Complexes of Formaldehyde and α-Dicarbonyls with Hydroxylamine: FTIR Matrix Isolation and Theoretical Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formaldehyde–hydroxylamine Complexes
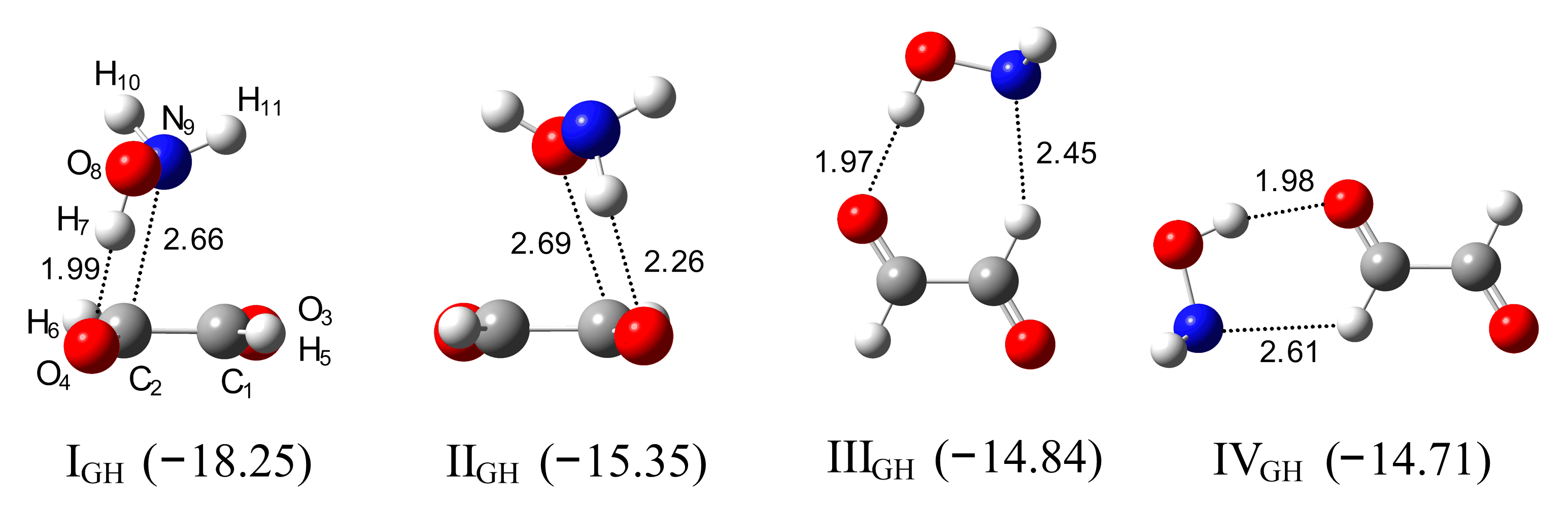
2.2. Glyoxal–hydroxylamine Complexes
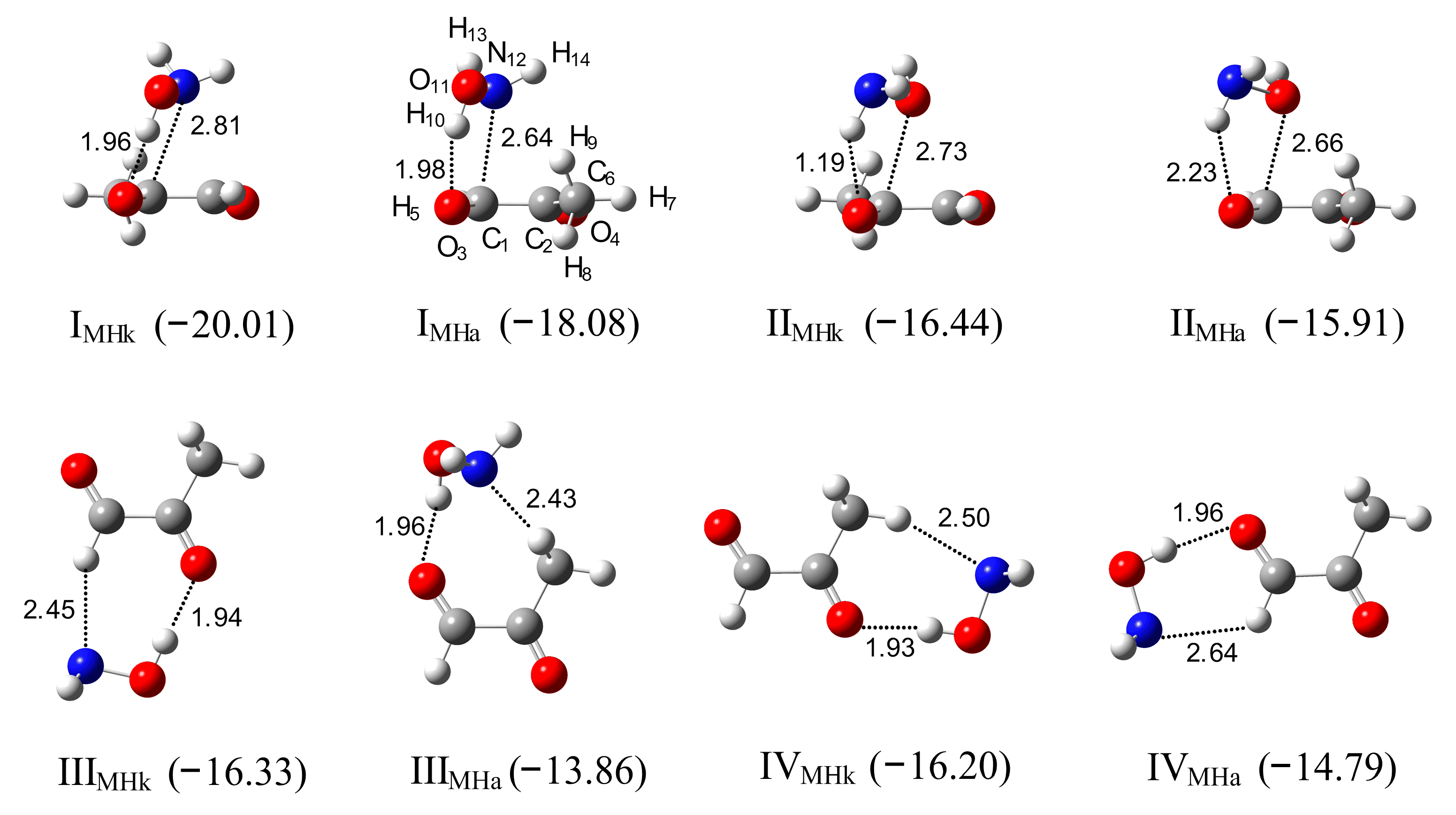
2.3. Methylglyoxal–Hydroxylamine Complexes
2.4. AIM Analysis
2.5. Hydrogen Bonding in the Carbonyl-Hydroxylamine Complexes
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Whittle, E.; Dows, D.A.; Pimentel, G.C. Matrix isolation method for the experimental study of unstable species. J. Chem. Phys. 1954, 22, 1943. [Google Scholar] [CrossRef]
- Ault, B.S. Matrix isolation studies of reactive intermediate complexes. Rev. Chem. Intermed. 1988, 9, 233–269. [Google Scholar] [CrossRef]
- Barnes, A.J. Matrix isolation studies of hydrogen bonding—An historical perspective. J. Mol. Struct. 2018, 1163, 77–85. [Google Scholar] [CrossRef]
- Rosenberg, S.; Silver, S.M.; Sayer, J.M.; Jencks, W.P. Evidence for two concurrent mechanisms and a kinetically significant proton transfer process in acid-catalyzed o-methyloxime formation. J. Am. Chem. Soc. 1974, 96, 7986–7998. [Google Scholar] [CrossRef]
- Sayer, J.M.; Pinsky, B.; Schonbrunn, A.; Washtien, W. Mechanism of carbinolamine formation. J. Am. Chem. Soc. 1974, 96, 7998–8009. [Google Scholar] [CrossRef]
- Lahann, J. Click Chemistry for Biotechnology and Materials Science; John Wiley and Sons Ltd.: Chichester, UK, 2009; ISBN 9780470699706. [Google Scholar]
- Gauthier, M.A.; Klok, H.A. Peptide/protein-polymer conjugates: Synthetic strategies and design concepts. Chem. Commun. 2008, 2591–2611. [Google Scholar] [CrossRef]
- Venkatesan, N.; Kim, B.H. Peptide conjugates of oligonucleotides: Synthesis and applications. Chem. Rev. 2006, 106, 3712–3761. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0-12-382239-0. [Google Scholar]
- Kubler-Kielb, J. Conjugation of LPS-derived oligosaccharides to proteins using oxime chemistry. Methods Mol. Biol. 2011, 751, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Kölmel, D.K.; Kool, E.T. Oximes and hydrazones in bioconjugation: Mechanism and catalysis. Chem. Rev. 2017, 117, 10358–10376. [Google Scholar] [CrossRef]
- Lowry, T.H.; Schueller Richardson, K. Mechanism and Theory in Organic Chemistry, 2nd ed.; HarperCollins Publishers: New York, NY, USA, 1987; ISBN 13-978-0063504288. [Google Scholar]
- Morokuma, K. Molecular orbital studies of hydrogen bonds. III. C=O···H–O hydrogen bond in H2CO···H2O and H2CO···2H2O. J. Chem. Phys. 1971, 55, 1236–1244. [Google Scholar] [CrossRef]
- Del Bene, J.E. Molecular orbital theory of hydrogen bond. VI. Effect of hydrogen bonding on n-π* transitions in dimers ROHOCH2. J. Am. Chem. Soc. 1973, 2042, 6517–6522. [Google Scholar] [CrossRef]
- Ventura, O.N.; Coitiño, E.L.; Irving, K.; Iglessias, A.; Lledós, A. Comparison of semiempirical and bsse corrected møller-plesset ab initio calculations on the direct addition of water to formaldehyde. J. Mol. Struct. Theochem. 1990, 210, 427–440. [Google Scholar] [CrossRef]
- Vos, R.J.; Hendriks, R.; Van Duijneveldt, F.B. SCF, MP2 and CEPA-1 calculations on the OH-O hydrogen bonded complexes (H2O)2 and (H2O-H2CO). J. Comput. Chem. 1990, 11, 1–18. [Google Scholar] [CrossRef]
- Nelander, B. A matrix isolation study of the water-formaldehyde complex. The far-infrared region. Chem. Phys. 1992, 159, 281–287. [Google Scholar] [CrossRef]
- Ventura, O.N.; Coitiño, E.L.; Lledós, A.; Bertran, J. Analysis of the gas-phase addition of water to formaldehyde: A semiempirical and ab initio study of bifunctional catalysis by H2O. J. Comput. Chem. 1992, 13, 1037–1046. [Google Scholar] [CrossRef]
- Ha, T.K.; Makarewicz, J.; Bauder, A. Ab initio study of the water-formaldehyde complex. J. Phys. Chem. 1993, 97, 11415–11419. [Google Scholar] [CrossRef]
- Dimitrova, Y.; Peyerimhoff, S.D. Theoretical study of the n→π* transitions in hydrogen-bonded formaldehyde complexes. Chem. Phys. Lett. 1994, 227, 384–389. [Google Scholar] [CrossRef]
- Sánchez, M.L.; Aguilar, M.A.; Del Valle, F.J.O. Solvent effects on optical emission and absorption spectra: Theoretical calculation of the 1(n, π*) transition of formaldehyde in solution. J. Phys. Chem. 1995, 99, 15758–15764. [Google Scholar] [CrossRef]
- Dimitrova, Y. Solvent effects on vibrational spectra of hydrogen-bonded complexes of formaldehyde and water: An ab initio study. J. Mol. Struct. Theochem. 1997, 391, 251–257. [Google Scholar] [CrossRef]
- Galano, A.; Narciso-Lopez, M.; Francisco-Marquez, M. Water complexes of important air pollutants: Geometries, complexation energies, concentrations, infrared spectra, and intrinsic reactivity. J. Phys. Chem. A 2010, 114, 5796–5809. [Google Scholar] [CrossRef]
- Ahlström, M.; Jönsson, B.; Karlström, G. Ab initio molecular orbital calculations on hydrogen- and non-hydrogen-bonded complexes. H2CO·H2O and H2CO·H2S. Mol. Phys 1979, 1051–1059. [Google Scholar] [CrossRef]
- Nelander, B. Infrared spectrum of the water formaldehyde complex in solid argon and solid nitrogen. J. Chem. Phys. 1980, 72, 77–84. [Google Scholar] [CrossRef]
- Butler, L.G.; Brown, T.L. Nuclear quadrupole coupling constants and hydrogen-bonding. A molecular-orbital study of O17 and deuterium field gradients in formaldehyde-water hydrogen bonding. J. Am. Chem. Soc. 1981, 103, 6541–6549. [Google Scholar] [CrossRef]
- Williams, I.H.; Spangler, D.; Femec, D.A.; Maggiora, G.M.; Schowen, R.L. Theoretical models for solvation and catalysis in carbonyl addition. J. Am. Chem. Soc. 1983, 105, 31–40. [Google Scholar] [CrossRef]
- Lewell, X.Q.; Hillier, I.H.; Field, M.J.; Morris, J.J.; Taylor, P.J. Theoretical studies of vibrational frequency shifts upon hydrogen bonding. The carbonyl stretching mode in complexes of formaldehyde. J. Chem. Soc. Faraday Trans. 2 1988, 84, 893–898. [Google Scholar] [CrossRef]
- Blair, J.T.; Krogh-Jespersen, K.; Levy, R.M. Solvent effects on optical absorption spectra: The 1A1—1A2 transition of formaldehyde in water. J. Am. Chem. Soc. 1989, 111, 6948–6956. [Google Scholar] [CrossRef]
- Blair, J.T.; Westbrook, J.D.; Levy, R.M.; Kroghjespersen, K. Simple-models for solvation effects on electronic-transition energies. Formaldehyde and water. Chem. Phys. Lett. 1989, 154, 531–535. [Google Scholar] [CrossRef]
- Kumpf, R.A.; Damewood, J.R. Interaction of formaldehyde with water. J. Phys. Chem. 1989, 93, 4478–4486. [Google Scholar] [CrossRef]
- Nelander, B. Infrared spectra of formaldehyde complexes with hydrogen bromide, chlorine and iodine chloride. J. Mol. Struct. 1980, 69, 59–68. [Google Scholar] [CrossRef]
- Nelander, B. Infrared spectra and structure of formaldehyde complexes with ammonia and acetonitrile. J. Mol. Struct. 1982, 81, 223–228. [Google Scholar] [CrossRef]
- Baiocchi, F.A.; Klemperer, W. The rotational and hyperfine spectrum and structure of H2CO-HF. J. Chem. Phys. 1983, 78, 3509–3520. [Google Scholar] [CrossRef]
- Bach, S.B.H.; Ault, B.S. Infrared matrix isolation study of the hydrogen-bonded complexes between formaldehyde and the hydrogen halides and cyanide. J. Phys. Chem. 1984, 88, 3600–3604. [Google Scholar] [CrossRef]
- Rivelino, R. Lewis acid-base interactions in weakly bound formaldehyde complexes with CO2, HCN, and FCN: Considerations on the cooperative H-bonding effects. J. Phys. Chem. A 2008, 112, 161–165. [Google Scholar] [CrossRef]
- Mitchell, J.B.O.; Price, S.L. The nature of the N-H O-C hydrogen bond—An intermolecular perturbation theory study of the formamide/formaldehyde complex. J. Comput. Chem. 1990, 11, 1217–1233. [Google Scholar] [CrossRef]
- Tolosa, S.; Hidalgo, A.; Sansón, J.A. Thermodynamic, structural, and dynamic study of the N-H···O=C hydrogen bond association in aqueous solution. Chem. Phys. 2000, 255, 73–84. [Google Scholar] [CrossRef]
- Müller, R.P.; Russegger, P.; Huber, J.R. Hydrogen-bonded complex between HNO and formaldehyde. Photolysis of methyl nitrite in an argon matrix. Chem. Phys. 1982, 70, 281–290. [Google Scholar] [CrossRef]
- Moore, K.B.; Sadeghian, K.; Sherrill, C.D.; Ochsenfeld, C.; Schaefer, H.F. C-H···O Hydrogen bonding. the prototypical methane-formaldehyde system: A critical assessment. J. Chem. Theory Comput. 2017, 13, 5379–5395. [Google Scholar] [CrossRef]
- Brumer, Y.; Shapiro, M.; Brumer, P.; Baldridge, K.K. Controlled alcohol-ketone interconversion by dihydrogen transfer: An ab initio study of the methanol-formaldehyde complex. J. Phys. Chem. A 2002, 106, 9512–9519. [Google Scholar] [CrossRef]
- Mucha, M.; Mielke, Z. Complexes of atmospheric α-dicarbonyls with water: FTIR Matrix isolation and theoretical study. J. Phys. Chem. A 2007, 111, 2398–2406. [Google Scholar] [CrossRef]
- Mielke, Z.; Mucha, M.; Bil, A.; Golec, B.; Coussan, S.; Roubin, P. Photo-induced hydrogen exchange reaction between methanol and glyoxal: Formation of hydroxyketene. ChemPhysChem 2008, 9, 1774–1780. [Google Scholar] [CrossRef]
- Mucha, M.; Mielke, Z. Structure and photochemistry of the methanol complexes with methylglyoxal and diacetyl: FTIR matrix isolation and theoretical study. Chem. Phys. 2009, 361, 27–34. [Google Scholar] [CrossRef]
- Mucha, M.; Mielke, Z. Photochemistry of the glyoxal–hydrogen peroxide complexes in solid argon: Formation of 2-hydroxy-2-hydroperoxyethanal. Chem. Phys. Lett. 2009, 482, 87–92. [Google Scholar] [CrossRef]
- Mucha, M. Faculty of Chemistry. Ph.D. Thesis, University of Wrocław, Wrocław, Poland, 2008. [Google Scholar]
- Aloisio, S.; Francisco, J.S. Complexes of hydroperoxyl radical with glyoxal, methylglyoxal, methylvinyl ketone, acrolein, and methacrolein: Possible new sinks for HO2 in the atmosphere? J. Phys. Chem. A 2003, 107, 2492–2496. [Google Scholar] [CrossRef]
- Yeo, G.A.; Ford, T.A. The infrared spectrum of the hydroxylamine dimer. J. Mol. Struct. 1990, 217, 307–323. [Google Scholar] [CrossRef]
- Yeo, G.A.; Ford, T.A. Matrix isolation infrared spectrum of the water-hydroxylamine complex. Vib. Spectrosc. 1991, 253, 173–181. [Google Scholar] [CrossRef]
- Yeo, G.A.; Ford, T.A. The matrix isolation infrared spectrum of the hydroxylamine-ammonia complex. Spectrochim. Acta Part A 1991, 47, 919–925. [Google Scholar] [CrossRef]
- Sałdyka, M.; Mielke, Z. Photodecomposition of formohydroxamic acid. Matrix isolation FTIR and DFT studies. Phys. Chem. Chem. Phys. 2003, 5, 4790–4797. [Google Scholar] [CrossRef]
- Sałdyka, M. Photodecomposition of N-hydroxyurea in argon matrices. FTIR and theoretical studies. RSC Adv. 2013, 3, 1922–1932. [Google Scholar] [CrossRef]
- Khoshkhoo, H.; Nixon, E.R. Infrared and Raman spectra of formaldehyde in argon and nitrogen matrices. Spectrochim. Acta Part A 1973, 29, 603–612. [Google Scholar] [CrossRef]
- Nelander, B. Infrared spectrum of formaldehyde in solid nitrogen. I. Monomer absorption. J. Chem. Phys. 1980, 73, 1026–1033. [Google Scholar] [CrossRef]
- Nelander, B. Infrared spectrum of formaldehyde in solid nitrogen. II. Dimer spectrum and dimer structure. J. Chem. Phys. 1980, 73, 1034–1039. [Google Scholar] [CrossRef]
- Withnall, R.; Andrews, L. Matrix infrared spectra and normal-coordinate analysis of isotopic hydroxylamine molecules. J. Phys. Chem. 1988, 92, 2155–2161. [Google Scholar] [CrossRef]
- Kuchttsu, K.; Fukuyama, T.; Morino, Y. Average structures of butadiene, acrolein, and glyoxal determined by gas electron diffraction and spectroscopy. J. Mol. Struct. 1968, 1, 463–479. [Google Scholar] [CrossRef]
- Osamura, Y.; Schaefer, H.F. Internal rotation barrier and transition state for glyoxal. J. Chem. Phys. 1981, 74, 4576–4580. [Google Scholar] [CrossRef]
- Diem, M.; MacDonald, B.G.; Lee, E.K.C. Photolysis and laser-excited fluorescence and phosphorescence emission of trans-glyoxal in an argon matrix at 13 K. J. Phys. Chem. 1981, 85, 2227–2232. [Google Scholar] [CrossRef]
- Profeta, L.T.M.; Sams, R.L.; Johnson, T.J.; Williams, S.D. Quantitative infrared intensity studies of vapor-phase glyoxal, methylglyoxal, and 2,3-butanedione (diacetyl) with vibrational assignments. J. Phys. Chem. A 2011, 115, 9886–9900. [Google Scholar] [CrossRef]
- Hobza, P.; Havlas, Z. Blue-shifting hydrogen bonds. Chem. Rev. 2000, 100, 4253–4264. [Google Scholar] [CrossRef]
- Hermansson, K. Blue-shifting hydrogen bonds. J. Phys. Chem. A 2002, 106, 4695–4702. [Google Scholar] [CrossRef]
- Karpfen, A.; Kryachko, E.S. Blue-shifted hydrogen-bonded complexes. II. CH3F···(HF)1≤n≤3 and CH2F2···(HF)1≤n≤3. Chem. Phys. 2005, 310, 77–84. [Google Scholar] [CrossRef]
- Rutkowski, K.S.; Rodziewicz, P.; Melikova, S.M.; Koll, A. Theoretical study of Hal3CH/F2CD2 (Hal=F,Cl) and F3CH/FH heterodimers with blue shifted hydrogen bonds. Chem. Phys. 2006, 327, 193–201. [Google Scholar] [CrossRef]
- Melikova, S.M.; Rutkowski, K.S.; Rodziewicz, P.; Koll, A. Unusual spectroscopic properties of CF3H dissolved in liquified Ar, N2, CO and CO2. Chem. Phys. Lett. 2002, 352, 301–310. [Google Scholar] [CrossRef]
- Asfin, R.E.; Melikova, S.M.; Rutkowski, K.S. The infrared study of fluoroform+methyl fluoride mixtures in argon and nitrogen matrices. Evidence of nonlinear blue-shifting complex formation. Spectrochim. Acta Part A 2018, 208, 185–194. [Google Scholar] [CrossRef]
- Dyllick-Brenzinger, C.E.; Bauder, A. Microwave spectrum, dipole moment and barrier to internal rotation of trans-methyl glyoxal. Chem. Phys. 1978, 30, 147–153. [Google Scholar] [CrossRef]
- Reid, S.A.; Kim, H.L.; McDonald, J.D. A stimulated emission pumping study of jet-cooled methyl glyoxal. J. Chem. Phys. 1990, 92, 7079–7086. [Google Scholar] [CrossRef]
- Grabowski, S.J. What is the covalency of the hydrogen bonding? Chem. Rev. 2011, 111, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- Koch, U.; Popelier, P.L.A. Characterization of C-H-O hydrogen-bonds on the basis of the charge density. J. Phys. Chem. 1995, 99, 9747–9754. [Google Scholar] [CrossRef]
- Kraka, E.; Zou, W.; Tao, Y. Decoding chemical information from vibrational spectroscopy data: Local vibrational mode theory. WIREs Comput. Mol. Sci. 2020, 10, e1480. [Google Scholar] [CrossRef]
- Kalescky, R.; Zou, W.; Kraka, E.; Cremer, D. Local vibrational modes of the water dimer—Comparison of theory and experiment. Chem. Phys. Lett. 2012, 554, 243–247. [Google Scholar] [CrossRef]
- Tao, Y.; Zou, W.; Jia, J.; Li, W.; Cremer, D. Different ways of hydrogen bonding in water—Why does warm water freeze faster than cold water? J. Chem. Theory Comput. 2017, 13, 55–76. [Google Scholar] [CrossRef]
- Tao, Y.; Zou, W.; Kraka, E. Strengthening of hydrogen bonding with the push-pull effect. Chem. Phys. Lett. 2017, 685, 251–258. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. Calculation of small molecular interactions by differences of separate total energies—Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision, C.02; Gaussian Inc.: Wallingford, CT, USA, 2003. [Google Scholar]
- Keith, T.A. AIMAll, Version 12.09.23, Standard, 1997–2012. Available online: http://aim.tkgristmill.com (accessed on 19 February 2021).
- Barnes, A.J.; Mielke, Z. Matrix effects on hydrogen-bonded complexes trapped in low-temperature matrices. J. Mol. Struct. 2012, 1023, 216–221. [Google Scholar] [CrossRef]
- Gantenberg, M.; Halupka, M.; Sander, W. Dimerization of formic acid-an example of a “noncovalent” reaction mechanism. Chem. Eur. J. 2000, 6, 1865–1869. [Google Scholar] [CrossRef]
- Sałdyka, M.; Mielke, Z. Dimerization of the keto tautomer of acetohydroxamic acid—Infrared matrix isolation and theoretical study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Sałdyka, M. N-Hydroxyurea dimers: A matrix isolation and theoretical study. Vib. Spectrosc. 2016, 85, 149–156. [Google Scholar] [CrossRef]
- Sałdyka, M.; Mielke, Z.; Haupa, K. Structural and spectroscopic characterization of DMF complexes with nitrogen, carbon dioxide, ammonia and water. Infrared matrix isolation and theoretical studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 190, 423–432. [Google Scholar] [CrossRef]






| Approximate Description | Experimental | Calculated | ||||||
|---|---|---|---|---|---|---|---|---|
| Ar | N2 | Δν | ||||||
| ν M | νFH | Δν 1 | ν M | νFH | Δν 1 | IFH | IIFH | |
| Hydroxylamine | ||||||||
| ν(OH) | 3635.5 | 3522.2 3518.5 | −115.1 | 3637.6 | 3516.0 3510.0 | −124.6 | −141 (408) | −126 (117) |
| δ(NOH) | 1351.2 | 1423.4 1418.7 | +69.9 | 1367.4 | 1427.7 | +60.3 | +86 (36) | +62 (51) |
| ω(NH2) | 1118.3 | 1137.8 | +19.5 | 1133.0 | 1156.7 | +23.7 | +24 (117) | +13 (102) |
| ν(NO) | 895.6 | 900.5 | +4.9 | 895.3 | 897.0 901.8 | +4.1 | +6 (7) | +8 (4) |
| τ(OH) | 402 2 | 562.1 | +162.1 | 402 2 | 574.5 | +172.5 | +238 (115) | +106 (122) |
| Formaldehyde | ||||||||
| νas(CH) | 2864.1 | 2893.3 2891.1 | +28.1 | 2866.4 | 2892.3 | +25.9 | +56 (34) | +34 (72) |
| νs(CH) | 2798.0 | 2814.2 2811.0 | +14.6 | 2800.1 2798.0 | +25 (77) | +29 (56) | ||
| ν(C=O) | 1742.2 | 1727.1 1724.4 | −16.8 | 1740.7 1739.7 | 1723.1 | −17.1 | −20 (53) | −9 (66) |
| δ(CH2) | 1499.1 | 1493.9 | −5.2 | 1499.6 1496.4 | −6 (15) | −3 (14) | ||
| γ(CH2) | 1168.6 | 1179.8 | +11.2 | 1170.0 1167.9 | 1180.0 | +11.0 | +12 (5) | +8 (20) |
| Approximate Description | Experimental | Calculated | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ar | N2 | Δν | |||||||
| ν M | νGH | Δν 1 | ν M | νGH 2 | Δν 1 | IGH | IIIGH | IVGH | |
| Hydroxylamine | |||||||||
| ν(OH) | 3635.5 | 3521.0 3512.4 | −118.8 | 3637.6 | 3541.1 3520.9 3515.8 | −96.5 −119.2 | −145 (7) | −106 (383) | −103 (356) |
| δ(NOH) | 1351.2 | 1412.1 1410.2 | +60.9 | 1367.4 | 1416.6 1399.7 | +49.2 +32.3 | +61 (63) | +72 (29) | +71 (34) |
| ω(NH2) | 1118.3 | 1129.0 1125.8 | +9.1 | 1133.0 | 1142.6 | +9.6 | +14 (11) | +24 (112) | +22 (122) |
| ν(NO) | 895.6 | 895.3 | 898.8 | +3.5 | +14 (3) | +9 (8) | +6 (8) | ||
| Glyoxal | |||||||||
| ν(CH) | 2860.1 2854.9 | 2857.6 | −0.4 | 2857.1 | 2875.6 | +18.5 | −9 (60) +12 (39) | +15 (53) +20 (1) | +1 (52) +51 (8) |
| ν(C=O) | 1724.5 | 1719.0 | −5.5 | 1730.1 | 1720.2 1723.1 | −9.9 −7.0 | −4 (122) +4 (23) | −11 (126) −6 (20) | −13 (89) −6 (33) |
| γ(CH) | 812.1 807.8 | 807.4 | 820.8 | +13.4 | +37 (0) | +23 (8) | +16 (2) | ||
| Approximate Description | Experimental | Calculated | ||||||
|---|---|---|---|---|---|---|---|---|
| Ar | N2 | Δν | ||||||
| ν M | ν MH | Δν 1 | ν M | ν MH | Δν 1 | IMHk | IMHa | |
| Hydroxylamine | ||||||||
| ν(OH) | 3635.5 | 3511.7 3507.6 | −125.8 | 3637.6 | 3505.7 3495.2 | −137.1 | −148 (138) | −156 (73) |
| δ(NOH) 2 | 1351.2 | 1404.1 | +52.9 | 1367.4 | 1402.7 1400.3 | +34.1 | +64 (58) | +62 (51) |
| ω(NH2) | 1118.3 | 1134.1 1131.2 | +14.4 | 1133.0 | 1139.3 1140.6 | +7.0 | +17 (117) | +11 (109) |
| ν(NO) | 895.6 | 904.8 902.9 | +8.3 | 895.3 | +9 (5) | +13 (6) | ||
| Methylglyoxal | ||||||||
| ν(CH) | 2843.1 2840.7 | 2858.2 | +16.3 | 2844.7 2840.2 2835.9 | −1 (60) | +24 (51) | ||
| νket(C=O) | 1733.5 | 1737.7 | +4.2 | 1739.0 1737.2 1735.9 | 1741.3 | +3.9 | −5 (132) | +4 (61) |
| νald(C=O) | 1726.4 | 1705.4 | −21.0 | 1730.1 1727.9 | 1710.8 | −18.2 | +6 (27) | −10 (97) |
| δ(CH3) 2 | 1420.0 | 1416.1 | −3.9 | 1423.2 1420.8 | 0 (8) +3 (24) −3 (36) | +5 (6) +3 (25) −2 (31) | ||
| νas(C–C) | 1228.3 | 1233.7 | +5.4 | 1234.5 1229.9 | 1240.5 1237.1 | +6.6 | +5 (14) | +5 (15) |
| νs(C–C) | 777.1 | 784.7 | +7.6 | 780.9 | 785.8 | +4.8 | +6 (15) | +6 (15) |
| 779.5 | 782.3 | |||||||
| 777.6 | ||||||||
| Complex | BCP | ρb | ∇2ρb | Complex | BCP | ρb | ∇2ρb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Formaldehyde–Hydroxylamine Complexes | Glyoxal–Hydroxylamine Complexes | ||||||||||
| IFH | O4-H5 a | 0.0256 | 0.0855 | IGH | C2-N9 | 0.0203 | 0.0626 | ||||
| H2-N7 c | 0.0090 | 0.0268 | O4-H7 a | 0.0213 | 0.0800 | ||||||
| IIFH | O4-H5 a | 0.0207 | 0.0791 | IIGH | C1-O8 | 0.0158 | 0.0582 | ||||
| C1-N7 | 0.0160 | 0.0514 | O3-H10 b | 0.0129 | 0.0472 | ||||||
| IIIFH | O4-H5 a | 0.0226 | 0.0798 | IIIGH | O4-H7 a | 0.0225 | 0.0772 | ||||
| H5-N9 d | 0.0122 | 0.0342 | |||||||||
| IVFH | O4-H8 b | 0.0164 | 0.0560 | IVGH | O4-H7 a | 0.0227 | 0.0782 | ||||
| H2-O6 c | 0.0099 | 0.0337 | H6-N9 d | 0.0097 | 0.0290 | ||||||
| VFH | C1-O6 | 0.0131 | 0.0478 | VGH | O4-H7 a | 0.0194 | 0.0687 | ||||
| O4-H8 b | 0.0136 | 0.0513 | H5-O8 c | 0.0107 | 0.0404 | ||||||
| VIGH | O4-H10 b | 0.0143 | 0.0494 | ||||||||
| H5-O8 c | 0.0126 | 0.0414 | |||||||||
| VIIGH | H6-O8 c | 0.0108 | 0.0370 | ||||||||
| O4-H10 b | 0.0144 | 0.0499 | |||||||||
| VIIIGH | O4-H7 a | 0.0186 | 0.0690 | ||||||||
| Approximate Description | HCHO | CHOCHO | CH3COCHO | NH2OH 1 | CO 2 | H2O 3 | NH3 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ar (p) 5 | N2 (p) 5 | Ar (n) 5 | N2 (p,n) 5 | Ar (n) 5 | N2 (n) 5 | Ar | N2 | Ar | Ar | N2 | Ar | N2 | |
| ν(OH) | −115 | −125 | −119 | −97 (p) −119 (n) | −126 | −137 | −294 −345 | −289 −333 | −39 | −93 | −139 | −297 | −345 |
| δ(NOH) | +70 | +60 | +61 | +49 (p) +34 (n) | +53 | +34 | +119 | +105 | +10 | +54 | +121 | +108 | |
| ω(NH2) ωNH2 | +20 | +24 | +9 | +10 (p) | +14 | +7 | +34 | +31 | +4 | +19 | +18 | +12 | +13 |
| ν(NO) | +4 | +5 | +4 (p) | +8 | +14 | +9 | +12 | +19 | +10 | +8 | |||
| τ(OH) | +162 | +173 | +362 +32 | +340 +28 | +422 | +350 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golec, B.; Sałdyka, M.; Mielke, Z. Complexes of Formaldehyde and α-Dicarbonyls with Hydroxylamine: FTIR Matrix Isolation and Theoretical Study. Molecules 2021, 26, 1144. https://doi.org/10.3390/molecules26041144
Golec B, Sałdyka M, Mielke Z. Complexes of Formaldehyde and α-Dicarbonyls with Hydroxylamine: FTIR Matrix Isolation and Theoretical Study. Molecules. 2021; 26(4):1144. https://doi.org/10.3390/molecules26041144
Chicago/Turabian StyleGolec, Barbara, Magdalena Sałdyka, and Zofia Mielke. 2021. "Complexes of Formaldehyde and α-Dicarbonyls with Hydroxylamine: FTIR Matrix Isolation and Theoretical Study" Molecules 26, no. 4: 1144. https://doi.org/10.3390/molecules26041144






