Pentacyclic Triterpene Profile and Its Biosynthetic Pathway in Cecropia telenitida as a Prospective Dietary Supplement
Abstract
:1. Introduction
2. Results
2.1. Retrieving a Chemical DE16 Fraction (DE16-R) from Different Samplings
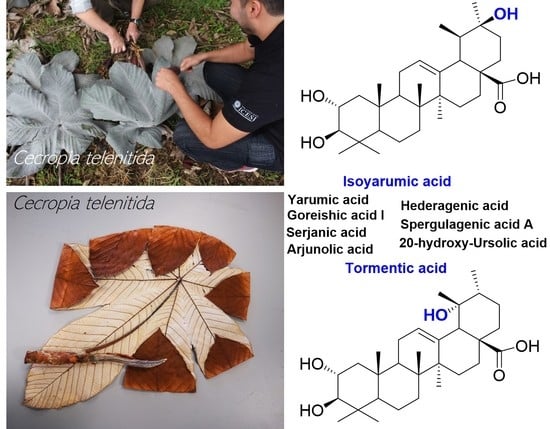
2.2. Classical Molecule-by-Molecule Isolation and NMR Analysis
2.3. Cell Viability in HepG2, C2C12 and 3T3-L1cell Lines
3. Discussion
3.1. Biosynthetic Pathway to Pentacyclic Triterpenes and Cecropia telenitida PT Profile
3.2. A Brief Overview of the Structure–Activity Relationship among PTs
3.3. Viability Cell Test and General Considerations
4. Materials and Methods
4.1. Reagents
4.2. Plant Treatment
4.3. Extraction Process and Flash and Preparative Chromatography
4.4. LC–MS and MALDI-TOF Analysis
4.5. Nuclear Magnetic Resonance Experiments
4.6. Cell Cultures and Cell Viability Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availbility
References
- De la Torre, R.; Carbó, M.; Pujadas, M.; Biel, S.; Mesa, M.D.; Covas, M.I.; Expósito, M.; Espejo, J.A.; Sanchez-Rodriguez, E.; Díaz-Pellicer, P.; et al. Pharmacokinetics of maslinic and oleanolic acids from olive oil—Effects on endothelial function in healthy adults. A randomized, controlled, dose–response study. Food Chem. 2020, 322, 126676. [Google Scholar] [CrossRef]
- Sheng, H.; Sun, H. Synthesis, biology and clinical significance of pentacyclic triterpenes: A multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat. Prod. Rep. 2011, 28, 543–593. [Google Scholar] [CrossRef]
- Sharma, H.; Kumar, P.; Deshmukh, R.R.; Bishayee, A.; Kumar, S. Pentacyclic triterpenes: New tools to fight metabolic syndrome. Phytomedicine 2018, 50, 166–177. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, W.; Zhou, Y.Y.; Zhang, Y.N.; Li, J.Y.; Hu, L.H.; Li, J. Corosolic acid stimulates glucose uptake via enhancing insulin receptor phosphorylation. Eur. J. Pharmacol. 2008, 584, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Kuo, Y.H.; Lin, C.H.; Ho, H.Y.; Shih, C.C. Tormentic Acid, a major component of suspension cells of eriobotrya japonica, suppresses high-fat diet-induced diabetes and hyperlipidemia by glucose transporter 4 and amp-activated protein kinase phosphorylation. J. Agric. Food Chem. 2014, 62, 10717–10726. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.-X.; Li, M.-Z.; Zheng, W.-Y.; He, Y.; Ren, Y.; Wu, Z.-M.; Fan, Q.-S.; Hu, Y.-H.; Li, C.-J. Tormentic acid inhibits LPS-induced inflammatory response in human gingival fibroblasts via inhibition of TLR4-mediated NF-κB and MAPK signalling pathway. Arch. Oral Biol. 2015, 60, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, G.; Giraldo-Dávila, D.; Combariza, M.Y.; Holzgrabe, U.; Tabares-Guevara, J.H.; Ramírez-Pineda, J.R.; Acín, S.; Muñoz, D.L.; Montoya, G.; Balcazar, N. Serjanic acid improves immunometabolic markers in a diet-induced obesity mouse model. Molecules 2020, 25, 1486. [Google Scholar] [CrossRef] [Green Version]
- Mabhida, S.E.; Dludla, P.V.; Johnson, R.; Ndlovu, M.; Louw, J.; Opoku, A.R.; Mosa, R.A. Protective effect of triterpenes against diabetes-induced β-cell damage: An overview of in vitro and in vivo studies. Pharmacol. Res. 2018, 137, 179–192. [Google Scholar] [CrossRef]
- Reeds, D.N.; Patterson, B.W.; Okunade, A.; Holloszy, J.O.; Polonsky, K.S.; Klein, S. Ginseng and ginsenoside re do not improve β-cell function or insulin sensitivity in overweight and obese subjects with impaired glucose tolerance or diabetes. Diabetes Care 2011, 34, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Pan, J.H.; Cho, H.T.; Kim, Y.J. Black ginseng extract counteracts streptozotocin-induced diabetes in mice. PLoS ONE 2016, 11, e0146843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmazyn, M.; Gan, X.T. Ginseng for the treatment of diabetes and diabetes-related cardiovascular complications: A discussion of the evidence. Can. J. Physiol. Pharmacol. 2019, 97, 265–276. [Google Scholar] [CrossRef]
- Gathercole, L.L.; Stewart, P.M. Targeting the pre-receptor metabolism of cortisol as a novel therapy in obesity and diabetes. J. Steroid Biochem. Mol. Biol. 2010, 122, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Sabbadin, C.; Bordin, L.; Donà, G.; Manso, J.; Avruscio, G.; Armanini, D. Licorice: From pseudohyperaldosteronism to therapeutic uses. Front. Endocrinol. (Lausanne) 2019, 10, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoya Peláez, G.L.; Sierra, J.A.; Alzate, F.; Holzgrabe, U.; Ramirez-Pineda, J.R. Pentacyclic triterpenes from Cecropia telenitida with immunomodulatory activity on dendritic cells. Brazilian J. Pharmacogn. 2013, 23, 754–761. [Google Scholar] [CrossRef] [Green Version]
- Duke, J.A.; Bogenschutz-Godwin, M.J.; Ottesen, A.R.C.N. Duke’s Handbook of Medicinal Plants of Latin America; CRC: London, UK; Taylor & Francis [distributor]: Boca Raton, FL, USA, 2009; ISBN 9781420043167. [Google Scholar]
- Mosquera, C.; Panay, A.; Montoya, G. Pentacyclic Triterpenes from Cecropia telenitida Can Function as Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1. Molecules 2018, 23, 1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, H.-J.; Kim, I.-T.; Park, H.-J.; Kim, H.-M.; Choi, J.-H.; Lee, K.-T. Tormentic acid, a triterpenoid saponin, isolated from Rosa rugosa, inhibited LPS-induced iNOS, COX-2, and TNF-α expression through inactivation of the nuclear factor-κb pathway in RAW 264.7 macrophages. Int. Immunopharmacol. 2011, 11, 504–510. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Kratschmar, D.V.; Schuster, D.; Pfisterer, P.H.; Gumy, C.; Aubry, E.M.; Brandstötter, S.; Stuppner, H.; Wolber, G.; Odermatt, A. 11β-Hydroxysteroid dehydrogenase 1 inhibiting constituents from Eriobotrya japonica revealed by bioactivity-guided isolation and computational approaches. Bioorg. Med. Chem. 2010, 18, 1507–1515. [Google Scholar] [CrossRef]
- Ramesh, A.S.; Christopher, J.G.; Radhika, R.; Setty, C.R.; Thankamani, V. Isolation, characterisation and cytotoxicity study of arjunolic acid from Terminalia arjuna. Nat. Prod. Res. 2012, 26, 1549–1552. [Google Scholar] [CrossRef]
- Ghosh, J.; Das, J.; Manna, P.; Sil, P.C. Acetaminophen induced renal injury via oxidative stress and TNF-α production: Therapeutic potential of arjunolic acid. Toxicology 2010, 268, 8–18. [Google Scholar] [CrossRef]
- Ghosh, J.; Das, J.; Manna, P.; Sil, P.C. Arjunolic acid, a triterpenoid saponin, prevents acetaminophen (APAP)-induced liver and hepatocyte injury via the inhibition of APAP bioactivation and JNK-mediated mitochondrial protection. Free Radic. Biol. Med. 2010, 48, 535–553. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, Y.-Y.; Jiao, Q.-Y.; Khan, A.; Li, F.; Han, D.-F.; Cao, G.-D.; Lou, H.-X. Triterpenoid saponins from the pulp of Sapindus mukorossi and their antifungal activities. Phytochemistry 2018, 147, 1–8. [Google Scholar] [CrossRef]
- Zorzan, M.; Collazuol, D.; Ribaudo, G.; Ongaro, A.; Scaroni, C.; Zagotto, G.; Armanini, D.; Barollo, S.; Galeotti, F.; Volpi, N.; et al. Biological effects and potential mechanisms of action of Pistacia lentiscus Chios mastic extract in Caco-2 cell model. J. Funct. Foods 2019, 54, 92–97. [Google Scholar] [CrossRef]
- International Standads Organization Biological Evaluation of Medical Devices. Biomed. Saf. Stand. 1996, 26, 54. [CrossRef]
- Nakamura, M.; Linh, T.M.; Lien, L.Q.; Suzuki, H.; Mai, N.C.; Giang, V.H.; Tamura, K.; van Thanh, N.; Suzuki, H.; Misaki, R.; et al. Transcriptome sequencing and identification of cytochrome p450 monooxygenases involved in the biosynthesis of maslinic acid and corosolic acid in Avicennia marina. Plant. Biotechnol. 2018, 35, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Sandeep; Misra, R.C.; Chanotiya, C.S.; Mukhopadhyay, P.; Ghosh, S. Oxidosqualene cyclase and CYP716 enzymes contribute to triterpene structural diversity in the medicinal tree banaba. New Phytol. 2019, 222, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Tan, K.L.; Zhang, C.L.; Tan, C.Y.; Chen, Y.Z.; Jiang, Y.Y. What Does It Take to Synergistically Combine Sub-Potent Natural Products into Drug-Level Potent Combinations? PLoS ONE 2012, 7, e49969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, H. Synergy research: Approaching a new generation of phytopharmaceuticals. Fitoterapia 2011, 82, 34–37. [Google Scholar] [CrossRef]
- Ma, X.; Lian, Q.Q.; Dong, Q.; Ge, R.S. Environmental inhibitors of 11β-hydroxysteroid dehydrogenase type 2. Toxicology 2011, 285, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.C.; Rooyackers, O.; Walker, B.R. Effects of the 11β-hydroxysteroid dehydrogenase inhibitor carbenoxolone on insulin sensitivity in men with type 2 diabetes. J. Clin. Endocrinol. Metab. 2003, 88, 285–291. [Google Scholar] [CrossRef]
- Christeller, J.T.; McGhie, T.K.; Johnston, J.W.; Carr, B.; Chagné, D. Quantitative trait loci influencing pentacyclic triterpene composition in apple fruit peel. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Liu, H.; Lv, H.; Zhang, J.; Zhou, J.; Zhao, Z. Preparation, characterization, and in vitro/vivo studies of oleanolic acid-loaded lactoferrin nanoparticles. Drug Des. Devel Ther. 2017, 11, 1417–1427. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Hang, T.J.; Wang, Y.; Jiang, L.; Wu, X.L.; Zhang, Z.; Shen, J.; Zhang, Y. Determination of oleanolic acid in human plasma and study of its pharmacokinetics in Chinese healthy male volunteers by HPLC tandem mass spectrometry. J. Pharm. Biomed. Anal. 2006, 40, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Kim, Y.H.; Kim, H.H.; Ji, H.Y.; Yoo, S.D.; Choi, W.R.; Lee, S.M.; Han, C.-K.; Lee, H.S. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm. Drug Dispos. 2007, 28, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic triterpene bioavailability: An overview of in vitro and in vivo studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [Green Version]
- Mullen, P.J.; Lüscher, B.; Scharnagl, H.; Krähenbühl, S.; Brecht, K. Effect of simvastatin on cholesterol metabolism in C2C12 myotubes and HepG2 cells, and consequences for statin-induced myopathy. Biochem. Pharmacol. 2010, 79, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Peiffer, C.; Craig, D.L.; Biden, T.J. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J. Biol. Chem. 1999, 274, 24202–24210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, G.; Valencia, L.M.; Giraldo-Dávila, D.; Combariza, M.Y.; Galeano, E.; Balcazar, N.; Panay, A.J.; Jerez, A.M.; Montoya, G. Pentacyclic Triterpene Profile and Its Biosynthetic Pathway in Cecropia telenitida as a Prospective Dietary Supplement. Molecules 2021, 26, 1064. https://doi.org/10.3390/molecules26041064
Gutiérrez G, Valencia LM, Giraldo-Dávila D, Combariza MY, Galeano E, Balcazar N, Panay AJ, Jerez AM, Montoya G. Pentacyclic Triterpene Profile and Its Biosynthetic Pathway in Cecropia telenitida as a Prospective Dietary Supplement. Molecules. 2021; 26(4):1064. https://doi.org/10.3390/molecules26041064
Chicago/Turabian StyleGutiérrez, Gustavo, Laura Marcela Valencia, Deisy Giraldo-Dávila, Marianny Y. Combariza, Elkin Galeano, Norman Balcazar, Aram J. Panay, Alejandra Maria Jerez, and Guillermo Montoya. 2021. "Pentacyclic Triterpene Profile and Its Biosynthetic Pathway in Cecropia telenitida as a Prospective Dietary Supplement" Molecules 26, no. 4: 1064. https://doi.org/10.3390/molecules26041064
APA StyleGutiérrez, G., Valencia, L. M., Giraldo-Dávila, D., Combariza, M. Y., Galeano, E., Balcazar, N., Panay, A. J., Jerez, A. M., & Montoya, G. (2021). Pentacyclic Triterpene Profile and Its Biosynthetic Pathway in Cecropia telenitida as a Prospective Dietary Supplement. Molecules, 26(4), 1064. https://doi.org/10.3390/molecules26041064