spectrometer at T = 293 K.
3.2. Synthesis
3.2.1. Synthesis of [Mn2(Piv)4(2,2′-bipy)2] (1) and [Mn2(Piv)4(phen)2] (2)
The syntheses were carried out under an argon atmosphere. [Mn(Piv)2(EtOH)]n (0.150 g, 0.50 mmol) was dissolved in MeCN (30 mL for 1 or 25 mL for 2), followed by the addition of 2,2′-bipyridine (0.078 g, 0.50 mmol) for 1 or 1,10-phenantroline (0.090 g, 0.50 mmol) for 2. The resulting colorless solution was heated at 80 °C during 30 min, then concentrated to 6–8 mL for 1 or 4–6 mL for 2 and kept at 5 °C during 24 h. Crystals were isolated by decantation, washed by cold MeCN and dried under argon stream. Yield of 1: 0.14 g (67%), yellow crystals; 2: 0.162 g (74%), colorless crystals. Anal, calc. for 1, C40H52Mn2N4O8/found, %: C 58.1/58.0, H 6.3/6.5, N 6.8/6.9. IR-spectrum of 1 (cm−1): 3434 m, 3058 w, 2955 s, 2923 m, 2865 w, 1597 vs, 1542 s, 1516 s, 1482 s, 1420 s, 1373 m, 1359 m, 1225 m, 1143 w, 1101 w, 894 w, 864 w, 846 m, 792 b.w, 729 s, 637 w, 601 w. Anal, calc. for 2, C44H52Mn2N4O8/found, %: C 60.4/59.8, H 6.0/6.2, N 6.4/6.4. IR-spectrum of 2 (cm−1): 3434 s, 2956 s, 2923 m, 2866 w, 1586 v.s, 1547 s, 1482 s, 1440 s, 1419 s, 1372 m, 1358 m, 1314 w, 1226 m, 1172 w, 1155 w, 1059 w, 1015 m, 893 m, 805 w, 792 w, 767 s, 738 m, 646 w, 625 w, 603 w, 558 w, 415 m.
3.2.2. Synthesis of [Mn4O2(Piv)6(2,2′-bipy)2]·MeCN (3·MeCN) (3) and [Mn4O2(Piv)6(phen)2]· 0.5MeCN (4·0.5MeCN) (4)
The syntheses were carried out under an argon atmosphere. [Mn(Piv)2(EtOH)]n (0.150 g, 0.50 mmol) was dissolved in 30 mL of MeCN followed by the addition of 2,2′-bipyridine (0.078 g, 0.50 mmol) for 3·MeCN or 1,10-phenantroline (0.090 g, 0.50 mmol) for 4·0.5MeCN. The colorless solution was heated at 80 °C during 30 min, then kept under air at room temperature during 24 h for 3·MeCN or 10 days for 4·0.5MeCN. The precipitate was isolated by decantation and dissolved in 30 mL of THF, filtered and then kept under air at room temperature during one week. Brown crystals were isolated by decantation, washed by cold MeCN and dried on air. Yield of 3·MeCN: 0.051 g (35%), brown microcrystals; yield of 4·0.5MeCN: 0.045 g (29%), dark-brown crystals. Anal, calc. for 3·MeCN, C50H70Mn4N4O14/found, %: C 51.3/51.1, H 6.0/6.2, N 4.8/4.9. IR-spectrum of 3·MeCN (cm−1): 3433 m, 2956 m, 2925 m, 2868 w, 1589 v.s., 1570 s, 1482 s, 1441 m, 1415 s, 1370 s, 1357 m, 1317 w, 1225 m, 1155 w, 1016 w, 890 w, 789 w, 766 m, 740 w, 640 b.m, 431 m, 410 m. Anal, calc. for 4·0.5MeCN, C55H71.5Mn4N4.5O14/found, %: C 53.3/53.4, H 5.8/5.9, N 5.1/5.2. IR-spectrum of 4·0.5MeCN (cm−1): 3435 w, 2956 s, 2924 m, 2868 m, 1590 s, 1560 s, 1514 s, 1482 s, 1456 m, 1411 s, 1371 s, 1358 s, 1261 w, 1226 s, 1141 w, 1101 m, 1028 w, 891 w, 864 m, 853 m, 790 m, 732 s, 656 s, 639 s, 616 s, 420 bm.
3.2.3. Synthesis of [Mn6(OH)2(Piv)10(pym)4] (5) and Synthesis of [Mn6O2(Piv)10(pym)2]n (6)
Syntheses were carried out under an argon atmosphere. [Mn(Piv)2(EtOH)]n (0.6 g, 1.98 mmol) was dissolved in 30 mL of EtOH, followed by the addition of pyrimidine (0.11 g, 1.40 mmol). The colorless solution was heated at 80 °C during 30 min. For the isolation of 5 the solution was concentrated to 2–3 mL and cooled at −18 °C during 24 h. For the isolation of 6 the solution was kept on air at room temperature during 24 h. The crystals were isolated by decantation, washed by cold MeCN and dried under argon stream. Yield of 5: 0.12 g (22%) colorless crystals; yield of 6: 0.43 g (85%), colorless crystals. Anal, calc. for 5, C66H108Mn6N8O22/found, %: C 46.8/46.7, H 6.4/6.2, N 6.6/6.8. IR-spectrum of 5 (cm−1): 3419 b.m, 2960 s, 2928 s, 1676 s, 1587 b.s, 1484 s, 1640 s, 1422 s, 1362 s, 1227 s, 1169 w, 1077 w, 1030 w, 937 w, 895 m, 790 m, 716 m, 636 m, 601s, 559 m, 540 m, 415 m. Anal, calc. for 6, C58H98Mn6N4O22/found, %: C 45.4/45.5, H 6.4/6.2, N 3.7/3.8. IR-spectrum of 6 (cm−1): 3434 b.m, 2958 m, 2926 w, 2870 w, 1582 v.s, 1569 s, 1482 s, 1467 w, 1417 v.s, 1374 s, 1359 s, 1228 s, 1163 w, 1076 w, 1029 w, 1003 w, 892 w, 795 w, 786 w, 715 w, 632 w, 612 m, 557 w, 437 w, 404 w.
3.2.4. Synthesis of [Mn4(OH)(Piv)7(pz)2]n∙2nMeCN (7∙2MeCN)
The synthesis was carried out under an argon atmosphere. [Mn(Piv)2(EtOH)]n (0.1 g, 0.33 mmol) was dissolved in 20 mL of MeCN, then a solution containing pyrazine (0.03 g, 0.33 mmol) in 5 mL MeCN was added dropwise. The colorless solution was kept at 25 °C during 48 h. Yellow precipitate with crystals was isolated by decantation, washed by cold MeCN and dried under argon stream. Yield 0.08 g (82%). Anal, calc. for C47H78Mn4N6O15/found, %: C 47.6/47.4, H 6.6/6.8, N 7.1/7.2. IR-spectra (cm−1): 3432 b.m, 2959 s, 2928 m, 2870 w, 1589 v.s, 1569 v.s, 1483 v.s, 1458 m, 1422 s, 1374 s, 1360 s, 1227 s, 1135 w, 1047 m, 893 w, 791 m, 600 m, 564 w, 450 w, 409 m.
3.2.5. Synthesis of [Mn4Fe2O2(Piv)10(MeCN)2(HPiv)2]·2MeCN (8·2MeCN)
The synthesis was carried out under an argon atmosphere. A reaction mixture of [Mn(Piv)2(EtOH)]n (0.8 g, 2.64 mmol) and FeCl3 (0.122 g, 0.75 mmol) in 100 mL MeCN was heated (80 °C) during 30 min to complete the dissolution of reagents. The brown solution obtained was concentrated to 50 mL and kept at room temperature during 6 h. The white and brown precipitates formed were separated from the solution. The solution was concentrated to 20 mL and kept at room temperature during 24 h. Brown crystals were isolated by decantation, washed by cold MeCN and dried under argon stream. Yield 0.16 g (15%). Anal, calc. for C64Fe2H116Mn4O26N2 (without solvent molecules)/found, %: C 46.3/46.4, H 7.0/6.9, N 1.7/1.6, Fe 6.7/6.6; Mn 13.2/13.0. IR-spectra (cm−1): 3192 w, 2962 s, 2930 s, 2873 m, 1692 m, 1570 s, 1484 s, 1460 m, 1420 s, 1376 s, 1360 s, 1314 w, 1227 s, 1206 m, 1031 w, 938 w, 895 m, 872 w, 787 m, 764 v.w, 604 m, 574 m, 516 m, 420 s.
3.2.6. Synthesis of [MnFe2O(OAc)6(4,4′-bipy)2]n·2nDMF (9·2DMF)
[Fe2MnO(OAc)6(H2O)3] (0.1 g, 0.169 mmol) was dissolved in 3 mL of DMF, followed by the addition of 15 mL of MeCN, then 0.1 mL of acetic acid was added, and then bipy (0.250 g, 0.423 mmol, a 25% excess) was dissolved in this solution. After one day black crystals formed, which were collected by filtration, washed with MeCN (2 portions by 3 mL) and dried on air. Yield 0.04 g (25%). Anal, calc. for C38H48N6O15Fe2Mn/found, %: C 45.9/45.6, H 4.86/4.50, N 8.44/8.50.
3.2.7. Synthesis of [MnFe2O(OAc)6(bpe)2]n·2nDMF (10·2DMF)
[Fe2MnO(OAc)6(H2O)3] (0.1 g, 0.169 mmol) was dissolved in 3 mL of DMF, followed by the addition of 15 mL of MeCN. Next 0.1 mL of acetic acid was added, and then bpe (0.185 g, 0.014 mmol, a 3-fold excess) was dissolved in this solution. After one day black crystals formed, which were collected by filtration, washed with MeCN (2 portions by 3 mL) and dried on air. Yield 0.07 g (40%). Anal, calc. for C42H52N6O15Fe2Mn / found, %: C 48.2/48.1, H 5.00/5.15, N 8.02/7.98.
3.2.8. Synthesis of [MnFe2O(OAc)6(bpe)(DMF)]n·3.5nDMF (11·3.5DMF)
[Fe2MnO(OAc)6(H2O)3] (0.1 g, 0.169 mmol) was dissolved in 3 mL of DMF, followed by the addition of 15 mL of MeCN. Next 0.1 mL of acetic acid was added, and then bpe (0.038 g, 0.211 mmol, a 25% excess) was dissolved in this solution. After one day black crystals formed, which were collected by filtration, washed with MeCN (two portions of 3 mL) and dried on air. Yield 0.02 g (10%). Anal, calc. for C34.5H52.5N5.5O16.5Fe2Mn/found, %: C 42.5/42.1, H 5.43/5.35, N 7.90/7.95.
3.3. X-ray Structure Determination
For X-ray structure determination single crystals of the compounds 1–8 were isolated from the mother liquors and mounted on a Bruker APEX II diffractometer equipped with a CCD camera and a graphite monochromated MoKα radiation source (λ = 0.71073 Å) at the N. S. Kurnakov Institute of General and Inorganic Chemistry (Moscow, Russia). X-ray structure determination for compounds 9–11 was performed using a a Kappa-Nonius four circle diffractometer equipped with a CCD camera and a graphite monochromated MoKα radiation source (λ = 0.71073 Å), located at the Centre de Diffractométrie (CDIFX), Université de Rennes 1 (Rennes, France).
Effective absorption correction was performed using SCALEPACK. Structures of the complexes were solved by the direct method using SHELXS-97 [85] or Sir-97 [86] software, and refined with a full matrix least squares method on F2 using SHELXL-97, SHELX-2014 or SHELX-2018 program [87]. H atoms were treated by a riding model. Solvent molecules, which could not be localized, were removed by SQUEEZE procedure for compounds 9–11 [88]. The structure of 6 was solved taking into account crystal twinning (Flack parameter is 0.42(4)). Crystallographic data and structure refinement parameters for 1–11 are presented in Table 2, Table 3 and Table 4. Supplementary crystallographic data for the compounds synthesized are given in CCDC numbers 2055493-2055503 for 1–11, respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.

Table 2.
Crystallographic data and structure refinement parameters for 1–4.

Table 3.
Crystallographic data and structure refinement parameters for 5–8.

Table 4.
Crystallographic data and structure refinement parameters for 9–11.
3.4. DFT Calculations
The signs and the magnitudes of exchange coupling parameters J were independently estimated by DFT calculations similar to the previously reported by us in details [89], brief description of the methodology is provided in this section.
The calculations were performed via ORCA software [90]. TPSSh [91,92,93] exchange-correlation potential was employed for the calculation together with LANLTZ basis sets [94] for 3d ions and def2-SVP [95] basis set for the rest of the elements.
The atomic coordinates were taken from the crystallographic data. For calculation of each J value, all Mn2+ ions in Mn4 core except the two ions taking part in the coupling were “substituted” by diamagnetic Zn2+ ions in order to simplify the system of spin states of the species. Broken symmetry DFT approach was applied for the calculation of J: first, a single-point calculation was performed for the high-spin state of the Mn2Zn2 species, then the broken symmetry state was constructed by artificial flipping the spin projections of the unpaired electrons localized on one of the Mn2+ ions, and a single-point calculation was performed again. J value was obtained using the energies EHS and EBS resulted from the two converged single-point calculations as J = − (EHS − EBS)/(<S2>HS−<S2>BS) [96] (<S2>HS and <S2>BS—the total spin operator expectation values derived from the calculations).
4. Conclusions
In this study a variety of transformations of Mn-containing complexes in reactions with N-donor heterocycles was shown. In was found that homometallic Mn pivalates underwent metamorphosis while heterometallic acetates with a Fe2MnO core preserved their structure, giving rise to coordination polymers. Different behavior of MnII or NiII and CoII pivalates in reactions with FeCl3 was also revealed: while the first complex produced a hexanuclear [MnII4FeIII2O2(Piv)10(MeCN)2(HPiv)2] compound, the latter (NiII and CoII) pivalates under similar conditions gave trinuclear pivalates with a Fe2MnO core.
Several new coordination polymers with bridging pyrimidine (i.e., [Mn6O2(Piv)10(pym)2]n), 4,4-bipyridine (i.e., [MnFe2O(OAc)6(4,4′-bipy)2]n) or 1,2-bis-trans-(4-pyridyl)ethylene (i.e., [MnFe2O(OAc)6(bpe)2]n and [MnFe2O(OAc)6(bpe)(DMF)]n) were prepared. Unexpectedly, the composition of the coordination polymers based on MnFe2O(OAc)6 was probably governed by a fine balance between formation and crystallization kinetics and solubility of certain species, in contrast to the ratio of potential vacations in the coordination spheres of metal ions and the number of donor atoms). The crystal lattice of [MnFe2O(OAc)6(bpe)(DMF)]n collapsed upon desolvatation, and the resulting compound was not porous in respect to N2, however the porosity was restored upon interaction with methanol and ethanol. Notably, the hexanuclear unit {MnII4FeIII2O2(O2CR)10} can be considered a promising new building block for the creation of coordination polymers.
Magnetic properties of the compounds were in line with those expected for Mn carboxylates. The temperature dependence of the magnetic susceptibility of [Mn4(μ3-OH)(Piv)7(μ-pz)2]n could be fitted with five exchange coupling parameters, and their reliability was independently checked by DFT calculations.
Supplementary Materials
The following are available online. Figure S1: PXRD data for 6; Figure S2: Visualization of solvent-accessible voids for 9.
Author Contributions
Conceptualization, L.O. and I.L.E.; Formal analysis, R.A.P., I.S.E., A.S.L., V.V.M., S.V.K. and M.A.K.; Investigation, R.A.P., I.S.E., O.C., S.G., K.S.G., A.S.L., N.N.E., V.V.M., A.S.B., S.V.K. and M.A.K.; Methodology, R.A.P., S.V.K. and M.A.K.; Writing—original draft, S.V.K.; Writing—review & editing, S.G., A.S.L., S.V.K. and M.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
S.V.K. thanks to the National Academy of Sciences of Ukraine and University of Rennes for support. I.S.E., N.N.E., V.V.M., M.A.K. and I.L.E. thank IGIC RAS state assignment for support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Single crystal X-ray analysis (1–8), IR-spectroscopy, elemental analysis, magnetic (1, 2, 7) and EPR (1, 2) measurements were performed using shared experimental facilities supported by IGIC RAS state assignment.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Glaser, F.; Wenger, O.S. Recent Progress in the Development of Transition-Metal Based Photoredox Catalysts. Coord. Chem. Rev. 2020, 405, 213129. [Google Scholar] [CrossRef]
- Hazari, N.; Melvin, P.R.; Beromi, M.M. Well-Defined Nickel and Palladium Precatalysts for Cross-Coupling. Nat. Rev. Chem. 2017, 1, 0025. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.G.; Napoline, J.W.; Thomas, C.M. Catalytic Applications of Early/Late Heterobimetallic Complexes. Cat. Rev. 2012, 54, 1–40. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Dronova, M.S.; Yalymov, A.I.; Lamaty, F.; Bantreil, X.; Martinez, J.; Bizet, C.; Shul’pina, L.S.; Korlyukov, A.A.; Arkhipov, D.E.; et al. Cage-like Copper(II) Silsesquioxanes: Transmetalation Reactions and Structural, Quantum Chemical, and Catalytic Studies. Chem. Eur. J. 2015, 21, 8758–8770. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, A.M.; Kirillova, M.V.; Pombeiro, A.J.L. Homogeneous Multicopper Catalysts for Oxidation and Hydrocarboxylation of Alkanes. Adv. Inorg. Chem. 2013, 65, 1–31. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A. Applications of Metal–Organic Coordination Polymers as Precursors for Preparation of Nano-Materials. Coord. Chem. Rev. 2012, 256, 2921–2943. [Google Scholar] [CrossRef]
- de la Rosa, L.A.G.; Méndez-Rojas, M.A. Direct Synthesis of Nanomaterials: Building Bridges between Metal Complexes and Nanomaterials. In Direct Synthesis of Metal Complexes; Elsevier: Amsterdam, The Neatherlands, 2018; pp. 317–337. ISBN 978-0-12-811061-4. [Google Scholar]
- Zauzolkova, N.; Dobrokhotova, Z.; Lermontov, A.; Zorina, E.; Emelina, A.; Bukov, M.; Chernyshev, V.; Sidorov, A.; Kiskin, M.; Bogomyakov, A.; et al. Step-by-Step Thermal Transformations of a New Porous Coordination Polymer [(H2O)5CuBa(Me2mal)2]n (Me2mal2−=dimethylmalonate): Thermal Degradation to Barium Cuprate. J. Solid State Chem. 2013, 197, 379–391. [Google Scholar] [CrossRef]
- Maniaki, D.; Pilichos, E.; Perlepes, S.P. Coordination Clusters of 3d-Metals That Behave as Single-Molecule Magnets (SMMs): Synthetic Routes and Strategies. Front. Chem. 2018, 6, 461. [Google Scholar] [CrossRef]
- Coronado, E. Molecular Magnetism: From Chemical Design to Spin Control in Molecules, Materials and Devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Gaita-Ariño, A.; Luis, F.; Hill, S.; Coronado, E. Molecular Spins for Quantum Computation. Nat. Chem. 2019, 11, 301–309. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shubina, E.S.; Long, J.; Guari, Y.; Larionova, J. Magnetic cage-like metallasilsesquioxanes. Coord. Chem. Rev. 2019, 398, 213015. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Nandasiri, M.I.; Jambovane, S.R.; McGrail, B.P.; Schaef, H.T.; Nune, S.K. Adsorption, Separation, and Catalytic Properties of Densified Metal-Organic Frameworks. Coord. Chem. Rev. 2016, 311, 38–52. [Google Scholar] [CrossRef]
- Jeon, I.-R.; Clérac, R. Controlled Association of Single-Molecule Magnets (SMMs) into Coordination 15.Networks: Towards a New Generation of Magnetic Materials. Dalton Trans. 2012, 41, 9569. [Google Scholar] [CrossRef]
- Polunin, R.A.; Kolotilov, S.V.; Kiskin, M.A.; Cador, O.; Mikhalyova, E.A.; Lytvynenko, A.S.; Golhen, S.; Ouahab, L.; Ovcharenko, V.I.; Eremenko, I.L.; et al. Topology Control of Porous Coordination Polymers by Building Block Symmetry. Eur. J. Inorg. Chem. 2010, 2010, 5055–5057. [Google Scholar] [CrossRef]
- Kolotilov, S.V.; Cador, O.; Gavrilenko, K.S.; Golhen, S.; Ouahab, L.; Pavlishchuk, V.V. Assembly of Dinuclear CuII Rigid Blocks by Bridging Azido or Poly(Thiocyanato)Chromates: Synthesis, Structures and Magnetic Properties of Coordination Polymers and Polynuclear Complexes. Eur. J. Inorg. Chem. 2010, 2010, 1255–1266. [Google Scholar] [CrossRef]
- Lytvynenko, A.S.; Kolotilov, S.V.; Kiskin, M.A.; Cador, O.; Golhen, S.; Aleksandrov, G.G.; Mishura, A.M.; Titov, V.E.; Ouahab, L.; Eremenko, I.L.; et al. Redox-Active Porous Coordination Polymers Prepared by Trinuclear Heterometallic Pivalate Linking with the Redox-Active Nickel(II) Complex: Synthesis, Structure, Magnetic and Redox Properties, and Electrocatalytic Activity in Organic Compound Dehalogenation in Heterogeneous Medium. Inorg. Chem. 2014, 53, 4970–4979. [Google Scholar] [CrossRef] [PubMed]
- Polunin, R.A.; Kiskin, M.A.; Cador, O.; Kolotilov, S.V. Coordination Polymers Based on Trinuclear Heterometallic Pivalates and Polypyridines: Synthesis, Structure, Sorption and Magnetic Properties. Inorg. Chim. Acta 2012, 380, 201–210. [Google Scholar] [CrossRef]
- Botezat, O.; van Leusen, J.; Kravtsov, V.C.; Filippova, I.G.; Hauser, J.; Speldrich, M.; Hermann, R.P.; Krämer, K.W.; Liu, S.-X.; Decurtins, S.; et al. Interpenetrated (8,3)-c and (10,3)-b Metal–Organic Frameworks Based on {FeIII3} and {FeIII2CoII} Pivalate Spin Clusters. Cryst. Growth Des. 2014, 14, 4721–4728. [Google Scholar] [CrossRef]
- Botezat, O.; van Leusen, J.; Kögerler, P.; Baca, S.G. Tuning the Condensation Degree of {FeIIIn} Oxo Clusters via Ligand Metathesis, Temperature, and Solvents. Inorg. Chem. 2018, 57, 7904–7913. [Google Scholar] [CrossRef]
- Botezat, O.; van Leusen, J.; Kravtsov, V.C.; Kögerler, P.; Baca, S.G. Ultralarge 3d/4f Coordination Wheels: From Carboxylate/Amino Alcohol-Supported {Fe4Ln2} to {Fe18Ln6} Rings. Inorg. Chem. 2017, 56, 1814–1822. [Google Scholar] [CrossRef]
- Taguchi, T.; Daniels, M.R.; Abboud, K.A.; Christou, G. Mn4, Mn6, and Mn11 Clusters from the Use of Bulky Diphenyl(pyridine-2-yl)methanol. Inorg. Chem. 2009, 48, 9325–9335. [Google Scholar] [CrossRef] [PubMed]
- Mondal, K.C.; Song, Y.; Mukherjee, P.S. Self-Assembly of a Mn9 Nanoscopic Mixed-Valent Cluster: Synthesis, Crystal Structure, and Magnetic Behavior. Inorg. Chem. 2007, 46, 9736–9742. [Google Scholar] [CrossRef]
- Wemple, M.W.; Tsai, H.-L.; Wang, S.; Claude, J.P.; Streib, W.E.; Huffman, J.C.; Hendrickson, D.N.; Christou, G. Tetranuclear and Octanuclear Manganese Carboxylate Clusters: Preparation and Reactivity of (NBun4)[Mn4O2(O2CPh)9(H2O)] and Synthesis of (Nbun4)2[Mn8O4(O2CPh)12(Et2Mal)2(H2O)2] with a “Linked-Butterfly” Structure. Inorg. Chem. 1996, 35, 6437–6449. [Google Scholar] [CrossRef]
- Boskovic, C.; Wernsdorfer, W.; Folting, K.; Huffman, J.C.; Hendrickson, D.N.; Christou, G. Single-Molecule Magnets: Novel Mn8 and Mn9 Carboxylate Clusters Containing an Unusual Pentadentate Ligand Derived from Pyridine-2,6-Dimethanol. Inorg. Chem. 2002, 41, 5107–5118. [Google Scholar] [CrossRef]
- Polunin, R.A.; Kolotilov, S.V.; Kiskin, M.A.; Gavrilenko, K.S.; Ouahab, L.; Eremenko, I.L.; Novotortsev, V.M.; Pavlishchuk, V.V. Structures and Sorption Properties of the Coordination Polymers Built up of 3d Metal Carboxylate Polynuclear Complexes. Russ. Chem. Bull. 2010, 59, 1217–1224. [Google Scholar] [CrossRef]
- Köberl, M.; Cokoja, M.; Herrmann, W.A.; Kühn, F.E. From Molecules to Materials: Molecular Paddle-Wheel Synthons of Macromolecules, Cage Compounds and Metal–Organic Frameworks. Dalton Trans. 2011, 40, 6834. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.F.; Kanekar, C.R.; Mok, K.F. Some Trinuclear Iron(III) Carboxylate Complexes. J. Chem. Soc. Inorg. Phys. Theor. 1969, 480–482. [Google Scholar] [CrossRef]
- Blake, A.B.; Yavari, A.; Hatfield, W.E.; Sethulekshmi, C.N. Magnetic and Spectroscopic Properties of Some Heterotrinuclear Basic Acetates of Chromium(III), Iron(III), and Divalent Metal Ions. J. Chem. Soc. Dalton Trans. 1985, 2509–2520. [Google Scholar] [CrossRef]
- Gavrilenko, K.S.; Vértes, A.; Vanko, G.; Kiss, L.F.; Addison, A.W.; Weyhermüller, T.; Pavlishchuk, V.V. Synthesis, Magnetochemistry, and Spectroscopy of Heterometallic Trinuclear Basic Trifluoroacetates [Fe2M(µ3-O)(CF3COO)6(H2O)3]·H2O (M = Mn, Co, Ni). Eur. J. Inorg. Chem. 2002, 2002, 3347–3355. [Google Scholar] [CrossRef]
- Lytvynenko, A.S.; Kolotilov, S.V.; Cador, O.; Gavrilenko, K.S.; Golhen, S.; Ouahab, L.; Pavlishchuk, V.V. Porous 2D Coordination Polymeric Formate Built up by Mn(II) Linking of Fe3O Units: Influence of Guest Molecules on Magnetic Properties. Dalton Trans. 2009, 3503–3509. [Google Scholar] [CrossRef]
- Kiskin, M.; Zorina-Tikhonova, E.; Kolotilov, S.; Goloveshkin, A.; Romanenko, G.; Efimov, N.; Eremenko, I. Synthesis, Structure, and Magnetic Properties of a Family of Complexes Containing a CoII2DyIII Pivalate Core and a Pentanuclear CoII4DyIII Derivative. Eur. J. Inorg. Chem. 2018, 2018, 1356–1366. [Google Scholar] [CrossRef]
- Burkovskaya, N.P.; Orlova, E.V.; Kiskin, M.A.; Efimov, N.N.; Bogomyakov, A.S.; Fedin, M.V.; Kolotilov, S.V.; Minin, V.V.; Aleksandrov, G.G.; Sidorov, A.A.; et al. Synthesis, Structure, and Magnetic Properties of Heterometallic Trinuclear Complexes {MII—LnIII—MII} (MII = Ni, Cu; LnIII = La, Pr, Sm, Eu, Gd). Russ. Chem. Bull. 2011, 60, 2490–2503. [Google Scholar] [CrossRef]
- Lutsenko, I.A.; Kiskin, M.A.; Nikolaevskii, S.A.; Starikova, A.A.; Efimov, N.N.; Khoroshilov, A.V.; Bogomyakov, A.S.; Ananyev, I.V.; Voronina, J.K.; Goloveshkin, A.S.; et al. Ferromagnetically Coupled Molecular Complexes with a CoII2GdIII Pivalate Core: Synthesis, Structure, Magnetic Properties and Thermal Stability. ChemistrySelect 2019, 4, 14261–14270. [Google Scholar] [CrossRef]
- Reynolds, R.A.; Dunham, W.R.; Coucouvanis, D. Kinetic Lability, Structural Diversity, and Oxidation Reactions of New Oligomeric, Anionic Carboxylate−Pyridine Complexes. Inorg. Chem. 1998, 37, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.S.; Sawyer, D.T. The Redox Chemistry of Manganese(III) and –(IV) Complexes. Isr. J. Chem. 1985, 25, 164–176. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Bendix, J.; Clérac, R. Single-Molecule Magnet Engineering: Building-Block Approaches. Chem. Commun. 2014, 50, 4396–4415. [Google Scholar] [CrossRef]
- Dincǎ, M.; Long, J.R. High-Enthalpy Hydrogen Adsorption in Cation-Exchanged Variants of the Microporous Metal−Organic Framework Mn3[(Mn4Cl)3(BTT)8(CH3OH)10]2. J. Am. Chem. Soc. 2007, 129, 11172–11176. [Google Scholar] [CrossRef]
- Kar, P.; Haldar, R.; Gómez-García, C.J.; Ghosh, A. Antiferromagnetic Porous Metal–Organic Framework Containing Mixed-Valence [MnII4MnIII2(μ4-O)2]10+ Units with Catecholase Activity and Selective Gas Adsorption. Inorg. Chem. 2012, 51, 4265–4273. [Google Scholar] [CrossRef]
- Li, C.-X.; Zeng, D.-F.; Chen, Y.-P.; Zhang, H.-H.; Sun, Y.-Q.; Chai, X.-C.; Lei, R.; Sun, R.-Q.; Huaxue, J. Syntheses and Crystal Structures of Two New Compounds: [Zn(2,2′-bipy)(L)Cl]2 and [Zn(phen)(L2)]2 (HL—3-Methylbenzoic Acid). Chin. J. Struct. Chem. 2009, 28, 1381–1386. [Google Scholar]
- Perlepes, S.P.; Libby, E.; Streib, W.E.; Folting, K.; Christou, G. The Reactions of Cu2(O2CMe)4(H2O)2 with 2,2′-Bipyridine (Bpy): Influence of the Cu: Bpy Ratio, and the Structure of a Linear Polymer Comprising Two Alternating Types of Cu2 Units. Polyhedron 1992, 11, 923–936. [Google Scholar] [CrossRef]
- Talismanova, M.O.; Sidorov, A.A.; Novotortsev, V.M.; Aleksandrov, G.G.; Nefedov, S.E.; Eremenko, I.L.; Moiseev, I.I. Unusual Transformation of the Urea Molecule Giving Rise to the NCO− Anion as a Bridging Ligand between Two CoII Atoms. Russ. Chem. Bull. 2001, 50, 2251–2253. [Google Scholar] [CrossRef]
- Zhang, Q.-Z.; Lu, C.-Z. Di-μ-Acetato-Bis[Bis(4-Aminobenzoato)(2,2′-Bipyridyl)Manganese(II)]. Acta Crystallogr. C 2005, 61, m78–m80. [Google Scholar] [CrossRef]
- Banerjee, S.; Rajakannu, P.; Butcher, R.J.; Murugavel, R. Auxiliary Ligand-Aided Tuning of Aggregation of Transition Metal Benzoates: Isolation of Four Different Types of Coordination Polymers. CrystEngComm 2014, 16, 8429–8441. [Google Scholar] [CrossRef]
- Goldberg, A.E.; Kiskin, M.A.; Kozyukhin, S.A.; Sidorov, A.A.; Eremenko, I.L. Binuclear Zinc Naphthoate Complex with 1,10-Phenanthroline: Synthesis, Structure, and Photoluminescence Properties. Russ. Chem. Bull. 2011, 60, 1012–1015. [Google Scholar] [CrossRef]
- Dey, D.; Roy, S.; Dutta Purkayastha, R.N.; Pallepogu, R.; McArdle, P. Zinc Carboxylates Containing Diimine: Synthesis, Characterization, Crystal Structure, and Luminescence. J. Mol. Struct. 2013, 1053, 127–133. [Google Scholar] [CrossRef]
- Li, X.-M.; Wang, Q.-W.; Liu, B.; Huaxue, J. Synthesis and Crystal Structure of a Zinc(II) Complex with 2-(4′-Chlorine-benzoyl)-benzoic Acid and 1,10-Phenanthroline. Chin. J. Struct. Chem. 2011, 30, 1646–1649. [Google Scholar]
- Baca, S.G.; Malaestean, I.L.; Keene, T.D.; Adams, H.; Ward, M.D.; Hauser, J.; Neels, A.; Decurtins, S. One-Dimensional Manganese Coordination Polymers Composed of Polynuclear Cluster Blocks and Polypyridyl Linkers: Structures and Properties. Inorg. Chem. 2008, 47, 11108–11119. [Google Scholar] [CrossRef]
- Evstifeev, I.S.; Kiskin, M.A.; Mironov, V.S.; Bogomyakov, A.S.; Sidorov, A.A.; Novotortsev, V.M.; Eremenko, I.L. 1D Nickel(II) Coordination Polymer with Pyrimidine and Pivalate Bridges: Synthesis, Structure and Magnetic Properties. Inorg. Chem. Commun. 2010, 13, 498–501. [Google Scholar] [CrossRef]
- Kiskin, M.A.; Fomina, I.G.; Aleksandrov, G.G.; Sidorov, A.A.; Novotortsev, V.M.; Rakitin, Y.V.; Dobrokhotova, Z.V.; Ikorskii, V.N.; Shvedenkov, Y.G.; Eremenko, I.L.; et al. New Antiferromagnetic Mn(II) Pivalate Polymer: Synthesis and Reactivity. Inorg. Chem. Commun. 2005, 8, 89–93. [Google Scholar] [CrossRef]
- Nakata, K.; Miyasaka, H.; Sugimoto, K.; Ishii, T.; Sugiura, K.; Yamashita, M. Construction of a One-Dimensional Chain Composed of Mn6 Clusters and 4,4′-Bipyridine Linkers: The First Step for Creation of “Nano-Dots-Wires”. Chem. Lett. 2002, 31, 658–659. [Google Scholar] [CrossRef]
- Kar, P.; Ida, Y.; Ishida, T.; Ghosh, A. Formation of Two Drastically Different MOFs Based on Mn(II)–Benzoate and Pyrazine with a Change in Seasonal Temperature: Structural Analysis and Magnetic Study. CrystEngComm 2012, 15, 400–410. [Google Scholar] [CrossRef]
- Kar, P.; Ida, Y.; Kanetomo, T.; Drew, M.G.B.; Ishida, T.; Ghosh, A. Synthesis of Mixed-Valence Hexanuclear Mn(II/III) Clusters from Its Mn(II) Precursor: Variations of Catecholase-like Activity and Magnetic Coupling. Dalton Trans. 2015, 44, 9795–9804. [Google Scholar] [CrossRef]
- Malaestean, I.L.; Kravtsov, V.C.; Speldrich, M.; Dulcevscaia, G.; Simonov, Y.A.; Lipkowski, J.; Ellern, A.; Baca, S.G.; Kögerler, P. One-Dimensional Coordination Polymers from Hexanuclear Manganese Carboxylate Clusters Featuring a {MnII4MnIII2(µ4-O)2} Core and Spacer Linkers. Inorg. Chem. 2010, 49, 7764–7772. [Google Scholar] [CrossRef] [PubMed]
- Sañudo, E.C.; Cauchy, T.; Ruiz, E.; Laye, R.H.; Roubeau, O.; Teat, S.J.; Aromí, G. Molecules Composed of Two Weakly Magnetically Coupled [MnIII4] Clusters. Inorg. Chem. 2007, 46, 9045–9047. [Google Scholar] [CrossRef] [PubMed]
- Abdulwahab, K.O.; Malik, M.A.; O’Brien, P.; Timco, G.A.; Tuna, F.; Muryn, C.A.; Winpenny, R.E.P.; Pattrick, R.A.D.; Coker, V.S.; Arenholz, E. A One-Pot Synthesis of Monodispersed Iron Cobalt Oxide and Iron Manganese Oxide Nanoparticles from Bimetallic Pivalate Clusters. Chem. Mater. 2014, 26, 999–1013. [Google Scholar] [CrossRef]
- Dulcevscaia, G.M.; Filippova, I.G.; Speldrich, M.; van Leusen, J.; Kravtsov, V.C.; Baca, S.G.; Kögerler, P.; Liu, S.-X.; Decurtins, S. Cluster-Based Networks: 1D and 2D Coordination Polymers Based on {MnFe2(µ3-O)}-Type Clusters. Inorg. Chem. 2012, 51, 5110–5117. [Google Scholar] [CrossRef]
- Novitchi, G.; Helm, L.; Anson, C.; Powell, A.K.; Merbach, A.E. NMR Study of Ligand Exchange and Electron Self-Exchange between Oxo-Centered Trinuclear Clusters [Fe3(µ3-O)(μ-O2CR)6(4-R′py)3]+/0. Inorg. Chem. 2011, 50, 10402–10416. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Wu, T. Crystal Structure of (2,2′-Bipyridine)-(Adamantane-1,3-Dicarboxylato)- Manganese(II) Hydrate, Mn(C10H8N2)(C12H14O4)·H2O. Z. Für Krist.—New Cryst. Struct. 2010, 225, 483–485. [Google Scholar] [CrossRef]
- Ma, Y.-S.; Tang, X.-Y.; Xue, F.-F.; Chen, B.; Dai, Y.-L.; Yuan, R.-X.; Roy, S. Structural Diversity and Magnetic Properties of the Manganese(II)/Carbazol-9-ylacetate/N,N′-Donor Reaction System. Eur. J. Inorg. Chem. 2012, 2012, 1243–1249. [Google Scholar] [CrossRef]
- Wu, D.-H.; Shi, J.; Shi, Y.-J.; Jiang, G.-Q. Tetrakis(μ-Phenoxyacetato-κ2O:O′)Bis[(1,10-Phenanthroline-κ2N,N′)Manganese(II)] Methanol Hemisolvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, m161. [Google Scholar] [CrossRef] [PubMed]
- Albela, B.; El Fallah, M.S.; Ribas, J.; Folting, K.; Christou, G.; Hendrickson, D.N. Two New Mixed-Valence Manganese Complexes of Formula [Mn4O2(X-Benzoato)7(Bpy)2] (X = 2-Cl, 2-Br) and the Crystal Structure of the 2-Cl Complex: Ground-State Spin Variability in the [Mn4O2]7+ Complexes. Inorg. Chem. 2001, 40, 1037–1044. [Google Scholar] [CrossRef]
- Pistilli, J.; Beer, R.H. A Tetranuclear Manganese Oxo Complex with Terminal and Bridging Trifluoroacetate Ligands: Synthesis and Structure of [Mn4O2(O2CCF3)8(Bpy)2]. Inorg. Chem. Commun. 2002, 5, 206–210. [Google Scholar] [CrossRef]
- Cañada-Vilalta, C.; Huffman, J.C.; Christou, G. Preparation, Crystal Structure and Chelate Substitution Reactions of [Mn4O2(O2CPh)6(Dpm)2] (Dpm=the Anion of Dipivaloylmethane). Polyhedron 2001, 20, 1785–1793. [Google Scholar] [CrossRef]
- Basler, R.; Chaboussant, G.; Cañada-Vilalta, C.; Christou, G.; Mutka, H.; Janssen, S.; Altorfer, F.; Güdel, H.-U. Magnetic and Inelastic Neutron Scattering Studies of a Frustrated Tetranuclear Mn3+ Butterfly-Type Cluster. Polyhedron 2003, 22, 2471–2479. [Google Scholar] [CrossRef]
- Saines, P.J.; Jain, P.; Cheetham, A.K. Evolution of the Structures and Magnetic Properties of the Manganese Dicarboxylates, Mn2(CO2(CH2)NCO2)(OH)2 and Mn4(CO2(CH2)NCO2)3(OH)2. Chem. Sci. 2011, 2, 1929–1939. [Google Scholar] [CrossRef]
- Mahata, P.; Prabu, M.; Natarajan, S. Role of Temperature and Time in the Formation of Infinite −M−O−M− Linkages and Isolated Clusters in MOFs: A Few Illustrative Examples. Inorg. Chem. 2008, 47, 8451–8463. [Google Scholar] [CrossRef] [PubMed]
- Kiskin, M.A.; Aleksandrov, G.G.; Dobrokhotova, Z.V.; Novotortsev, V.M.; Shvedenkov, Y.G.; Eremenko, I.L. Transformations of High Spin MnII and FeII Polymeric Pivalates in Reactions with Pivalic Acid and O-Phenylenediamines. Russ. Chem. Bull. 2006, 55, 806–820. [Google Scholar] [CrossRef]
- Reynolds, R.A.; Yu, W.O.; Dunham, W.R.; Coucouvanis, D. Synthesis and Characterization of a New Class of µ3-OH-Bridged Trimers That Contain Octahedrally Coordinated Divalent Metal Ions Bridged by Three Acetate Ligands and a Unique Catecholate Ligand. Solid State Molecular Structures of the [(Py)5MII3(OAc)3(µ3-OH)(Cat)] Complexes (M = Mn(II), Fe(II), Co(II), Ni(II)). Inorg. Chem. 1996, 35, 2721–2722. [Google Scholar] [CrossRef]
- Baikie, A.R.E.; Howes, A.J.; Hursthouse, M.B.; Quick, A.B.; Thornton, P. Preparation, Crystal Structure, Magnetic Properties, and Chemical Reactions of a Hexanuclear Mixed Valence Manganese Carboxylate. J. Chem. Soc. Chem. Commun. 1986, 1587. [Google Scholar] [CrossRef]
- Köhler, K.; Roesky, H.W.; Noltemeyer, M.; Schmidt, H.-G.; Freire-Erdbrügger, C.; Sheldrick, G.M. Neue Beiträge Zur Chemie Des Mangans: Synthese Und Strukturen Zweier Monomerer MnII1-Verbindungen Und Eines Hexanuklearen MnII/III1-Komplexes. Chem. Ber. 1993, 126, 921–926. [Google Scholar] [CrossRef]
- Murrie, M.; Parsons, S.; Winpenny, R.E.P. Deltahedra as Underlying Structural Motifs in Polynuclear Metal Chemistry: Structure of an Undecanuclear Manganese–Potassium Cage. J. Chem. Soc. Dalton Trans. 1998, 0, 1423–1424. [Google Scholar] [CrossRef]
- Gavrilenko, K.S.; Punin, S.V.; Cador, O.; Golhen, S.; Ouahab, L.; Pavlishchuk, V.V. Synthesis, Structure, and Magnetism of Heterometallic Carboxylate Complexes [MnIII2MII4O2(PhCOO)10(DMF)4], M = MnII, CoII, NiII. Inorg. Chem. 2005, 44, 5903–5910. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON, An Integrated Tool for the Analysis of the Results of a Single Crystal Structure Determination. Acta Crystallogr. Sect. A 1990, 46, c34. [Google Scholar] [CrossRef]
- Férey, G.; Serre, C. Large Breathing Effects in Three-Dimensional Porous Hybrid Matter: Facts, Analyses, Rules and Consequences. Chem. Soc. Rev. 2009, 38, 1380–1399. [Google Scholar] [CrossRef]
- Rakitin, Y.V.; Larin, G.M.; Minin, V.V. Interpretation of EPR Spectra of Coordination C ompounds; Nauka: Moscow, Russia, 1993. [Google Scholar]
- Belford, G.G.; Belford, R.L.; Burkhalter, J.F. Eigenfields: A Practical Direct Calculation of Resonance Fields and Intensities for Field-Swept Fixed-Frequency Spectrometers. J. Magn. Reason. 1969, 11, 251–265. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; Wiley: Hoboken, NJ, USA, 1993; ISBN 978-0-471-18838-4. [Google Scholar]
- Ma, C.; Chen, C.; Liu, Q.; Liao, D.; Li, L.; Sun, L. Structural Transformation Mediated by o-, m-, and p-Phthalates from Two to Three Dimensions for Manganese/Phthalate/4,4′-Bpy Complexes (4,4′-Bpy = 4,4′-Bipyridine). New J. Chem. 2003, 27, 890–894. [Google Scholar] [CrossRef]
- Ma, C.; Hu, M.; Chen, C.; Liu, Q. Designed Construction of a Non-Interpenetrated 2D Bilayer Framework with Large Guest-Clathrated Channels. Inorg. Chem. Commun. 2005, 8, 219–221. [Google Scholar] [CrossRef]
- Litvinenko, A.S.; Mikhaleva, E.A.; Kolotilov, S.V.; Pavlishchuk, V.V. Effect of Spin–Orbit Coupling on the Magnetic Susceptibility of Polynuclear Complexes of 3d Metals Containing a Co2+ Ion. Theor. Exp. Chem. 2011, 46, 422–428. [Google Scholar] [CrossRef]
- Shcherbakov, I.N.; Ivanova, T.M.; Kiskin, M.A.; Kolotilov, S.V.; Novotortsev, V.M.; Eremenko, I.L.; Kogan, V.A. Computational Study of Exchange Coupling in Homo- and Heterometallic Oxo- and Carboxylato Bridged Trinuclear Complexes with Triangular {FeIII2M(µ3-O)} (M=FeIII, NiII, CoII) Core. Inorg. Chim. Acta 2014, 421, 507–512. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.; Polidori, G.; Spagna, R. SIR97: A New Tool for Crystal Structure Determination and Refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- van der Sluis, P.; Spek, A.L. BYPASS: An Effective Method for the Refinement of Crystal Structures Containing Disordered Solvent Regions. Acta Crystallogr. Sect. A 1990, 46, 194–201. [Google Scholar] [CrossRef]
- Lutsenko, I.A.; Kiskin, M.A.; Efimov, N.N.; Ugolkova, E.A.; Maksimov, Y.V.; Imshennik, V.K.; Goloveshkin, A.S.; Khoroshilov, A.V.; Lytvynenko, A.S.; Sidorov, A.A.; et al. New Heterometallic Pivalates with FeIII and ZnII Ions: Synthesis, Structures, Magnetic, Thermal Properties. Polyhedron 2017, 137, 165–175. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Comparative Assessment of a New Nonempirical Density Functional: Molecules and Hydrogen-Bonded Complexes. J. Chem. Phys. 2003, 119, 12129–12137. [Google Scholar] [CrossRef]
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Erratum: “Comparative Assessment of a New Nonempirical Density Functional: Molecules and Hydrogen-Bonded Complexes” [J. Chem. Phys. 119, 12129 (2003)]. J. Chem. Phys. 2004, 121, 11507. [Google Scholar] [CrossRef]
- Jensen, K.P. Bioinorganic Chemistry Modeled with the TPSSh Density Functional. Inorg. Chem. 2008, 47, 10357–10365. [Google Scholar] [CrossRef]
- Roy, L.E.; Hay, P.J.; Martin, R.L. Revised Basis Sets for the LANL Effective Core Potentials. J. Chem. Theory Comput. 2008, 4, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Soda, T.; Kitagawa, Y.; Onishi, T.; Takano, Y.; Shigeta, Y.; Nagao, H.; Yoshioka, Y.; Yamaguchi, K. Ab Initio Computations of Effective Exchange Integrals for H–H, H–He–H and Mn2O2 Complex: Comparison of Broken-Symmetry Approaches. Chem. Phys. Lett. 2000, 319, 223–230. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).