Chiral Recognition for Chromatography and Membrane-Based Separations: Recent Developments and Future Prospects
Abstract
1. Introduction
2. Chromatographic Separation
2.1. Liquid Chromatography
2.2. Gas Chromatography
2.3. Capillary Electrochromatography
2.4. Supercritical Fluid Chromatography
2.5. High-Speed Countercurrent Chromatography
2.6. Thin-Layer Chromatography
3. Chiral Membrane Separation Technology
3.1. Liquid Membrane Separation
3.2. Solid Membrane Separation
4. Concluding Remarks and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Flack, H.D. Louis Pasteur’s Discovery of Molecular Chirality and Spontaneous Resolution in 1848, Together with a Complete Review of His Crystallographic and Chemical Work. Acta Cryst. A. 2010, 53, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Dogan, A.; Płotka-Wasylka, J.; Kempińska-Kupczyk, D.; Namieśnik, J.; Kot-Wasik, A. Detection, Identification and Determination of Chiral Pharmaceutical Residues in Wastewater: Problems and Challenges. TrAC Trends Anal. Chem. 2020, 122, 115710. [Google Scholar] [CrossRef]
- Xiao, L.; An, T.; Wang, L.; Xu, X.; Sun, H. Novel Properties and Applications of Chiral Inorganic Nanostructures. Nano Today 2020, 30, 100824. [Google Scholar] [CrossRef]
- Murashima, H.; Fujihara, A. Wavelength Dependence of Chiral Recognition Using Ions between Photoexcited Tryptophan and Sugars. Chem. Phys. 2020, 536, 110818. [Google Scholar] [CrossRef]
- Fernandes, C.; Phyo, Y.Z.; Silva, A.S.; Tiritan, M.E.; Kijjoa, A.; Pinto, M. Chiral Stationary Phases Based on Small Molecules: An Update of the Last 17 Years. Sep. Purif. Rev. 2017, 47, 89–123. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, Y.; Yan, L.; Wang, J.H.; Lu, C.S.; Zhang, Q. Risk Assessment of the Endocrine-Disrupting Effects of Nine Chiral Pesticides. J. Hazard. Mater. 2017, 338, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Shen, G.; Huang, Y.; Luo, J.; Zhu, L.; Qi, S.; Li, Y.; Wang, C.; Li, X. The Enantioselective Toxicity and Oxidative Stress of Beta-Cypermethrin on Zebrafish. Environ. Pollut. 2017, 229, 312–320. [Google Scholar] [CrossRef]
- Harner, T.; Wiberg, K.; Norstrom§, R. Enantiomer Fractions Are Preferred to Enantiomer Ratios for Describing Chiral Signatures in Environmental Analysis. Environ. Sci. Technol. 2000, 34, 218–220. [Google Scholar] [CrossRef]
- Lang, J.C.; Armstrong, D.W. Chiral Surfaces: The Many Faces of Chiral Recognition. Curr. Opin. Colloid Interface Sci. 2017, 32, 94–107. [Google Scholar] [CrossRef]
- Lämmerhofer, M. Chiral Recognition by Enantioselective Liquid Chromatography: Mechanisms and Modern Chiral Stationary Phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Chiral Recognition in Separation Science—An Update. J. Chromatogr. A 2016, 1467, 56–78. [Google Scholar] [CrossRef]
- Pirkle, W.H.; Lee, W. Separation of the Enantiomers of β-Blockers Using Brush Type Chiral Stationary Phase Derived from Con-for-Mationally Rigid α-Amino β-Lactam. Bull. Korean Chem. Soc. 2010, 31, 620. [Google Scholar] [CrossRef][Green Version]
- Chankvetadze, B. Recent Trends in Preparation, Investigation and Application of PolysacchariDe-based Chiral Stationary Phases for Separation of Enantiomers in High-Performance Liquid Chromatography. TrAC Trends Anal. Chem. 2020, 122, 115709. [Google Scholar] [CrossRef]
- Echevarría, R.N.; Keunchkarian, S.; Villarroel-Rocha, J.; Sapag, K.; Reta, M. Organic Monolithic Capillary Columns Coated with Cellulose Tris(3,5-Dimethylphenyl Carbamate) for Enanti-Oseparations by Capillary HPLC. Microchem. J. 2019, 149, 104011. [Google Scholar] [CrossRef]
- Wang, X.; Jameson, C.J.; Murad, S. Modeling Enantiomeric Separations as an Interfacial Process Using Amylose Tris (3,5-dimethylphenyl carbamate) (ADMPC) Polymers Coated on Amorphous Silica. Langmuir 2020, 36, 1113–1124. [Google Scholar] [CrossRef]
- Okamoto, Y. Chiral Polymers for Resolution of Enantiomers. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1731–1739. [Google Scholar] [CrossRef]
- Ogasawara, M.; Enomoto, Y.; Uryu, M.; Yang, X.; Kataoka, A.; Ohnishi, A. Application of Polysaccharide-Based Chiral HPLC Columns for Separation of Nonenantiomeric Isomeric Mixtures of Organometallic Compounds. Organometallics 2019, 38, 512–518. [Google Scholar] [CrossRef]
- Zhang, G.-H.; Fu, K.-Q.; Xi, J.; Chen, W.; Tang, S.; Bai, Z. Structure Screening and Performance Restoration of Chiral Separation Materials Based on Chitosan Derivatives. Carbohydr. Polym. 2019, 214, 259–268. [Google Scholar] [CrossRef] [PubMed]
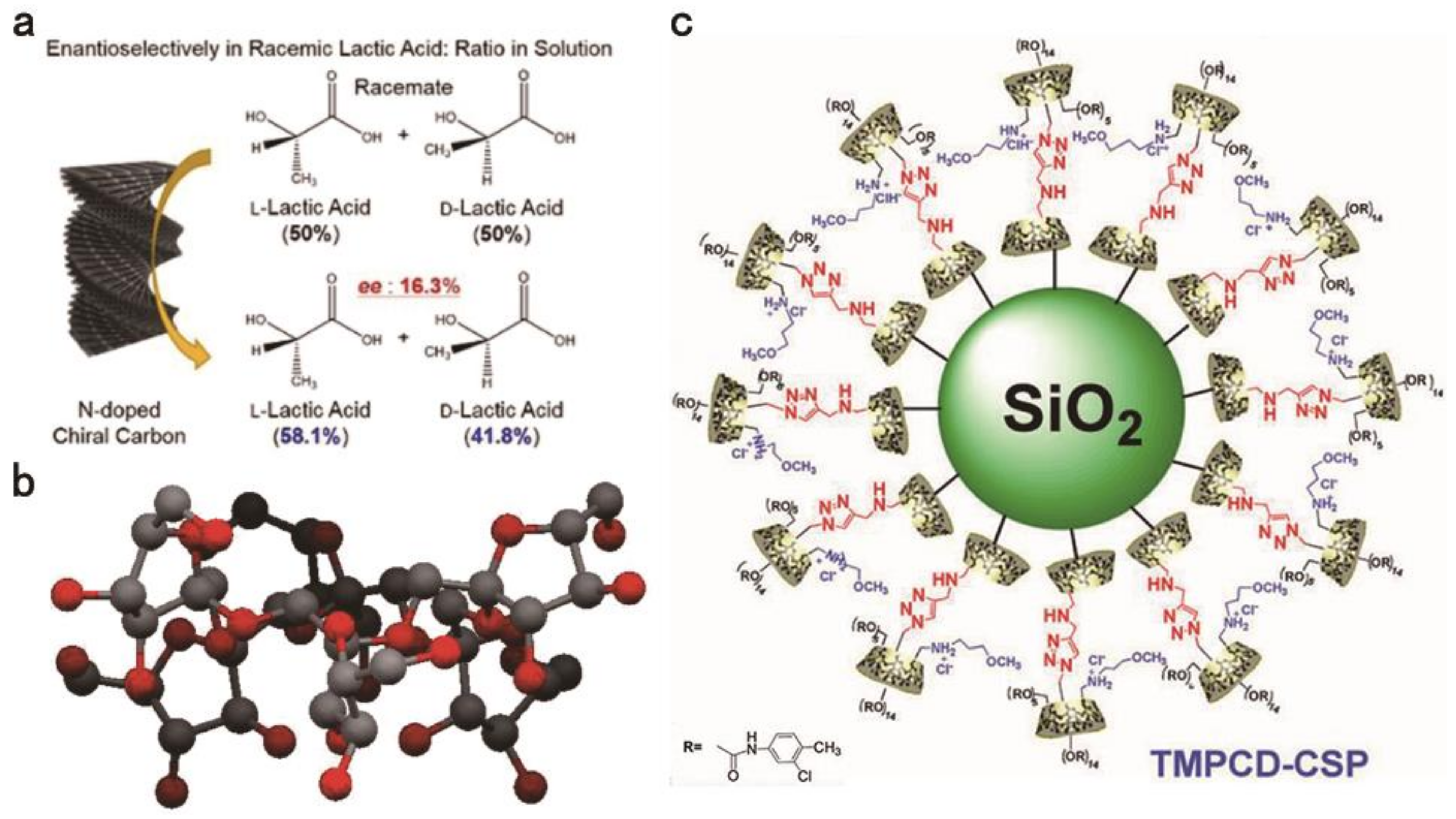
- Nguyen, H.; Ju, S.; Hao, L.T.; Tran, T.H.; Gil Cha, H.; Cha, Y.J.; Park, J.Y.; Hwang, S.Y.; Yoon, D.K.; Hwang, D.S.; et al. The Renewable and Sustainable Conversion of Chitin into a Chiral Nitrogen-Doped Carbon-Sheath Nanofiber for Enantioselective Adsorption. ChemSusChem 2019, 12, 3236–3242. [Google Scholar] [CrossRef]
- Tang, S.; Mei, X.; Chen, W.; Huang, S.; Bai, Z. A High-Performance Chiral Selector Derived from Chitosan (P-Methylbenzylurea) for Efficient Enantiomer Separation. Talanta 2018, 185, 42–52. [Google Scholar] [CrossRef]
- Wang, J.; Xi, J.-B.; Chen, W.; Huang, S.; Bai, Z. High Performance Chiral Separation Materials Based on Chitosan Bis(3,5-Dimethylphenylcarbamate)-(Alkyl Urea)s. Carbohydr. Polym. 2017, 156, 481–489. [Google Scholar] [CrossRef]
- Feng, Z.-W.; Qiu, G.-S.; Mei, X.-M.; Liang, S.; Yang, F.; Huang, S.; Chen, W.; Bai, Z. Structural Dependence on the Property of Chiral Stationary Phases Derived from Chitosan Bis(Arylcarbamate)-(Amide)s. Carbohydr. Polym. 2017, 168, 301–309. [Google Scholar] [CrossRef]
- Xie, S.M.; Yuan, L.M. Recent Development Trends for Chiral Stationary Phases Based on Chitosan Derivatives, Cyclofructan Derivatives and Chiral Porous Materials in High Performance Liquid Chromatography. J. Sep. Sci. 2019, 42, 6–20. [Google Scholar] [CrossRef]
- Frink, L.A.; Berthod, A.; Xu, Q.-L.; Gao, H.; Kurti, L.; Armstrong, D.W. Separation of 2-Naphthol Atropisomers on Cyclofructan-Based Chiral Stationary Phases. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 710–717. [Google Scholar] [CrossRef]
- Sun, P.; Wang, C.; Breitbach, Z.S.; Zhang, Y.; Armstrong, D.W. Development of New HPLC Chiral Stationary Phases Based on Native and Derivatized Cyclofructans. Anal. Chem. 2009, 81, 10215–10226. [Google Scholar] [CrossRef]
- Breitbach, A.S.; Lim, Y.; Xu, Q.-L.; Kürti, L.; Armstrong, D.W.; Breitbach, Z.S. Enantiomeric Separations of α-Aryl Ketones with Cyclofructan Chiral Stationary Phases via High Performance Liquid Chromatography and Supercritical Fluid Chromatography. J. Chromatogr. A 2016, 1427, 45–54. [Google Scholar] [CrossRef]
- Moskaľová, M.; Kozlov, O.; Gondová, T.; Budovská, M.; Armstrong, D.W. HPLC Enantioseparation of Novel Spirobrassinin Analogs on the Cyclofructan Chiral Stationary Phases. Chromatographia 2017, 80, 53–62. [Google Scholar] [CrossRef]
- Khan, M.M.; Breitbach, Z.S.; Berthod, A.; Armstrong, D.W. Chlorinated Aromatic Derivatives of Cyclofructan 6 as HPLC Chiral Stationary Phases. J. Liq. Chromatogr. Rel. Technol. 2016, 39, 497–503. [Google Scholar] [CrossRef]
- Chankvetadze, B. Contemporary Theory of Enantioseparations in Capillary Electrophoresis. J. Chromatogr. A 2018, 1567, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Benkovics, G.; Darcsi, A.; Várnai, B.; Sohajda, T.; Malanga, M.; Béni, S. Comparative Analysis of the Full Set of Methylated β-Cyclodextrins as Chiral Selectors in Capillary Electrophoresis. Electrophoresis 2019, 40, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cheng, B.; Zhou, R.; Cao, Z.; Zeng, C.; Li, L. Preparation and Evaluation of a Novel N-Benzyl-Phenethylamino-β-Cyclodextrin-Bonded Chiral Stationary Phase for HPLC. Talanta 2017, 174, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ma, S.; Liu, B.; Yu, J.; Guo, X. A Fully Derivatized 4-Chlorophenylcarbamate-β-Cyclodextrin Bonded Chiral Stationary Phase for Enhanced en-Antioseparation in HPLC. Talanta 2019, 204, 817–825. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, B.; Tang, J.; Tang, W.-H. A Cationic Cyclodextrin Clicked Bilayer Chiral Stationary Phase for Versatile Chiral Separation in HPLC. New J. Chem. 2018, 42, 3526–3533. [Google Scholar] [CrossRef]
- Li, X.X.; Wang, Y. HPLC Enantioseparation on Cyclodextrin-Based Chiral Stationary Phases. Chiral Separations; Springer: Berlin/Heidelberg, Germany, 2019; pp. 159–169. [Google Scholar]
- Chu, C.; Liu, R. Application of Click Chemistry on Preparation of Separation Materials for Liquid Chromatography. Chem. Soc. Rev. 2011, 40, 2177–2188. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, B.; Tang, J.; Tang, W.-H. Cationic Cyclodextrin Clicked Chiral Stationary Phase for Versatile Enantioseparations in High-Performance Liq-Uid Chromatography. J. Chromatogr. A 2016, 1467, 169–177. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, J.; Wang, Y.; Tang, J.; Tang, W.-H. Enantioseparation of Isoxazolines with Functionalized Perphenylcarbamate Cyclodextrin Clicked Chiral Stationary Phases in HPLC. Electrophoresis 2017, 38, 1939–1947. [Google Scholar] [CrossRef]
- Tang, J.; Lin, Y.; Yang, B.; Zhou, J.; Tang, W.-H. Functionalities Tuned Enantioselectivity of Phenylcarbamate Cyclodextrin Clicked Chiral Stationary Phases in HPLC. Chirality 2017, 29, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Shuang, Y.Z.; Liao, Y.Q.; Wang, H.; Wang, Y.X.; Li, L.S. Preparation and Evaluation of a Triazole-Bridged Bis(β-Cyclodextrin)–Bonded Chiral Stationary Phase For HPLC. Chirality 2020, 32, 1681–1684. [Google Scholar] [CrossRef]
- Shuang, Y.; Liao, Y.; Zhang, T.; Li, L. Preparation and Evaluation of an Ethylenediamine Dicarboxyethyl Diamido-Bridged Bis(β-Cyclodextrin)-Bonded Chiral Stationary Phase for High Performance Liquid Chromatography. J. Chromatogr. A 2020, 1619, 460937. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Zhu, N.; Gao, Z.-X.; Li, T.-J.; Zhou, T.; Ma, Y.-L. Preparation of Cyclodextrin Type Stationary Phase Based on Graphene Oxide and Its Application in Enantioseparation and Hydrophilic Interaction Chromatography. Chin. J. Anal. Chem. 2018, 46, 1455–1463. [Google Scholar] [CrossRef]
- Tang, Q.; Yu, B.; Gao, L.; Cong, H.; Zhang, S. Light-Assisted Preparation of a Cyclodextrin-Based Chiral Stationary Phase and Its Separation Performance in Liquid Chromatography. New J. Chem. 2018, 42, 1115–1120. [Google Scholar] [CrossRef]
- Ilisz, I.; Bajtai, A.; Lindner, W.; Péter, A. Liquid Chromatographic Enantiomer Separations Applying Chiral Ion-Exchangers Based on Cinchona Alkaloids. J. Pharm. Biomed. Anal. 2018, 159, 127–152. [Google Scholar] [CrossRef] [PubMed]
- Rosini, C.; Bertucci, C.; Pini, D.; Altemura, P.; Salvadori, P. Cinchona Alkaloids for Preparing New, Easily Accessible Chiral Stationary phases.I. 11-(10,11-Dihydro-6′-Methoxy-Cinchonan-9-Ol)-Tiopropylsilanized Silica. Tetrahedron Lett. 1985, 26, 3361–3364. [Google Scholar] [CrossRef]
- Lämmerhofer, M.; Lindner, W. Quinine and Quinidine Derivatives as Chiral Selectors I. Brush Type Chiral Stationary Phases for High-Performance Liquid Chromatography Based on Cinchonan Carbamates and Their Application as Chiral Ani-on Exchangers. J. Chromatogr. A 1996, 741, 33–48. [Google Scholar] [CrossRef]
- Ianni, F.; Sardella, R.; Carotti, A.; Natalini, B.; Lindner, W.; Lämmerhofer, M. Quinine-Based Zwitterionic Chiral Stationary Phase as a Complementary Tool for Peptide Analysis: Mobile Phase Effects on Enantio-and Stereoselectivity of Underivatized Oligopeptides. Chirality 2015, 28, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Pataj, Z.; Berkecz, R.; Szatmari, I.; Fülöp, F.; Péter, A. Comparison of Separation Performances of Cellulose-Based Chiral Stationary Phases in LC Enantioseparation of Aminonaphthol Analogues. Chromatography 2009, 70, 723–729. [Google Scholar] [CrossRef]
- Sugimoto, H.; Kakehi, M.; Jinno, F. Method Development for the Determination of d-and l-Isomers of Leucine in Human Plasma by High-Performance Liquid Chromatography Tandem Mass Spectrometry and Its Application to Animal Plasma Samples. Anal. Bioanal. Chem. 2015, 407, 7889–7898. [Google Scholar] [CrossRef]
- Woiwode, U.; Neubauer, S.; Lindner, W.; Buckenmaier, S.; Lämmerhofer, M. Enantioselective Multiple Heartcut Two-Dimensional Ultra-high-Performance Liquid Chromatography Method with a Coreshell Chiral Stationary Phase in the Second Dimension for Analysis of All Proteinogenic Amino Acids in a Single Run. J. Chromatogr. A 2018, 1562, 69–77. [Google Scholar] [CrossRef]
- Beridze, N.; Tsutskiridze, E.; Takaishvili, N.; Farkas, T.; Chankvetadze, B. Comparative Enantiomer-Resolving Ability of Coated and Covalently Immobilized Versions of Two Polysaccharide-Based Chiral Selectors in High-Performance Liquid Chromatography. Chromatography 2018, 81, 611–621. [Google Scholar] [CrossRef]
- Padró, J.M.; Keunchkarian, S. State-of-the-Art and Recent Developments of Immobilized PolysacchariDe-based Chiral Stationary Phases for Enantioseparations by High-Performance Liquid Chromatography (2013–2017). Microchem. J. 2018, 140, 142–157. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, N.; Ma, Y.; Li, Q.; Li, P. Preparation of Polysaccharide-Based Chiral Stationary Phases on SiO2@AG Core–Shell Particles by Means of Coating and Intermolecular Polycondensation and Comparative Liquid Chromatography Enantioseparations. Anal. Bioanal. Chem. 2017, 410, 441–449. [Google Scholar] [CrossRef]
- Bezhitashvili, L.; Bardavelidze, A.; Ordjonikidze, T.; Chankvetadze, L.; Chity, M.; Farkas, T.; Chankvetadze, B. Effect of Pore-Size Optimization on the Performance of Polysaccharide-Based Superficially Porous Chiral Stationary Phases for the Separation of Enantiomers in High-Performance Liquid Chromatography. J. Chromatogr. A 2017, 1482, 32–38. [Google Scholar] [CrossRef]
- Gil-Av, E.; Feibush, B. Resolution of Enantiomers by Gas Liquid Chromatography with Optically Active Stationary Phases. Separation on Packed Columns. Tetrahedron Lett. 1967, 8, 3345–3347. [Google Scholar] [CrossRef]
- Schurig, V. Chiral Separations Using Gas Chromatography. TrAC Trends Anal. Chem. 2002, 21, 647–661. [Google Scholar] [CrossRef]
- Adly, F.G.; Antwi, N.Y.; Ghanem, A. Cyclodextrin-Functionalized Monolithic Capillary Columns: Preparation and Chiral Applications. Chirality 2015, 28, 97–109. [Google Scholar] [CrossRef]
- Smolková-Keulemansová, E. Cyclodextrins as Stationary Phases in Chromatography. J. Chromatogr. A 1982, 251, 17–34. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Chiral Recognition Mechanisms in Analytical Separation Sciences. Chromatography 2012, 75, 815–838. [Google Scholar] [CrossRef]
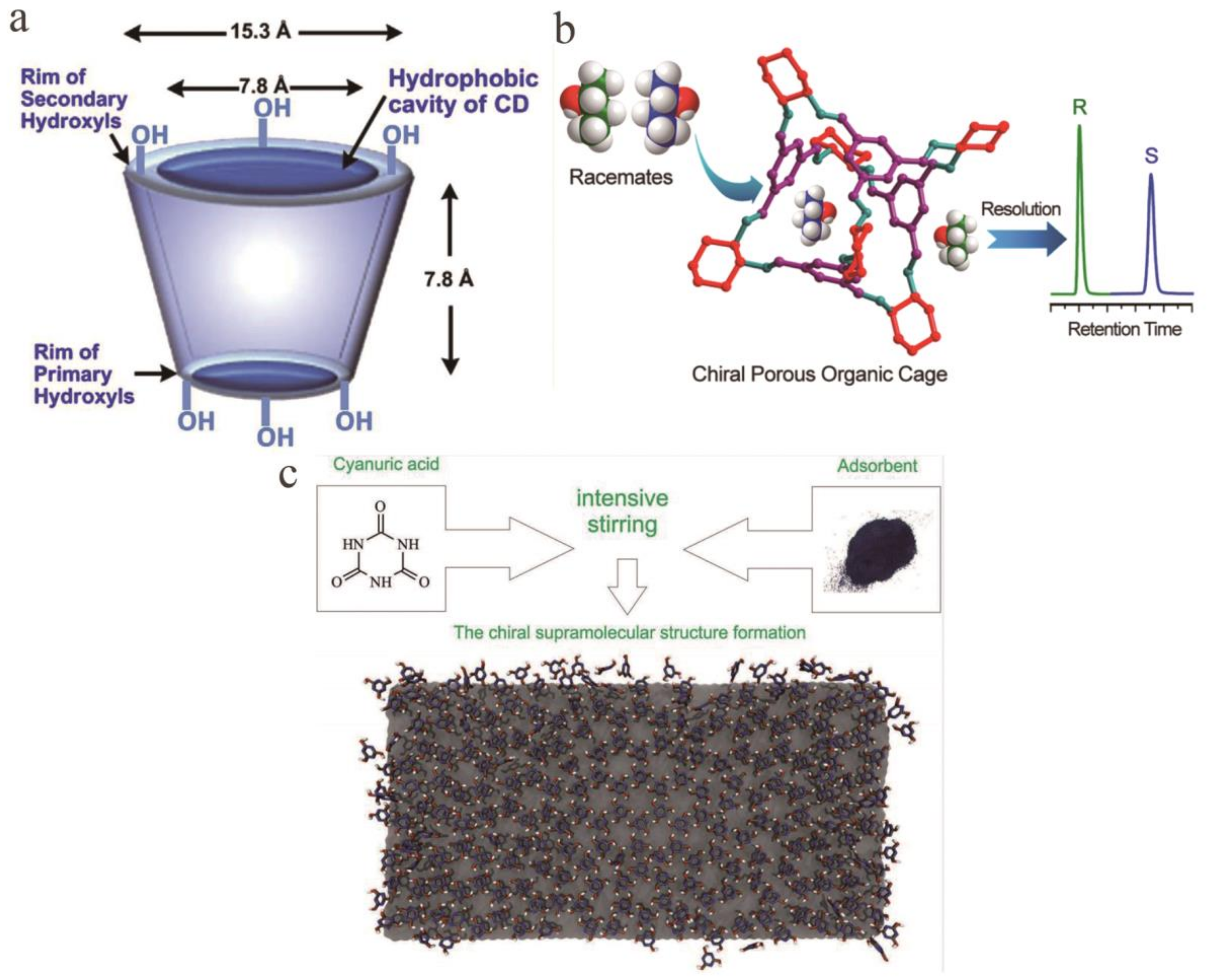
- Wang, S.-Y.; Li, L.; Xiao, Y.; Wang, Y. Recent Advances in Cyclodextrins-Based Chiral-Recognizing Platforms. TrAC Trends Anal. Chem. 2019, 121, 115691. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular Polymeric Materials via Cyclodextrin–Guest Interactions. Acc. Chem. Res. 2014, 47, 2128–2140. [Google Scholar] [CrossRef] [PubMed]
- Onuchak, L.A.; Kuraeva, Y.G.; Evdokimova, M.A.; Golov, A.A. Space Filling of Permethylated β-Cyclodextrin by Volatile Hydrophobic and Hydrophilic Guests in Polyethylene Glycol. J. Chin. Chem. Soc. 2019, 66, 157–163. [Google Scholar] [CrossRef]
- Xie, S.M.; Chen, X.X.; Zhang, J.H.; Yuan, L.M. Gas Chromatographic Separation of Enantiomers on Novel Chiral Stationary Phases. TrAC Trends Anal. Chem. 2020, 124, 115808. [Google Scholar] [CrossRef]
- Qian, H.-L.; Yang, C.-X.; Yan, X.-P. Bottom-up Synthesis of Chiral Covalent Organic Frameworks and Their Bound Capillaries for Chiral Separation. Nat. Commun. 2016, 7, 12104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-H.; Xie, S.-M.; Chen, L.; Wang, B.-J.; He, P.-G.; Yuan, L.-M. Homochiral Porous Organic Cage with High Selectivity for the Separation of Racemates in Gas Chromatography. Anal. Chem. 2015, 87, 7817–7824. [Google Scholar] [CrossRef]
- Xie, S.-M.; Fu, N.; Li, L.; Yuan, B.-Y.; Zhang, J.-H.; Li, Y.-X.; Yuan, L. Homochiral Metal–Organic Cage for Gas Chromatographic Separations. Anal. Chem. 2018, 90, 9182–9188. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, J.; Pu, Q.; Xie, S.; Li, Y.; Luo, L.; Chen, X.; Yuan, L. A Novel Chiral Inorganic Mesoporous Silica Used as a Stationary Phase in GC. Chirality 2019, 31, 1053–1059. [Google Scholar] [CrossRef]
- Hifsudheen, M.; Mishra, R.; Vedhanarayanan, B.; Praveen, V.K.; Ajayaghosh, A. The Helix to Super-Helix Transition in the Self-Assembly of π-Systems: Superseding of Molecular Chirality at Hierarchical Level. Angew Chem. 2017, 56, 12634–12638. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef] [PubMed]
- Lomenova, A.; Hroboňova, K. Comparison of HPLC Separation of Phenylalanine Enantiomers on Different Types of Chiral Stationary Phases. Food Anal. Method 2018, 11, 3314–3323. [Google Scholar] [CrossRef]
- West, C.; Konjaria, M.-L.; Shashviashvili, N.; Lemasson, E.; Bonnet, P.; Kakava, R.; Volonterio, A.; Chankvetadze, B. Enantioseparation of Novel Chiral Sulfoxides on Chlorinated Polysaccharide Stationary Phases in Supercritical Fluid Chromatography. J. Chromatogr. A 2017, 1499, 174–182. [Google Scholar] [CrossRef]
- Zhu, B.; Zhao, F.; Yu, J.; Wang, Z.; Song, Y.; Li, Q. Chiral Separation and a Molecular Modeling Study of Eight Azole Antifungals on the Cellulose Tris(3,5-Dichlorophenylcarbamate) Chiral Stationary Phase. New J. Chem. 2018, 42, 13421–13429. [Google Scholar] [CrossRef]
- Karakka, K.A.K.; Karatt, T.K.; Sayed, R.; Philip, M.; Meissir, S.; Nalakath, J. Separation of Ephedrine and Pseudoephedrine Enantiomers Using a PolysacchariDe-based Chiral Column: A Normal Phase Liquid Chromatography–High-Resolution Mass Spectrometry Approach. Chirality 2019, 31, 568–574. [Google Scholar] [CrossRef]
- Gainullina, Y.Y.; Timofeeva, D.V.; Ivanov, S.P.; Gus’Kov, V.Y. Separating Enantiomers of Haloalkanes and Alcohols on a Stationary Phase Based on the Supramolecular Structure of Melamine with Induced Chirality. Russ. J. Phys. Chem. A 2019, 93, 1165–1170. [Google Scholar] [CrossRef]
- Gus’Kov, V.Y.; Sukhareva, D.A.; Gainullina, Y.Y.; Hamitov, E.M.; Galkin, Y.G.; Maistrenko, V.N. Chiral Recognition Capabilities of Melamine and Cyanuric Acid Supramolecular Structures. Supramol. Chem. 2018, 30, 940–948. [Google Scholar] [CrossRef]
- Nafikova, A.R.; Allayarova, D.A.; Gus’Kov, V.Y. Separation of 2-Bromobutane, 2-Chlorobutane, 2-Chloropentane, and 2-Butanol Enantiomers Using a Stationary Phase Based on a Supramolecular Uracil Structure. J. Anal. Chem. 2019, 74, 565–569. [Google Scholar] [CrossRef]
- Scriba, G.K. Chiral Recognition in Separation Sciences. Part II: Macrocyclic Glycopeptide, Donor-Acceptor, Ion-Exchange, Ligand-Exchange and Micellar Selectors. TrAC Trends Anal. Chem. 2019, 119. [Google Scholar] [CrossRef]
- Chalavi, S.; Fakhari, A.R.; Nojavan, S. Development of a Modified Partial Filling Method in Capillary Electrophoresis Using Two Chiral Plugs for the Simultaneous Enantioseparation of Chiral Drugs: Comparison with Mixed Chiral Selector Capillary Electrophoresis. J. Chromatogr. A 2018, 1567, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lancioni, C.; Keunchkarian, S.; Castells, C.B.; Gagliardi, L.G. Enantiomeric Separations by Capillary Electrophoresis: Theoretical Method to Determine Optimum Chiral Selector Concentration. J. Chromatogr. A 2018, 1539, 71–77. [Google Scholar] [CrossRef]
- Nawała, J.; Dawidziuk, B.; Dziedzic, D.; Gordon, D.; Popiel, S. Applications of Ionic Liquids in Analytical Chemistry with a Particular Emphasis on Their Use in Solid-Phase Microextraction. TrAC Trends Anal. Chem. 2018, 105, 18–36. [Google Scholar] [CrossRef]
- Zhang, Q.; Qi, X.Y.; Feng, C.L.; Tong, S.S.; Rui, M.J. Three Chiral Ionic Liquids as Additives for Enantioseparation in Capillary Electro-Phoresis and Their Comparison with Conventional Modifiers. J. Chromatogr A. 2016, 1462, 146–152. [Google Scholar] [CrossRef]
- Ali, I.; Suhail, M.; Sanagi, M.M.; Aboul-Enein, H.Y. Ionic Liquids in HPLC and CE: A Hope for Future. Crit. Rev. Anal. Chem. 2017, 47, 332–339. [Google Scholar] [CrossRef]
- Greño, M.; Marina, M.L.; Castro-Puyana, M. Enantioseparation by Capillary Electrophoresis Using Ionic Liquids as Chiral Selectors. Crit. Rev. Anal. Chem. 2018, 48, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Alajmi, M.F.; Hussain, I.; Ali, I. Future of Ionic Liquids for Chiral Separations in High-Performance Liquid Chromatography and Capillary Electrophoresis. Crit. Rev. Anal. Chem. 2018, 49, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Ionic Liquids in Capillary Electrophoresis for Enantioseparation. TrAC Trends Anal. Chem. 2018, 100, 145–154. [Google Scholar] [CrossRef]
- Xu, H.; Du, Y.; Feng, Z.; Sun, X.; Liu, J. Synthesis of a Chiral Ionic Liquid, Cholinium-Clindamycin Phosphate, as Sole Chiral Selector in Capillary Electrophoresis. J. Chromatogr. A 2020, 1615, 460721. [Google Scholar] [CrossRef]
- Yu, R.B.; Quirino, J.P. Chiral Selectors in Capillary Electrophoresis: Trends During 2017–2018. Molecules 2019, 24, 1135. [Google Scholar] [CrossRef]
- Benkovics, G.; Fejős, I.; Darcsi, A.; Varga, E.; Malanga, M.; Fenyvesi, É.; Sohajda, T.; Szente, L.; Béni, S.; Szeman, J. Single-Isomer Carboxymethyl-γ-Cyclodextrin as Chiral Resolving Agent for Capillary Electrophoresis. J. Chromatogr. A 2016, 1467, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, V.; Messina, M.; Contino, A.; Maccarrone, G.; Orlandini, S.; Giuffrida, A. Chiral Separation of Terbutaline and Non-Steroidal Anti-Inflammatory Drugs by Using a New Lysine-Bridged Hemispherodextrin in Capillary Electrophoresis. J. Pharm. Biomed. Anal. 2017, 145, 734–741. [Google Scholar] [CrossRef]
- Ilisz, I.; Berkecz, R.; Péter, A. Retention Mechanism of High-Performance Liquid Chromatographic Enantioseparation on Macrocyclic GlyCo-Peptide-Based Chiral Stationary Phases. J. Chromatogr. A 2009, 1216, 1845–1860. [Google Scholar] [CrossRef]
- Ilisz, I.; Grecsó, N.; Forró, E.; Fülöp, F.; Armstrong, D.W.; Péter, A. High-Performance Liquid Chromatographic Separation of Paclitaxel Intermediate Phenylisoserine Derivatives on Macrocyclic Glycopeptide and Cyclofructan-Based Chiral Stationary Phases. J. Pharm. Biomed. Anal. 2015, 114, 312–320. [Google Scholar] [CrossRef]
- Vargas-Caporali, J.; Juaristi, E. Fundamental Developments of Chiral Phase Chromatography in Connection with Enantioselective Synthesis of β-Amino Acids. Isr. J. Chem. 2017, 57, 896–912. [Google Scholar] [CrossRef]
- Arumugam, V.; Venkatesan, M.; Ramachandran, S.; Sundaresan, U. Bioactive Peptides from Marine Ascidians and Future Drug Development–A Review. Int. J. Pept. Res. Ther. 2018, 24, 13–18. [Google Scholar] [CrossRef]
- Calcaterra, A.; D’Acquarica, I. The Market of Chiral Drugs: Chiral Switches versus de Novo Enantiomerically Pure Compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, S.; Liu, C.; Zhao, X. Enantiomeric Separation of Five Acidic Drugs via Capillary Electrophoresis Using Streptomycin as Chiral Selector. J. Chromatogr. B 2017, 1063, 31–35. [Google Scholar] [CrossRef]
- Dixit, S.; Park, J.H. Application of Rifampicin as a Chiral Selector for Enantioresolution of Basic Drugs Using Capillary Electrophoresis. J. Chromatogr. A 2016, 1453, 138–142. [Google Scholar] [CrossRef]
- Hyun, M.H. Liquid Chromatographic Enantioseparations on Crown Ether-Based Chiral Stationary Phases. J. Chromatogr. A 2016, 1467, 19–32. [Google Scholar] [CrossRef]
- Hägele, J.S.; Schmid, M.G. Enantiomeric Separation of Novel Psychoactive Substances by Capillary Electrophoresis Using (+)-18-Crown-6-Tetracarboxylic Acid as Chiral Selector. Chirality 2018, 30, 1019–1026. [Google Scholar] [CrossRef]
- Paik, M.; Kim, K.-R.; Lee, W. Chiral Profiling Analysis of β-Blockers by Capillary Electrophoresis Using Dual Chiral Selectors. Bull. Korean Chem. Soc. 2015, 36, 1340–1344. [Google Scholar] [CrossRef]
- Tohala, L.; Oukacine, F.; Ravelet, C.; Peyrin, E. Sequence Requirements of Oligonucleotide Chiral Selectors for the Capillary Electrophoresis Resolution of Low-Affinity DNA Binders. Electrophoresis 2017, 38, 1383–1390. [Google Scholar] [CrossRef]
- Quintana, S.; García, M. Ángeles; Marina, M.L.; Ramírez, R.G.; De La Mata, F.J.; Ortega, P. Synthesis of Chiral Carbosilane Dendrimers with l-Cysteine and N-Acetyl-l-Cysteine on Their Surface and Their Application as Chiral Selectors for Enantiomer Separation by Capillary Electrophoresis. Tetrahedron Asymmetry 2017, 28, 1797–1802. [Google Scholar] [CrossRef]
- An, N.; Wang, L.; Zhao, J.; Lv, L.; Wang, N.; Guo, H. Enantioseparation of Fourteen Amino Alcohols by Nonaqueous Capillary Electrophoresis Using Lactobionic AC-Id/d-(+)-Xylose–Boric Acid Complexes as Chiral Selectors. Anal. Methods 2016, 8, 1127–1134. [Google Scholar] [CrossRef]
- Bao, J.J.; Jia, F.; Li, Y.; Liang, Q.; Wang, Y. Synthesis and Applications of Sulfopropyl Ether γ-Cyclodextrin Polymer as Chiral Selector in Capillary Electrophoresis. Anal. Bioanal. Chem. 2016, 408, 3639–3649. [Google Scholar] [CrossRef]
- Naghdi, E.; Fakhari, A.R. Simultaneous Chiral Separation of Tramadol and Methadone in Tablets, Human Urine, and Plasma by Capillary Electrophoresis Using Maltodextrin as the Chiral Selector. Chirality 2018, 30, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, J.; Zhang, X.; Zhao, L.; Li, S. Enantiomeric Separation of Adrenaline, Noradrenaline, and Isoprenaline by Capillary Electrophoresis Using Streptomycin-Modified Gold Nanoparticles. Microchim. Acta 2018, 185, 227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Du, Y.; Chen, J.; Xu, G.; Yu, T.; Hua, X.; Zhang, J. Investigation of Chondroitin Sulfate D and Chondroitin Sulfate E as Novel Chiral Selectors in Capillary Electrophoresis. Anal. Bioanal. Chem. 2013, 406, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, Y.; Sun, X.; Feng, Z.; Ma, X.; Li, J. Synthesis and Application of Amino Triazolium-Modified Lactobionic Acid as Chiral Selector in Capillary Electrophoresis. J. Chromatogr. A 2019, 1594, 199–207. [Google Scholar] [CrossRef]
- Kong, D.; Bao, T.; Chen, Z. In Situ Synthesis of the Imine-Based Covalent Organic Framework LZU1 on the Inner Walls of Capillaries for Electrochromatographic Separation of Nonsteroidal Drugs and Amino Acids. Microchim. Acta 2017, 184, 1169–1176. [Google Scholar] [CrossRef]
- Song, W.-F.; Zhao, Q.-L.; Zhou, X.-J.; Zhang, L.-S.; Huang, Y.-P.; Liu, Z.-S. A Star-Shaped Molecularly Imprinted Polymer Derived from Polyhedral Oligomeric Silsesquioxanes with Improved Site Accessibility and Capacity for Enantiomeric Separation via Capillary Electrochromatography. Microchim. Acta 2019, 186, 22. [Google Scholar] [CrossRef] [PubMed]
- Kartsova, L.; Makeeva, D.; Davankov, V. Nano-Sized Polymer and Polymer-Coated Particles in Electrokinetic Separations. TrAC Trends Anal. Chem. 2019, 120, 115656. [Google Scholar] [CrossRef]
- Khater, S.; Canault, B.; Azzimani, T.; Bonnet, P.; West, C. Thermodynamic Enantioseparation Behavior of Phenylthiohydantoin-Amino Acid Derivatives in Supercritical Fluid Chromatography on Polysaccharide Chiral Stationary Phases. J. Sep. Sci. 2018, 41, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Desfontaine, V.; Capetti, F.; Nicoli, R.; Kuuranne, T.; Veuthey, J.-L.; Guillarme, D. Systematic Evaluation of Matrix Effects in Supercritical Fluid Chromatography versus Liquid Chromatography Coupled to Mass Spectrometry for Biological Samples. J. Chromatogr. B 2018, 1079, 51–61. [Google Scholar] [CrossRef]
- Kalíková, K.; Martínková, M.; Schmid, M.G.; Tesařová, E. Cellulose Tris-(3,5-Dimethylphenylcarbamate)-Based Chiral Stationary Phase for the Enantioseparation of Drugs in Supercritical Fluid Chromatography: Comparison With HPLC. J. Sep. Sci. 2018, 41, 1471–1478. [Google Scholar] [CrossRef]
- Kolderova, N.; Neveselý, T.; Sturala, J.; Kuchař, M.; Holakovský, R.; Kohout, M. Enantioseparation of Chiral Sulfoxides on Amylose-Based Columns: Comparison of Normal Phase Liquid Chro-Matography and Supercritical Fluid Chromatography. Chromatographia 2017, 80, 547–557. [Google Scholar] [CrossRef]
- Chen, L.; Dean, B.; Liang, X. Evaluation of PolysacchariDe-based Chiral Stationary Phases in Modern SFC–MS/MS for Enantioselective Bioa-Nalysis. Bioanalysis 2019, 11, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Orosz, T.; Bajtai, A.; Le, T.M.; Tanács, D.; Szakonyi, Z.; Fülöp, F.; Péter, A.; Ilisz, I. Chiral High-Performance Liquid and Supercritical Fluid Chromatographic Enantioseparations of Limonene-Based Bicyclic Aminoalcohols and Aminodiols on Polysaccharide-Based Chiral Stationary Phases. Biomed. Chromatogr. 2019, 33, e4517. [Google Scholar] [CrossRef] [PubMed]
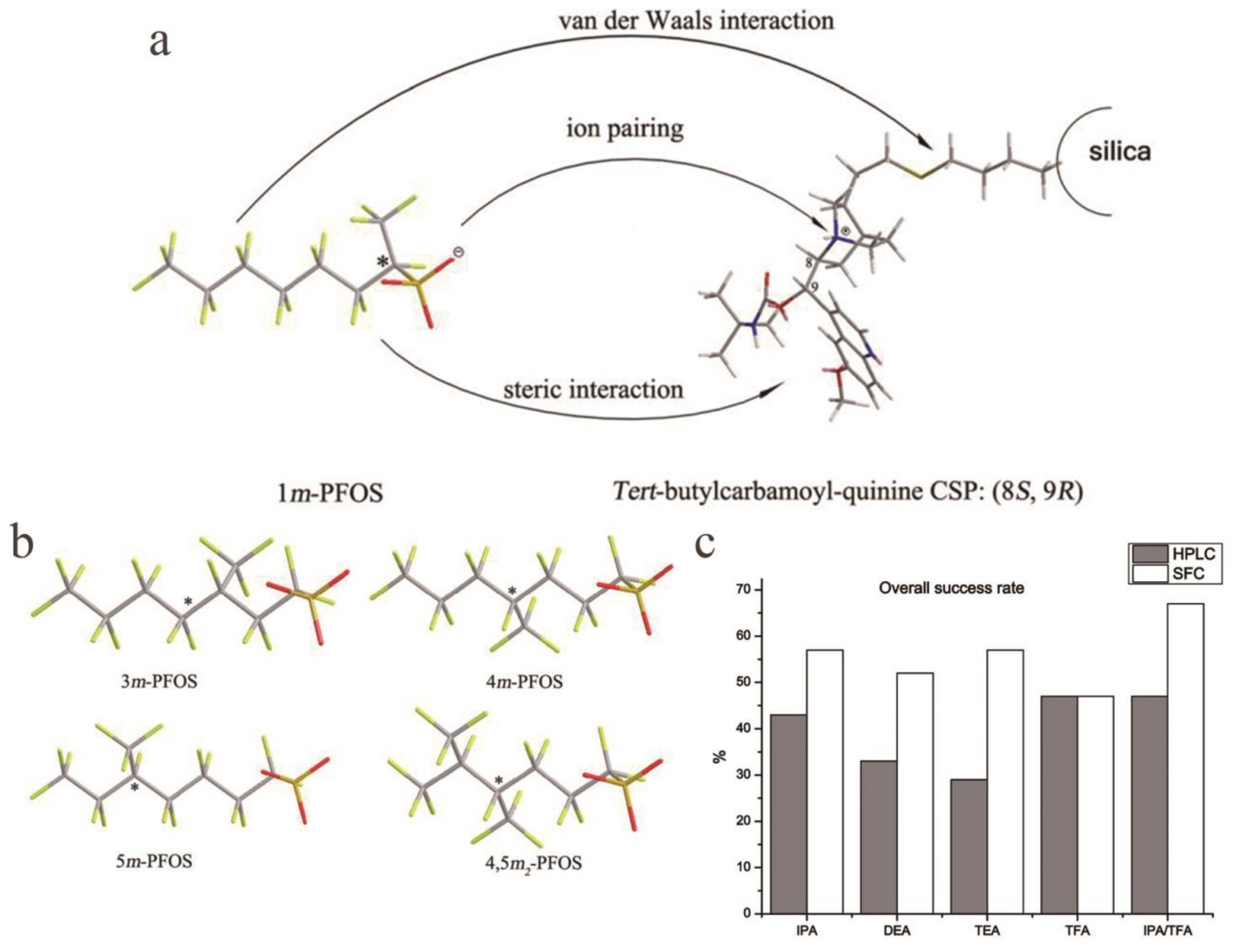
- Zhao, L.; Chen, F.; Guo, F.; Liu, W.; Liu, K. Enantioseparation of Chiral Perfluorooctane Sulfonate (PFOS) by Supercritical Fluid Chromatography (SFC): Effects of the Chromatographic Conditions and Separation Mechanism. Chirality 2019, 31, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Shen, M.; Cheng, D.; Zhang, Y.; Ito, Y.; Yan, J. Chiral Ligand Exchange High-Speed Countercurrent Chromatography: Mechanism and Application in Enanti-Oseparation of Aromatic α-Hydroxyl Acids. J. Chromatogr. A 2014, 1360, 110–118. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Di, D. Chiral Separation by Counter-current Chromatography. TrAC Trends Anal. Chem. 2015, 67, 128–133. [Google Scholar] [CrossRef]
- Han, C.; Luo, J.-G.; Li, Z.; Zhang, Y.; Zhao, H.; Kong, L.-Y. Synergetic Mechanism and Enantioseparation of Aromatic β-Amino Acids by Biphasic Chiral High-Speed Counter-Current Chromatography. J. Sep. Sci. 2016, 39, 2413–2421. [Google Scholar] [CrossRef]
- Lv, L.; Bu, Z.; Sun, W.; Wang, C.; Xu, C.; Tong, S. Application of pH-Zone-Refining Countercurrent Chromatography in the Chiral Separation of Two β-Adrenergic Blocking Agents. J. Sep. Sci. 2018, 41, 1433–1441. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, X.; Yang, G.; Zhan, J.; Li, M.; Long, T.; Ho, C.-T.; Li, S. Simultaneous Separation of Six Pure Polymethoxyflavones from Sweet Orange Peel Extract by High Performance Counter Current Chromatography. Food Chem. 2019, 292, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.J.; Sun, W.Y.; Wang, C.Y.; Yan, J.Z.; Tong, S.Q. Enantioseparation of Acetyltropic Acid by Countercurrent Chromatography with Sulfobutyl Ether-β-Cyclodextrin as Chiral Selector. J. Sep. Sci. 2020, 43, 681–688. [Google Scholar] [CrossRef]
- Wang, S.S.; Han, C.; Wang, S.S.; Bai, L.J.; Li, S.S.; Luo, J.G.; Kong, L. Development of a High Speed Counter-current Chromatography System with CU(II)-Chiral Ionic Liquid Complexes and Hydroxypropyl-β-Cyclodextrin as Dual Chiral Selectors for Enantioseparation of Naringenin. J. Chromatogr. A 2016, 1471, 155–163. [Google Scholar] [CrossRef]
- Park, S.B.; Kim, Y.S. Simultaneous Separation of Three Isomeric Sennosides from Senna Leaf (Cassia acutifolia) Using Counter-Current Chromatography. J. Sep. Sci. 2015, 38, 3502–3507. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, L.; Bu, Z.; Yan, J.; Tong, S. Separation of Epimeric Aromatic Acid(−)-Menthol Esters by Countercurrent Chromatography Using Hydroxy-Propyl-β-Cyclodextrin as an Additive. J. Sep. Sci. 2017, 40, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Sajewicz, M.; Kowalska, T. Chiral Thin-Layer Chromatography in Dynamic Studies: A Short Review. J. Planar Chromatogr. Mod. TLC 2017, 30, 333–339. [Google Scholar] [CrossRef]
- Singh, M.; Malik, P.; Bhushan, R. Resolution of Enantiomers of (RS)-Baclofen by Ligand-Exchange Thin-Layer Chromatography. J. Chromatogr. Sci. 2016, 54, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Sobańska, A.W. Impregnated Silica-Based Layers in Thin Layer Chromatography. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 319–327. [Google Scholar] [CrossRef]
- Singh, M.; Bhushan, R. Thin-Layer Chromatographic Enantioresolution of (RS)-Ketorolac Using l-Amino Acids as Chiral Additive in Stationary Phase. J. Planar Chromatogr. Mod. TLC 2019, 32, 475–479. [Google Scholar] [CrossRef]
- Malik, P.; Bhushan, R. Thin Layer Chromatographic Resolution of Some β-Adrenolytics and a β2-Agonist Using Bovine Serum Albumin as Chiral Additive in Stationary Phase. J. Chromatogr. Sci. 2017, 56, 1–7. [Google Scholar] [CrossRef]
- Malik, P.; Bhushan, R. Ligand Exchange Thin Layer Chromatographic Enantioresolution of (RS)-Ketorolac and (RS)-Etodolac and Recovery of Native Enantiomers. J. Chromatogr. Sci. 2019, 57, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, F.; Wang, B.; Shan, J.; Zhang, Z. Enantioselective Analysis of Naproxen Using Chiral Molecular Imprinting Polymers Based Thin-Layer Chroma-Tography. e-Polymers 2013, 13, 180–188. [Google Scholar] [CrossRef]
- Khatri, S.; Memon, N.; Khatri, Z.; Ahmed, F. TLC-Based Enantiomeric Separation of Amino Acids onto β-CD-Incorporated Glutaraldehyde-Crosslinked PVA Elec-Trospun Fiber Stationary Phase. Acta Chromatogr. 2020, 32, 210–213. [Google Scholar] [CrossRef]
- Ingole, P.G.; Ingole, N.P. Methods for Separation of Organic and Pharmaceutical Compounds by Different Polymer Materials. Korean J. Chem. Eng. 2014, 31, 2109–2123. [Google Scholar] [CrossRef]
- Izake, E.L. Chiral Discrimination and Enantioselective Analysis of Drugs: An Overview. J. Pharm. Sci. 2007, 96, 1659–1676. [Google Scholar] [CrossRef] [PubMed]
- Kryscio, D.R.; Peppas, N.A. Critical Review and Perspective of Macromolecularly Imprinted Polymers. Acta Biomater. 2012, 8, 461–473. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Tharpa, K.; Dima, Ş.O. Molecularly Imprinted Membranes: Past, Present, and Future. Chem. Rev. 2016, 116, 11500–11528. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Tiritan, M.E.; Pinto, M. Chiral Separation in Preparative Scale: A Brief Overview of Membranes as Tools for Enantiomeric Separation. Symmetry 2017, 9, 206. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Yoshikawa, M. Polymeric Pseudo-Liquid Membranes from Poly (Octadecyl Methacrylate). J. Membr. Sci. 2013, 445, 8–14. [Google Scholar] [CrossRef]
- Szabó-Szentjóbi, H.; Bagi, P.; Müller, J.; Balogh, G.T.; Tóth, T.; Huszthy, P. Synthesis and Enantioselective Transport Studies of Both Enantiomers of New Chiral Proton-Ionizable Crown Ethers Containing a Diarylphosphinic Acid Unit. Tetrahedron 2019, 75, 1275–1281. [Google Scholar] [CrossRef]
- Gössi, A.; Riedl, W.; Schuur, B. Enantioseparation with Liquid Membranes. J. Chem. Technol. Biotechnol. 2017, 93, 629–644. [Google Scholar] [CrossRef]
- Dahi, A.; Fatyeyeva, K.; Langevin, D.; Chappey, C.; Poncin-Epaillard, F.; Marais, S. Effect of Cold Plasma Surface Treatment on the Properties of Supported Ionic Liquid Membranes. Sep. Purif. Technol. 2017, 187, 127–136. [Google Scholar] [CrossRef]
- Rehn, G.; Ayres, B.M.; Adlercreutz, P.; Grey, C. An Improved Process for Biocatalytic Asymmetric Amine Synthesis by in Situ Product Removal Using a Support-Ed Liquid Membrane. J. Mol. Catal. B Enzym. 2016, 123, 1–7. [Google Scholar] [CrossRef]
- Zhang, F.; He, L.; Sun, W.; Cheng, Y.; Liu, J.; Ren, Z. Chiral Liquid Membrane for Enantioselective Separation of Racemic Ibuprofen by l-Tartaric Acid Derivatives. RSC Adv. 2015, 5, 41729–41735. [Google Scholar] [CrossRef]
- Kong, D.; Zhou, Z.; Zhu, H.; Mao, Y.; Guo, Z.; Zhang, W.; Ren, Z. Selective Separation of Salbutamol Enantiomers with Simultaneously Synergistic Extraction and Stripping Method. J. Membr. Sci. 2016, 499, 343–351. [Google Scholar] [CrossRef]
- Gaálová, J.; Yalcinkaya, F.; Cuřínová, P.; Kohout, M.; Yalcinkaya, B.; Koštejn, M.; Jirsák, J.; Stibor, I.; Bara, J.E.; Van Der Bruggen, B.; et al. Separation of Racemic Compound by Nanofibrous Composite Membranes with Chiral Selector. J. Membr. Sci. 2020, 596. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, F.; Luo, Y. Preparation and Evaluation of Chiral Selective Cation-Exchange PMMA–PNIPAm Thermal-Sensitive Membranes. Iran. Polym. J. 2014, 23, 679–687. [Google Scholar] [CrossRef]
- Ingole, P.G.; Bajaj, H.C.; Singh, K. Membrane Separation Processes: Optical Resolution of Lysine and Asparagine Amino Acids. Desalination 2014, 343, 75–81. [Google Scholar] [CrossRef]
- Meng, C.; Sheng, Y.; Chen, Q.; Tan, H.; Liu, H. Exceptional Chiral Separation of Amino Acid Modified Graphene Oxide Membranes with High-Flux. J. Membr. Sci. 2017, 526, 25–31. [Google Scholar] [CrossRef]
- Miao, L.; Yang, Y.; Tu, Y.; Lin, S.; Hu, J.; Du, Z.; Zhang, M.; Li, Y. Chiral Resolution by Polysulfone-Based Membranes Prepared via Mussel-Inspired Chemistry. React. Funct. Polym. 2017, 115, 87–94. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Jiang, Y.; Li, S.; Liu, W. Molecularly Imprinted Polymers for the Identification and Separation of Chiral Drugs and Biomolecules. Polymers 2016, 8, 216. [Google Scholar] [CrossRef]
- Li, H.; Huang, Q.; Li, D.; Li, S.; Wu, X.; Wen, L.; Ban, C. Generation of a Molecular Imprinted Membrane by Coating Cellulose Acetate onto a ZrO2-Modified Alumina Membrane for the Chiral Separation of Mandelic Acid Enantiomers. Org. Process Res. Dev. 2018, 22, 278–285. [Google Scholar] [CrossRef]







| Types of CSPs | Basic Material | Target Characteristics | Commercial Column |
|---|---|---|---|
| Polysaccharides | Amylose or cellulose | Widely applicable, such as compounds containing amide groups, aromatic ring substituents, carbonyl nitro, sulfonyl, cyano, hydroxyl, amino and other groups, and amino derivatives | Chiralcel®OD, Chiralpak®IB, Lux®Amylose-1, Lux®i-Amylose-1 |
| Cyclodextrins | β-cyclodextrin | Widely applicable, such as hydrocarbon compounds, sterols, phenol esters and their derivatives, aromatic amines, polyheterocycles | B-DEXTM225, Astec Cyclobond®, I 2000 RSP, LiChroCART®250-4, ChiraDex® |
| Proteins | Enzymes, plasma proteins, receptor proteins | Water-soluble medicine | Chiralpak®HAS, Resolvosil®BSA, Chiralpak®AGP |
| Crown ethers | Macrocyclic polyether | Amino acids, amino alcohols, primary amines | Crownpak® CR(+)/CR(−), Chirosil® RCA(+)/RAC(−) |
| Pirkle type | Amine, amino alcohol, amino acid derivative Compounds, anthrone derivatives | Widely applicable, CSPs designed by analyzing the target | Whelk-O1®, ULMO®, Chirex® |
| Ion exchange type | Cinchona alkaloids, sulfamic acid | N-protected amino acid, N-protected amino group, sulfamic acid, amino phosphoric acid, aromatic carbonyl acid | Chiralpak®QN-AX, Chiralpak®QD-AX, Chiralpak®ZWIX(+), Chiralpak®ZWIX(−) |
| Macrocyclic glycopeptides | Avomycin, Ristomycin A, Vancomycin, Teicoplanin and Teicoplanin aglycone | Widely applicable, such as amino acids, peptides, non-steroidal anti-inflammatory drugs | Astec® CHIROBIOTIC® V, Astec® CHIROBIOTIC® R, Astec® CHIROBIOTIC® TAG |
| Cyclofructans | Cyclic oligosaccharides | Primary amine, acid, secondary amine, tertiary amine, alcohol | Larihc® CF6-RN |
| Porous organic materials | Covalent organic framework, metal organic framework, metal organic cage, mesoporous silica | Halogenated hydrocarbons, ketones, esters, ethers, organic acids, alkylene oxides, alcohols and sulfoxides | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhu, X.; Jiang, W.; Liu, H.; Sun, B. Chiral Recognition for Chromatography and Membrane-Based Separations: Recent Developments and Future Prospects. Molecules 2021, 26, 1145. https://doi.org/10.3390/molecules26041145
Zhao Y, Zhu X, Jiang W, Liu H, Sun B. Chiral Recognition for Chromatography and Membrane-Based Separations: Recent Developments and Future Prospects. Molecules. 2021; 26(4):1145. https://doi.org/10.3390/molecules26041145
Chicago/Turabian StyleZhao, Yuan, Xuecheng Zhu, Wei Jiang, Huilin Liu, and Baoguo Sun. 2021. "Chiral Recognition for Chromatography and Membrane-Based Separations: Recent Developments and Future Prospects" Molecules 26, no. 4: 1145. https://doi.org/10.3390/molecules26041145
APA StyleZhao, Y., Zhu, X., Jiang, W., Liu, H., & Sun, B. (2021). Chiral Recognition for Chromatography and Membrane-Based Separations: Recent Developments and Future Prospects. Molecules, 26(4), 1145. https://doi.org/10.3390/molecules26041145