Reversible Humidity-Driven Transformation of a Bimetallic {EuCo} Molecular Material: Structural, Sorption, and Photoluminescence Studies
Abstract
1. Introduction
2. Results and Discussion
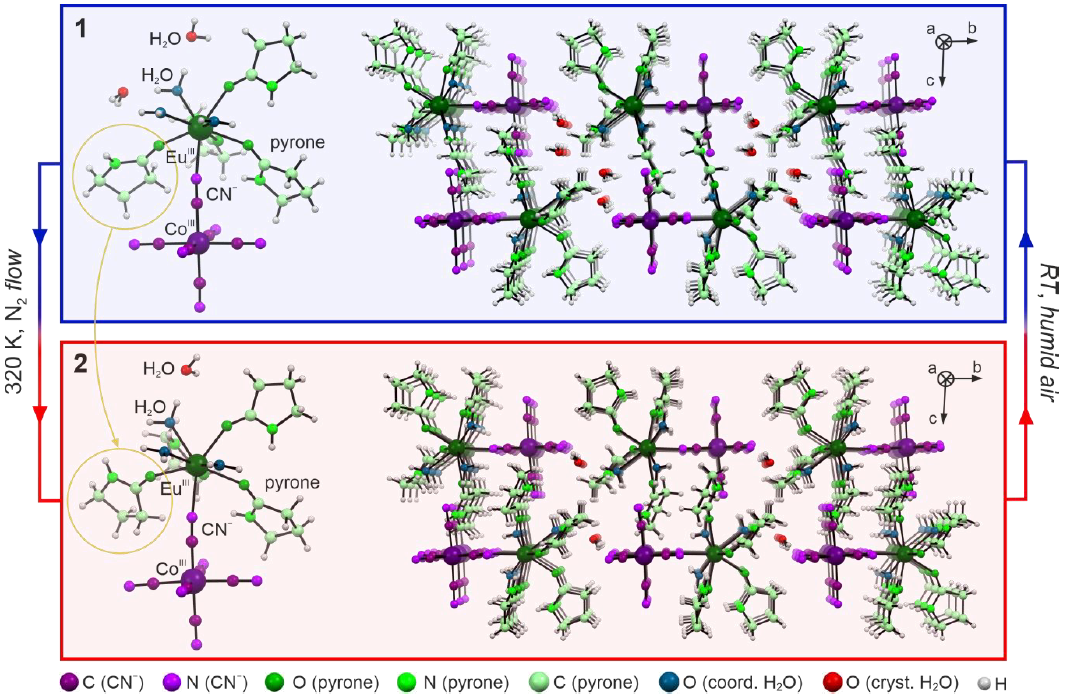
2.1. Basic Characterization and Structural Studies
2.2. Sorption Properties
2.3. Photoluminescent Properties
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Synthesis and Basic Characterization of 1
4.3. Preparation and Basic Characterization of 2
4.4. Crystallographic Studies
4.5. Physical Techniques
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef]
- Sato, O. Dynamic molecular crystals with switchable physical properties. Nat. Chem. 2016, 8, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liao, W.-Q.; Fu, D.-W.; Ye, H.-Y.; Liu, C.-M.; Chen, Z.-N.; Xiong, R.-G. The first organic-inorganic hybrid luminescent multiferroic: (Pyrrolidinium)MnBr3. Adv. Mater. 2015, 27, 3942–3946. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Z.; Wen, T.; Zhou, Y.; Li, N.; Han, F.; Xiao, Y.; Chow, P.; Sun, J.; Pravica, M.; et al. Pressure-driven cooperative spin-crossover, large-volume collapse, and semiconductor-to-metal transition in manganese(II) honeycomb lattices. J. Am. Chem. Soc. 2016, 138, 15751–15757. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, S.; Tsunobuchi, Y.; Matsuda, T.; Hashimoto, K.; Namai, A.; Hakoe, F.; Tokoro, H. Synthesis of a metal oxide with a room-temperature photoreversible phase transition. Nat. Chem. 2010, 2, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular luminescent sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef]
- Fittipaldi, M.; Cini, A.; Annino, G.; Vindigni, A.; Caneschi, A.; Sessoli, R. Electric field modulation of magnetic exchange in molecular helices. Nat. Mater. 2019, 18, 329–334. [Google Scholar] [CrossRef]
- Kimura, T.; Goto, T.; Shintani, H.; Ishizaka, K.; Arima, T.; Tokura, Y. Magnetic control of ferroelectric polarization. Nature 2003, 426, 55–58. [Google Scholar] [CrossRef]
- Vogelsberg, C.S.; Garcia-Garibay, M.A. Crystalline molecular machines: Function, phase order, dimentionality, and composition. Chem. Soc. Rev. 2012, 41, 1892–1910. [Google Scholar] [CrossRef]
- Bigdeli, F.; Lollar, C.T.; Morsali, A.; Zhou, H.-C. Switching of metal-organic frameworks. Angew. Chem. Int. Ed. 2020, 59, 4652–4669. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Imoto, K.; Tsunobuchi, Y.; Takano, S.; Tokoro, H. Light-induced spin-crossover magnet. Nat. Chem. 2011, 3, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Brites, C.D.S.; Carlos, L.D. Lanthanide organic framework luminescent thermometers. Chem. Eur. J. 2016, 22, 14782–14795. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-W.; Kitagawa, H. Proton transport in metal-organic frameworks. Chem. Rev. 2020, 120, 8416–8467. [Google Scholar] [CrossRef] [PubMed]
- Decker, G.E.; Lorzing, G.R.; Deegan, M.M.; Bloch, E.D. MOF-mimetic molecules: Carboxylate-based supramolecular complexes as molecular metal-organic framework analogues. J. Mater. Chem. A 2020, 8, 4217–4229. [Google Scholar] [CrossRef]
- Hang, T.; Zhang, W.; Ye, H.-Y.; Xiong, R.-G. Metal-organic complex ferroelectrics. Chem. Soc. Rev. 2011, 40, 3577–3598. [Google Scholar] [CrossRef]
- Létard, J.-F. Photomagnetism of iron(II) spin crossover complexes–the T(LIESST) approach. J. Mater. Chem. 2006, 16, 2550–2559. [Google Scholar] [CrossRef]
- Chorazy, S.; Stanek, J.J.; Nogaś, W.; Majcher, A.M.; Rams, M.; Kozieł, M.; Juszyńska-Gałązka, E.; Nakabayashi, K.; Ohkoshi, S.; Sieklucka, B.; et al. Tuning of charge transfer assisted phase transition and slow magnetic relaxation functionalities in {Fe9-xCox[W(CN)8]6} (x = 0–9) molecular solid solution. J. Am. Chem. Soc. 2016, 138, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Pointillart, F.; Flores Gonzalez, J.; Montigaud, V.; Tesi, L.; Cherkasov, V.; Le Guennic, B.; Cador, O.; Ouahab, L.; Sessoli, R.; Kuropatov, V. Redox- and solvato-magnetic switching in a tetrathiafulvalene-based triad single-molecule magnet. Inorg. Chem. Front. 2020, 7, 2322–2334. [Google Scholar] [CrossRef]
- Otto, S.; Harris, J.P.; Heinze, K.; Reber, C. Molecular ruby under pressure. Angew. Chem. Int. Ed. 2018, 57, 11069–11973. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Avendaño, C.; Dunbar, K.R. Molecular magnetic materials based on 4d and 5d transition metals. Chem. Soc. Rev. 2011, 40, 3213–3228. [Google Scholar] [CrossRef]
- Chorazy, S.; Zakrzewski, J.J.; Magott, M.; Korzeniak, T.; Nowicka, B.; Pinkowicz, D.; Podgajny, R.; Sieklucka, B. Octacyanidometallates for multifunctional molecule-based materials. Chem. Soc. Rev. 2020, 49, 5945–6001. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Pillet, S.; de Graaf, C.; Magott, M.; Bendeif, E.-E.; Guionneau, P.; Rouzières, M.; Marvaud, V.; Stefańczyk, O.; Pinkowicz, D.; et al. Photoinduced Mo-CN bond breakage in octacyanomolybdate leading to spin triplet trapping. Angew. Chem. Int. Ed. 2020, 59, 3117–3121. [Google Scholar] [CrossRef] [PubMed]
- Chorazy, S.; Wyczesany, M.; Sieklucka, B. Lanthanide photoluminescence in heterometallic polycyanidometallates-based coordination networks. Molecules 2017, 22, 1902. [Google Scholar] [CrossRef]
- Ikeda, H.; Ito, A.; Sakuda, E.; Kitamura, N.; Takayama, T.; Sekine, T.; Shinohara, A.; Yoshimura, T. Excited-state characteristics of tetracyanidonitridorhenium(V) and -technetium(V) complexes with N-heteroaromatic ligands. Inorg. Chem. 2013, 52, 6319–6327. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Takano, S.; Imoto, K.; Yoshikiyo, M.; Namai, A.; Tokoro, H. 90-degree optical switching of output second-harmonic light in chiral photomagnet. Nat. Photonics 2014, 8, 65–71. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Rams, M.; Mišek, M.; Kamenev, K.V.; Tomkowiak, H.; Katrusiak, A.; Sieklucka, B. Enforcing multifunctionality: A pressure-pressure induces spin-crossover photomagnet. J. Am. Chem. Soc. 2015, 137, 8795–8802. [Google Scholar] [CrossRef]
- Yersin, H.; Trümbach, D.; Strasser, J.; Patterson, H.H.; Assefa, Z. Tunable radiationless energy transfer in Eu[Au(CN)2]3·3H2O by high pressure. Inorg. Chem. 1998, 37, 3209–3216. [Google Scholar] [CrossRef]
- Mahfoud, T.; Molnár, G.; Bonhommeau, S.; Cobo, S.; Salmon, L.; Demont, P.; Tokoro, H.; Ohkoshi, S.; Boukheddaden, K.; Bousseksou, A. Electric-field-induced charge-transfer phase transition: A promising approach toward electrically switchable devices. J. Am. Chem. Soc. 2009, 131, 15049–15054. [Google Scholar] [CrossRef]
- Jankowski, R.; Reczyński, M.; Chorazy, S.; Zychowicz, M.; Arczyński, M.; Kozieł, M.; Ogorzały, K.; Makowski, W.; Pinkowicz, D.; Sieklucka, B. Guest-dependent pressure-induced spin crossover in FeII4[MIV(CN)8]2 (M=Mo,W) cluster-based material showing persistent solvent-driven structural transformations. Chem. Eur. J. 2020, 26, 11187–11198. [Google Scholar] [CrossRef]
- Milon, J.; Daniel, M.-C.; Kaiba, A.; Guionneau, P.; Brandès, S.; Sutter, J.-P. Nanoporous magnets of chiral and racemic [{Mn(HL)}2Mn{Mo(CN)7}2] with switchable ordering temperatures (TC = 85 K ⇿ 106 K) driven by H2O sorption (L = N,N-Dimethylalaninol). J. Am. Chem. Soc. 2007, 129, 13872–13878. [Google Scholar] [CrossRef] [PubMed]
- Pinkowicz, D.; Southerland, H.I.; Avendaño, C.; Prosvirin, A.; Sanders, C.; Wernsdorfer, W.; Pedersen, K.S.; Dreiser, J.; Clérac, R.; Nehrkom, J.; et al. Cyanide single-molecule magnets exhibiting solvent dependent reversible “on” and “off” exchange bias behavior. J. Am. Chem. Soc. 2015, 137, 14406–14422. [Google Scholar] [CrossRef] [PubMed]
- Magott, M.; Reczyński, M.; Gaweł, B.; Sieklucka, B.; Pinkowicz, D. A photomagnetic sponge: High-temperature light-induced ferrimagnet controlled by water sorption. J. Am. Chem. Soc. 2018, 140, 15876–15882. [Google Scholar] [CrossRef]
- Tokoro, H.; Ohkoshi, S.; Matsuda, T.; Hashimoto, K. A large thermal hysteresis loop produced by a charge-transfer phase transition in a rubidium manganese hexacyanoferrate. Inorg. Chem. 2004, 43, 5231–5236. [Google Scholar] [CrossRef]
- Zakrzewski, J.J.; Liberka, M.; Zychowicz, M.; Chorazy, S. Diverse physical functionalities of rare-earth hexacyanidometallate frameworks and their molecular analogues. Inorg. Chem. Front. 2021, 8, 452–483. [Google Scholar] [CrossRef]
- Zakrzewski, J.J.; Sieklucka, B.; Chorazy, S. Europium(III) photoluminescence governed by d8–d10 heterometallophilic interactions in trimetallic cyanido-bridged coordination frameworks. Inorg. Chem. 2020, 59, 1393–1404. [Google Scholar] [CrossRef]
- Jankowski, R.; Zakrzewski, J.J.; Surma, O.; Ohkoshi, S.; Chorazy, S.; Sieklucka, B. Near-infrared emissive Er(III) and Yb(III) molecular nanomagnets in metal-organic chains functionalized by octacyanidometallates(IV). Inorg. Chem. Front. 2019, 6, 2423–2434. [Google Scholar] [CrossRef]
- Davies, G.M.; Pope, S.J.A.; Adams, H.; Faulkner, S.; Ward, M.D. Structural and photophysical properties of coordination networks combining [Ru(bipy)(CN)4]2− anions and lanthanide(III) cations: Rates of photoinduced Ru-to-lanthanide energy transfer and sensitized near-infrared luminescence. Inorg. Chem. 2005, 44, 4656–4665. [Google Scholar] [CrossRef]
- Lazarides, T.; Davies, G.M.; Adams, H.; Sabatini, C.; Barigelletti, F.; Barbieri, A.; Pope, S.J.A.; Faulkner, S.; Ward, M.D. Ligand-field excited states of hexacyanochromate and hexacyanocobaltate as sensitizers for near-infrared luminescence from Nd(III) and Yb(III) in cyanide-bridged d-f assemblies. Photochem. Photobiol. Sci. 2007, 6, 1152–1157. [Google Scholar] [CrossRef]
- Rawashdeh-Omary, M.A.; Larochelle, C.L.; Petterson, H.H. Tunable energy transfer from dicyanoaurate(I) and dicyanoargentate(I) donor ions to terbium(III) acceptor ions in pure crystals. Inorg. Chem. 2000, 39, 4527–4534. [Google Scholar] [CrossRef]
- Maynard, B.A.; Kalachnikova, K.; Whitehead, K.; Assefa, Z.; Sykora, R.E. Intramolecular energy transfer in a one-dimensional europium tetracyanoplatinate. Inorg. Chem. 2008, 47, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Wang, J.; Zychowicz, M.; Zakrzewski, J.J.; Nakabayashi, K.; Sieklucka, B.; Chorazy, S.; Ohkoshi, S. Dehydration-hydration switching of single-molecule magnet behavior and visible photoluminescence in a cyanido-bridged DyIIICoIII framework. J. Am. Chem. Soc. 2019, 141, 18211–18220. [Google Scholar] [CrossRef]
- Wang, J.; Zakrzewski, J.J.; Heczko, M.; Zychowicz, M.; Nakagawa, K.; Nakabayashi, K.; Sieklucka, B.; Chorazy, S.; Ohkoshi, S. Proton conductive luminescent thermometer based on near-infrared emissive {YbCo2} molecular nanomagnets. J. Am. Chem. Soc. 2020, 142, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zakrzewski, J.J.; Zychowicz, M.; Vieru, V.; Chibotaru, L.F.; Nakabayashi, K.; Chorazy, S.; Ohkoshi, S. Holmium(III) molecular nanomagnets for optical thermometry exploring the luminescence re-absorption effect. Chem. Sci. 2021, 12, 730–741. [Google Scholar] [CrossRef]
- Errulat, D.; Marin, R.; Gálico, D.A.; Harriman, K.L.M.; Pialat, A.; Gabidullin, B.; Iikawa, F.; Couto, O.D.D., Jr.; Moilanen, J.O.; Hemmer, E.; et al. A luminescent thermometer exhibiting slow relaxation of the magnetization: Toward self-monitored building blocks for next-generation optomagnetic devices. ACS Cent. Sci. 2019, 5, 1187–1198. [Google Scholar] [CrossRef]
- Gao, J.-X.; Zhang, W.-Y.; Wu, Z.-G.; Zheng, Y.-X.; Fu, D.-W. Enantiomorphic perovskite ferroelectrics with circularly polarized luminescence. J. Am. Chem. Soc. 2020, 142, 4756–4761. [Google Scholar] [CrossRef]
- Kobayashi, A.; Imada, S.; Shigeta, Y.; Nagao, Y.; Yoshida, M.; Kato, M. Vapochromic luminescent proton conductors: Switchable vapochromism and proton conduction of luminescent Pt(II) complexes with proton-exchangeable sites. J. Mater. Chem. C 2019, 7, 14923–14931. [Google Scholar] [CrossRef]
- Chorazy, S.; Zakrzewski, J.J.; Reczyński, M.; Nakabayashi, K.; Ohkoshi, S.; Sieklucka, B. Humidity driven molecular switch based on photoluminescent DyIIICoIII single-molecule magnets. J. Mater. Chem. C 2019, 7, 4164–4172. [Google Scholar] [CrossRef]
- Petoud, S.; Cohen, S.M.; Bünzli, J.-C.G.; Raymond, K.N. Stable lanthanide luminescence agents highly emissive in aqueous solution: Multidentate 2-hydroxyisophthalamide complexes of Sm3+, Eu3+, Tb3+, Dy3+. J. Am. Chem. Soc. 2003, 125, 13324–13325. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.B.D.; Gonçalves, S.M.C.; Júnior, S.A.; Simas, A.M. A comprehensive strategy to boost the quantum yield of luminescence of europium complexes. Sci. Rep. 2013, 3, 2395. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Zhou, X.; Duan, C.-K.; Tanner, P.A. Luminescence and crystal field analysis of Eu3+ in Eu[Co(CN)6]·4H2O. J. Phys. Chem. Sol. 2007, 68, 1921–1925. [Google Scholar] [CrossRef]
- Kumar, K.; Chorazy, S.; Nakabayashi, K.; Sato, H.; Sieklucka, B.; Ohkoshi, S. TbCo and Tb0.5Dy0.5Co layered cyanido-bridged frameworks for construction of colorimetric and ratiometric luminescent thermometers. J. Mater. Chem. C 2018, 6, 8372–8384. [Google Scholar] [CrossRef]
- Kunkely, H.; Vogler, A. Optical properties of GdIII[MIII(CN)6] with M = Cr and Co. Phosphorescence form ligand-field states of [M(CN)6]3− under ambient conditions. Inorg. Chem. Commun. 2004, 7, 770–772. [Google Scholar] [CrossRef]
- Pandey, P.; Samanta, A.K.; Bandyopadhyay, B.; Chakraborty, T. Infrared spectroscopy of 2-pyrrolidinone and its hydrogen bonded dimers in a cold (8 K) inert gas matrix. Vib. Spectrosc. 2011, 50, 126–131. [Google Scholar] [CrossRef]
- Chorazy, S.; Rams, M.; Nakabayashi, K.; Sieklucka, B.; Ohkoshi, S. White Light Emissive DyIII Single-Molecule Magnets Sensitized by Diamagnetic [CoIII(CN)6]3− Linkers. Chem. Eur. J. 2016, 22, 7371–7375. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Casanova, D.; Cirera, J.; Llunell, M.; Alemany, P.; Avnir, D.; Alvarez, S. Minimal Distortion Pathways in Polyhedral Rearrangements. J. Am. Chem. Soc. 2004, 126, 1755–1763. [Google Scholar] [CrossRef]
- Chorazy, S.; Zakrzewski, J.J.; Wang, J.; Ohkoshi, S.; Sieklucka, B. Incorporation of hexacyanidoferrate(III) ion in photoluminescent trimetallic Eu(3-pyridone)[Co1−xFex(CN)6] chains exhibiting tunable visible light absorption and emission properties. CrystEngComm 2018, 20, 5695–5706. [Google Scholar] [CrossRef]



| Compound | 1 | 2 |
|---|---|---|
| formula | Eu1Co1C22H38N10O9 | Eu1Co1C22H36N10O8 |
| formula weight/g·mol−1 | 797.51 | 779.5 |
| T/K | 100(2) | |
| λ/Å | 0.71073 (Mo Kα) | |
| crystal system | triclinic | |
| space group | ||
| a/Å | 9.0135(5) | 9.032(1) |
| b/Å | 12.0910(7) | 12.089(2) |
| c/Å | 14.3010(8) | 14.023(2) |
| α/° | 90.199(2) | 95.815(4) |
| β/° | 92.429(2) | 90.186(4) |
| γ/° | 90.912(2) | 90.138(4) |
| V/Å3 | 1556.9 (2) | 1523.2(4) |
| Z | 2 | |
| ρcalc/g·cm−3 | 1.701 | 1.700 |
| μ/cm−1 | 2.592 | 2.645 |
| F(000) | 804 | 784 |
| crystal type | colorless plate | |
| crystal size/mm × mm × mm | 0.35 × 0.23 × 0.05 | |
| θ range/° | 2.725–25.026 | 2.345–25.027 |
| limiting indices | −10 < h < 10−14 < k < 14−15 < l < 17 | −10 < h < 10−14 < k < 14−16 < l < 16 |
| collected reflections | 15,970 | 16,135 |
| unique reflections | 5485 | 5354 |
| Rint | 0.0185 | 0.0346 |
| completeness/% | 99.7 | 99.5 |
| data/restraints/parameters | 5485/15/428 | 5354/13/411 |
| GOF on F2 | 1.097 | 1.153 |
| R1 [I ≥ 2σ(I)] | 0.0179 | 0.0319 |
| wR2 (all data) | 0.0444 | 0.0891 |
| largest diff. peak/hole/e∙Å−3 | 0.842/−0.408 | 1.896/−1.306 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakrzewski, J.J.; Heczko, M.; Jankowski, R.; Chorazy, S. Reversible Humidity-Driven Transformation of a Bimetallic {EuCo} Molecular Material: Structural, Sorption, and Photoluminescence Studies. Molecules 2021, 26, 1102. https://doi.org/10.3390/molecules26041102
Zakrzewski JJ, Heczko M, Jankowski R, Chorazy S. Reversible Humidity-Driven Transformation of a Bimetallic {EuCo} Molecular Material: Structural, Sorption, and Photoluminescence Studies. Molecules. 2021; 26(4):1102. https://doi.org/10.3390/molecules26041102
Chicago/Turabian StyleZakrzewski, Jakub J., Michal Heczko, Robert Jankowski, and Szymon Chorazy. 2021. "Reversible Humidity-Driven Transformation of a Bimetallic {EuCo} Molecular Material: Structural, Sorption, and Photoluminescence Studies" Molecules 26, no. 4: 1102. https://doi.org/10.3390/molecules26041102
APA StyleZakrzewski, J. J., Heczko, M., Jankowski, R., & Chorazy, S. (2021). Reversible Humidity-Driven Transformation of a Bimetallic {EuCo} Molecular Material: Structural, Sorption, and Photoluminescence Studies. Molecules, 26(4), 1102. https://doi.org/10.3390/molecules26041102






