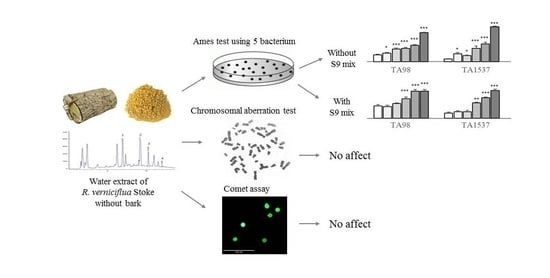
Genotoxicity of Water Extract from Bark-Removed Rhus verniciflua Stokes
Abstract
:1. Introduction
2. Results
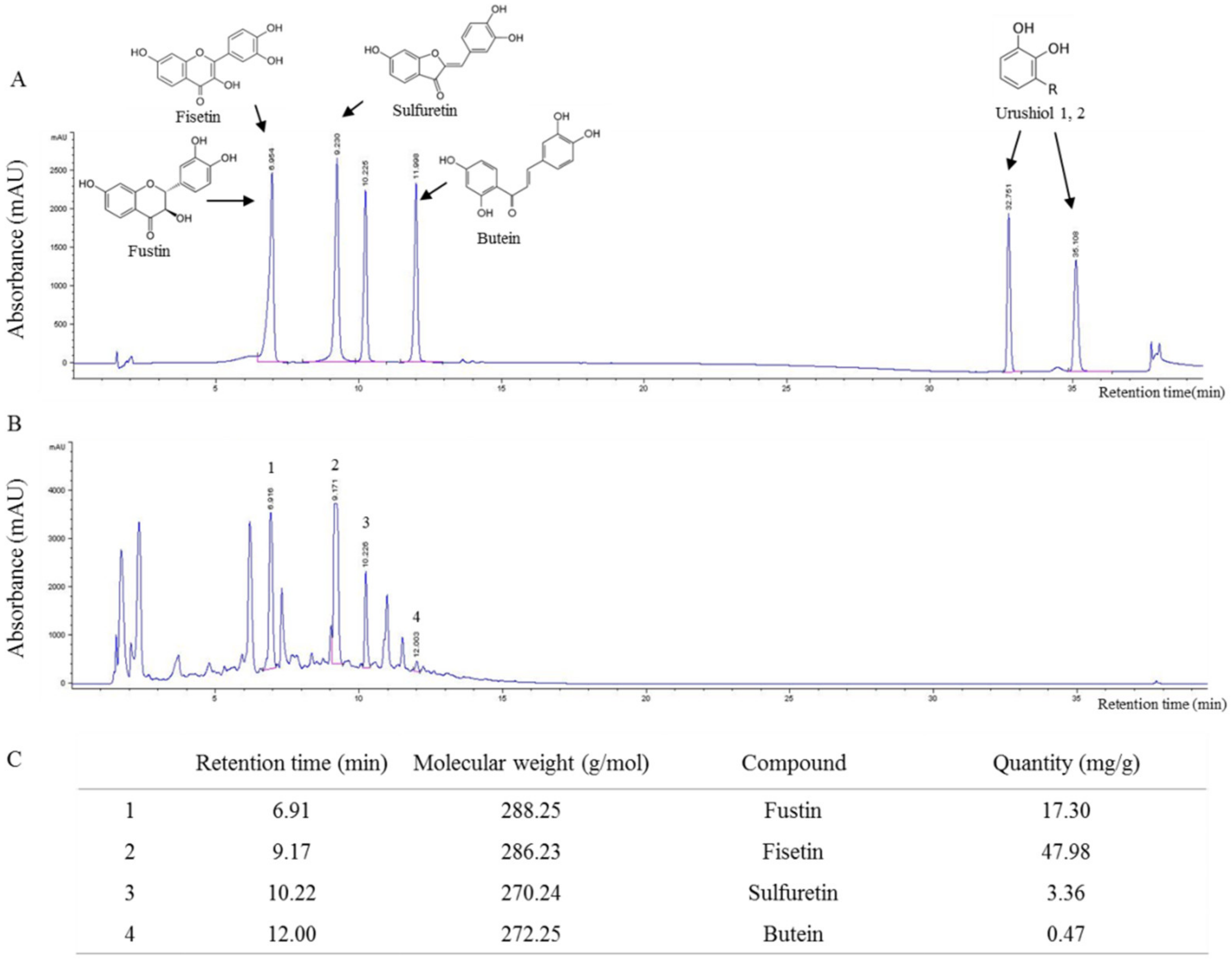
2.1. Fingerprint Analysis of RVX
2.2. Determination of Reverse Mutation in Bacterium
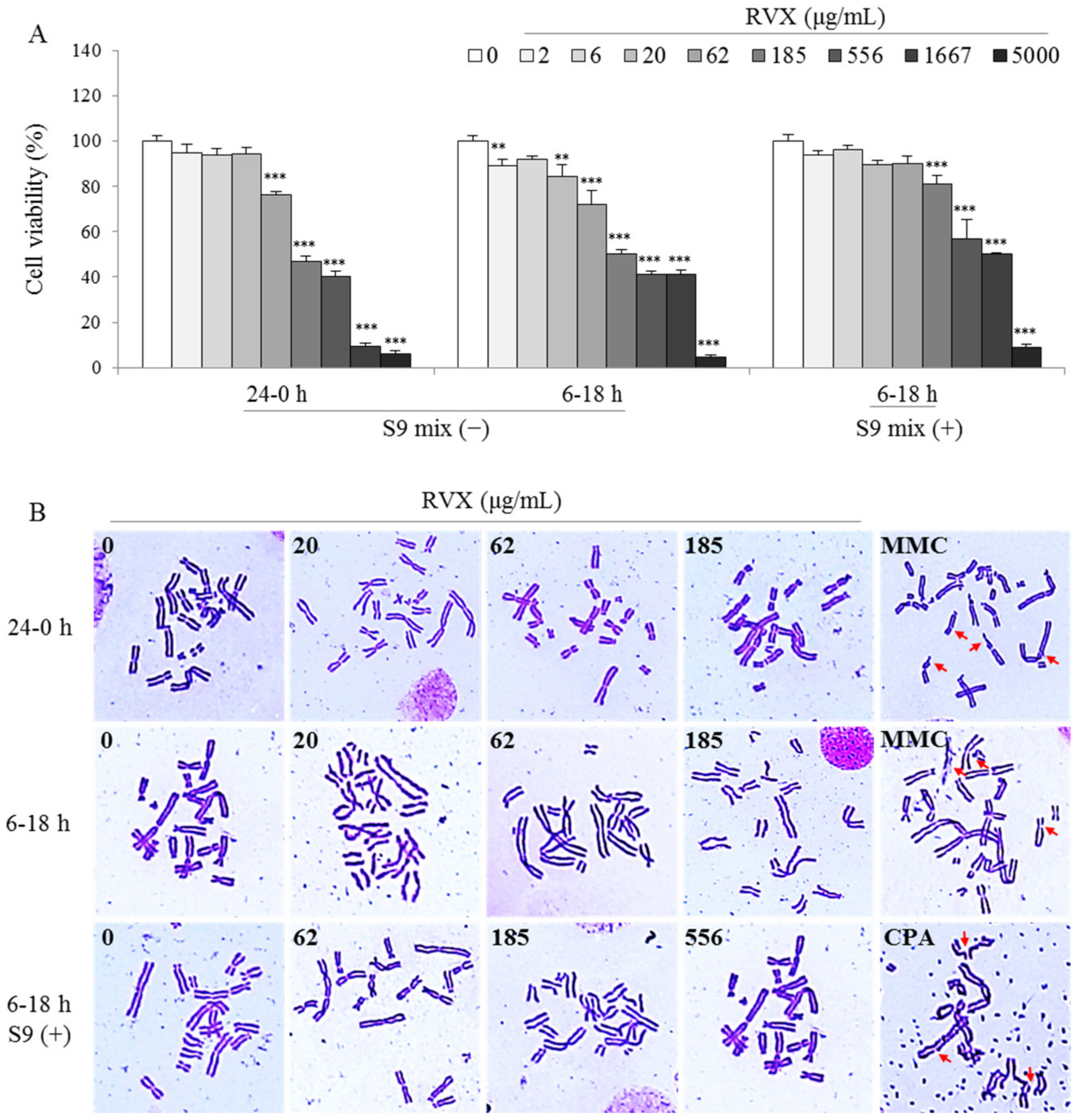
2.3. Determination of Chromosomal Aberration in a Hamster Ovary Cell Line
2.4. Determination of DNA Damage in Human and Mouse Cell Lines
3. Discussion
4. Materials and Methods
4.1. Preparation of RVS and Fingerprinting Analysis
4.2. Bacterial Reverse Mutation Test
4.3. Cell Cultures
4.4. Cell Viability Assay
4.5. In Vitro Mammalian Chromosomal Aberration Test
4.6. Comet Assay in Human Liver and Mouse Muscle Cells
4.7. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar] [CrossRef]
- Medium. Available online: https://medium.com/@faisal_44563/global-herbal-medicine-market-growth-2019-2024-ee649dfc3867 (accessed on 21 February 2019).
- Jordan, S.A.; Cunningham, D.G.; Marles, R.J. Assessment of herbal medicinal products: Challenges, and opportunities to increase the knowledge base for safety assessment. Toxicol. Appl. Pharmacol. 2010, 243, 198–216. [Google Scholar] [CrossRef]
- Posadzki, P.; Watson, L.K.; Ernst, E. Adverse effects of herbal medicines: An overview of systematic reviews. Clin. Med. 2013, 13, 7–12. [Google Scholar] [CrossRef]
- Abt, A.B.; Oh, J.Y.; Huntington, R.A.; Burkhart, K.K. Chinese Herbal Medicine Induced Acute Renal Failure. Arch. Intern. Med. 1995, 155, 211–212. [Google Scholar] [CrossRef]
- Xu, L.; Lin, Q. [Hepatic toxicity of Chinese herbal medicine]. Zhonghua Gan Zang Bing Za Zhi 2007, 15, 534–535. [Google Scholar]
- Zhang, J.; Wider, B.; Shang, H.; Li, X.; Ernst, E. Quality of herbal medicines: Challenges and solutions. Complementary Ther. Med. 2012, 20, 100–106. [Google Scholar] [CrossRef]
- Zhou, J.; Ouedraogo, M.; Qu, F.; Duez, P. Potential genotoxicity of traditional Chinese medicinal plants and phytochemicals: An overview. Phytother. Res. 2013, 27, 1745–1755. [Google Scholar] [CrossRef]
- Schins, R.P.F. Mechanisms of Genotoxicity of Particles and Fibers. Inhal. Toxicol. 2002, 14, 57–78. [Google Scholar] [CrossRef]
- Mei, N.; Guo, L.; Fu, P.P.; Fuscoe, J.C.; Luan, Y.; Chen, T. Metabolism, Genotoxicity, and Carcinogenicity of Comfrey. J. Toxicol. Environ. Health B Crit. Rev. 2010, 13, 509–526. [Google Scholar] [CrossRef] [Green Version]
- Bruno, L.O.; Simoes, R.S.; de Jesus Simoes, M.; Girão, M.J.B.C.; Grundmann, O. Pregnancy and herbal medicines: An unnecessary risk for women’s health—A narrative review. Phytother. Res. 2018, 32, 796–810. [Google Scholar] [CrossRef]
- Holst, L.; Nordeng, H.; Haavik, S. Use of herbal drugs during early pregnancy in relation to maternal characteristics and pregnancy outcome. Pharmacoepidemiol. Drug Saf. 2008, 17, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Korean Ministry of Food and Drug Safety. Pre-Clinical Study Guideline of Herbal Medicine. Volume 290. Available online: http://www.nifds.go.kr/brd/m_15/view.do?seq=11381&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=74 (accessed on 2 April 2019).
- Yoo, H.; Roh, J. Compendium of Prescriptions from the Countryside (Hyangyakjipseongbang); Hangrimchulpansa: Seoul, Korea, 1977; Volume 1433. [Google Scholar]
- Luo, X.; Li, S. Compendium of Materia Medica: Bencao Gangmu; Foreign Languages Press: Beijing, China, 2003. [Google Scholar]
- Lim, K.-T.; Hu, C.; Kitts, D.D. Antioxidant activity of a Rhus verniciflua Stokes ethanol extract. Food Chem. Toxicol. 2001, 39, 229–237. [Google Scholar] [CrossRef]
- Kim, M.-J.; Choi, W.-C.; Barshinikov, A.M.; Kobayashi, A. Anticancer and Antioxidant Activity of Allergen-Removed Extract in Rhus verniciflua Stokes. Korean J. Med. Crop Sci. 2002, 10, 288–293. [Google Scholar]
- Kim, J.-S.; Kwon, Y.-S.; Chun, W.-J.; Kim, T.-Y.; Sun, J.; Yu, C.-Y.; Kim, M.-J. Rhus verniciflua Stokes flavonoid extracts have anti-oxidant, anti-microbial and α-glucosidase inhibitory effect. Food Chem. 2010, 120, 539–543. [Google Scholar] [CrossRef]
- Suk, K.T.; Baik, S.K.; Kim, H.S.; Park, S.M.; Paeng, K.J.; Uh, Y.; Jang, I.H.; Cho, M.Y.; Choi, E.H.; Kim, M.J.; et al. Antibacterial effects of the urushiol component in the sap of the lacquer tree (Rhus verniciflua Stokes) on Helicobacter pylori. Helicobacter 2011, 16, 434–443. [Google Scholar] [CrossRef]
- Moon, J.E.; Shin, J.-H.; Kwon, O.; Kim, J.Y. A Standardized Extract of Rhus verniciflua Stokes Protects Wistar Rats Against Lipopolysaccharide-Induced Acute Inflammation. J. Med. Food 2015, 18, 1223–1230. [Google Scholar] [CrossRef]
- Lee, J.-D.; Huh, J.-E.; Jeon, G.; Yang, H.-R.; Woo, H.-S.; Choi, D.-Y.; Park, D.-S. Flavonol-rich RVHxR from Rhus verniciflua Stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritic fibroblast-like synovial cells and in vivo models. Int. Immunopharmacol. 2009, 9, 268–276. [Google Scholar] [CrossRef]
- Kang, S.-H.; Hwang, I.-H.; Son, E.; Cho, C.-K.; Choi, J.-S.; Park, S.-J.; Jang, B.-C.; Lee, K.-B.; Lee, Z.-W.; Lee, J.H.; et al. Allergen-Removed Rhus verniciflua Extract Induces Ovarian Cancer Cell Death via JNK Activation. Am. J. Chin. Med. 2016, 44, 1719–1735. [Google Scholar] [CrossRef]
- Kang, Y.; Yoon, S.W.; Park, B. Allergen-removed Rhus verniciflua Stokes suppresses invasion and migration of pancreatic cancer cells through downregulation of the JAK/STAT and Src/FAK signaling pathways. Oncol. Rep. 2018, 40, 3060–3068. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Lee, C.W.; Kim, J.-H.; Lee, J.-C.; An, W.G. Extract of Rhus verniciflua Stokes Induces p53-Mediated Apoptosis in MCF-7 Breast Cancer Cells. Evid. Based Complement. Alternat. Med. 2019, 2019, 9407340. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Jung, H.; Kim, K.; Lee, S.; Yoon, S.; Park, J.; Kim, S.; Cheon, S.; Eo, W.; Lee, S. Rhus Verniciflua Stokes against Advanced Cancer: A Perspective from the Korean Integrative Cancer Center; BioMed Research International 2011; Hindawi: London, UK, 2012. [Google Scholar]
- Kim, H.-S.; Kim, H.-G.; Im, H.-J.; Lee, J.-S.; Lee, S.-B.; Kim, W.-Y.; Lee, H.-W.; Lee, S.-K.; Byun, C.K.; Son, C.-G. Antiemetic and Myeloprotective Effects of Rhus verniciflua Stoke in a Cisplatin-Induced Rat Model. Evid. Based Complement. Alternat. Med. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.Y.; Shin, H.; Lim, J.-W.; Ahn, J.H.; Jo, Y.H.; Lee, K.Y.; Hwang, B.Y.; Jung, S.-J.; Kang, S.Y.; Lee, M.K. Comparison of antibacterial activity and phenolic constituents of bark, lignum, leaves and fruit of Rhus verniciflua. PLoS ONE 2018, 13, e0200257. [Google Scholar] [CrossRef]
- National Institute of Food and Drug Safety Evaluation. Standard and criteria of food Korean Ministry of Food and Drug Safety. Volume 31. Available online: https://www.mfds.go.kr/brd/m_211/view.do?seq=14324 (accessed on 26 April 2019).
- Chae, J.; Lee, S.; Lee, S. Potential Efficacy of Allergen Removed Rhus Verniciflua Stokes Extract to Maintain Progression-Free Survival of Patients With Advanced Hepatobiliary Cancer. Explore (NY) 2018, 14, 300–304. [Google Scholar] [CrossRef]
- Jeong, H.J.; Park, J.-H.; Kim, M.-J. Optimization of the extraction process for fermented Rhus verniciflua stokes using response surface methodology. Food Sci. Biotechnol. 2016, 25, 179–184. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kang, J.-Y.; Oh, M.-J. Antiviral activities of flavonoids isolated from the bark of Rhus verniciflua stokes against fish pathogenic viruses. In Vitro J. Microbiol. 2012, 50, 293–300. [Google Scholar] [CrossRef]
- Lee, J.-C.; Lee, K.-Y.; Kim, J.; Na, C.-S.; Jung, N.-C.; Chung, G.-H.; Jang, Y.-S. Extract from Rhus verniciflua Stokes is capable of inhibiting the growth of human lymphoma cells. Food Chem. Toxicol. 2004, 42, 1383–1388. [Google Scholar] [CrossRef]
- Shin, S.-H.; Koo, K.-H.; Bae, J.-S.; Cha, S.-B.; Kang, I.-S.; Kang, M.-S.; Kim, H.-S.; Heo, H.-S.; Park, M.-S.; Gil, G.-H.; et al. Single and 90-day repeated oral dose toxicity studies of fermented Rhus verniciflua stem bark extract in Sprague-Dawley rats. Food Chem. Toxicol. 2013, 55, 617–626. [Google Scholar] [CrossRef]
- Cheon, S.H.; Kim, K.S.; Kim, S.; Jung, H.S.; Choi, W.C.; Eo, W.K. Efficacy and safety of Rhus verniciflua stokes extracts in patients with previously treated advanced non-small cell lung cancer. Forsch Komplementmed 2011, 18, 77–83. [Google Scholar] [CrossRef]
- Kim, S.H.; Seo, H.-S.; Jang, B.-H.; Shin, Y.-C.; Ko, S.-G. The effect of Rhus verniciflua Stokes (RVS) for anti-aging and whitening of skin. Orient. Pharm. Exp. Med. 2014, 14, 213–222. [Google Scholar] [CrossRef]
- Singh, N.; Manshian, B.; Jenkins, G.J.; Griffiths, S.M.; Williams, P.M.; Maffeis, T.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef]
- Sugiyama, K.-I.; Yamada, M.; Awogi, T.; Hakura, A. The strains recommended for use in the bacterial reverse mutation test (OECD guideline 471) can be certified as non-genetically modified organisms. Genes Environ. 2016, 38, 2. [Google Scholar] [CrossRef] [Green Version]
- Clare, G. The in vitro mammalian chromosome aberration test. In Genetic Toxicology; Springer: Berlin, Germany, 2012; pp. 69–91. [Google Scholar]
- Ishidate, M., Jr. The in vitro chromosomal aberration test using Chinese hamster lung (CHL) fibroblast cells in culture. Prog. Mutat. Res. 1985, 5, 427–432. [Google Scholar]
- Nandhakumar, S.; Parasuraman, S.; Shanmugam, M.M.; Rao, K.R.; Chand, P.; Bhat, B.V. Evaluation of DNA damage using single-cell gel electrophoresis (Comet Assay). J. Pharmacol. Pharmacother. 2011, 2, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Ghazalla, M. Benhusein, Elaine Mutch, Suher Aburawi, and Faith, M. Williams. Genotoxic effect induced by hydrogen peroxide in human hepatoma cells using comet assay. Libyan J. Med. 2010, 5. [Google Scholar] [CrossRef]
- Walmsley, R.M.; Billinton, N. How accurate is in vitro prediction of carcinogenicity? Br. J. Pharmacol. 2011, 162, 1250–1258. [Google Scholar] [CrossRef]
- Kirkland, D.; Reeve, L.; Gatehouse, D.; Vanparys, P. A core in vitro genotoxicity battery comprising the Ames test plus the in vitro micronucleus test is sufficient to detect rodent carcinogens and in vivo genotoxins. Mutat. Res. /Genet. Toxicol. Environ. Mutagenesis 2011, 721, 27–73. [Google Scholar] [CrossRef]
- Fei, C.; Zhang, J.; Lin, Y.; Wang, X.; Zhang, K.; Zhang, L.; Zheng, W.; Wang, M.; Li, T.; Xiao, S.; et al. Safety evaluation of a triazine compound nitromezuril by assessing bacterial reverse mutation, sperm abnormalities, micronucleus and chromosomal aberration. Regul. Toxicol. Pharmacol. 2015, 71, 585–589. [Google Scholar] [CrossRef]
- Jeong, M.H.; Yang, K.; Lee, C.G.; Jeong, D.H.; Park, Y.S.; Choi, Y.J.; Kim, J.S.; Oh, S.J.; Jeong, S.K.; Jo, W.S. In Vitro Genotoxicity Assessment of a Novel Resveratrol Analogue, HS-1793. Toxicol. Res. 2014, 30, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.; Han, D.U. Genotoxicicological safety estimate for the Rhus-II. J. Food Hyg. Saf. 2005, 20, 18–21. [Google Scholar]
- Choi, W.C. Study on the Safety and Antitumor Activity of Rhus Verniciflua Extract (Nexia); Graduate School of East-West Medicine, Kyunghee University: Seoul, Korea, 2006. [Google Scholar]
- Jeong, J.-S.; Park, J.-W.; Yoon, S.-W.; Choi, W.-C. Case Report Carcinostatic effect of allergen removed Rhus Verniciflua stokes based Traditional Korean Medicine on a patient with lung adenocarcinoma; single case report. Orient. Pharm. Exp. Med. 2008, 7, 573–578. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Mei, N.; Fu, P.P. Genotoxicity of pyrrolizidine alkaloids. J. Appl. Toxicol. Int. J. 2010, 30, 183–196. [Google Scholar] [CrossRef]
- Li, M.-C.; Zhang, Y.-Q.; Meng, C.-W.; Gao, J.-G.; Xie, C.-J.; Liu, J.-Y.; Xu, Y.N. Traditional uses, phytochemistry, and pharmacology of Toxicodendron vernicifluum (Stokes) F.A. Barkley—A review. J. Ethnopharmacol. 2020, 113476. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Oh, S.-W.; Han, D.; Lee, M. Evaluation of Genotoxicity of Water and Ethanol Extracts from Rhus Verniciflua Stokes (RVS). Toxicol. Res. 2008, 24, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Jeon, W.K.; Lee, J.H.; Kim, H.K.; Lee, A.Y.; Lee, S.O.; Kim, Y.S.; Ryu, S.Y.; Kim, S.Y.; Lee, Y.J.; Ko, B.S. Anti-Platelet Effects of Bioactive Compounds Isolated from the Bark of Rhus Verniciflua Stokes. J. Ethnopharmacol. 2006, 106, 62–69. [Google Scholar] [CrossRef]
- Lee, J.-S.; Cho, J.-H.; Lee, D.-S.; Son, C.-G. Genotoxicity Evaluation of an Ethanol Extract Mixture of Astragali Radix and Salviae miltiorrhizae Radix. Evid. Based Complement. Alternat. Med. 2018, 5684805. [Google Scholar] [CrossRef] [Green Version]
- Kucharova, M.; Hronek, M.; Rybakova, K.; Zadak, Z.; Stetina, R.; Joskova, V.; Patkova, A. Comet assay and its use for evaluating oxidative DNA damage in some pathological states. Physiol. Res. 2019, 68, 1–15. [Google Scholar] [CrossRef]


| Metabolic Activation | Dose (μg/plate) | Number of Colonies/Plate | ||||
|---|---|---|---|---|---|---|
| Base-Pair Substitution Type | Frameshift Type | |||||
| TA100 | TA1535 | WP2uvrA | TA98 | TA1537 | ||
| S9 mix ( − ) | Positive | AF-2 | NaN3 | 4-NQO | AF-2 | 9-AA |
| Dose | 0.01 | 0.5 | 0.25 | 0.1 | 80 | |
| Mean ± SD | 545 ± 31.1 *** | 281 ± 39.3 *** | 318 ± 83.0 *** | 499 ± 9.0 *** | 1185 ± 146 *** | |
| S9 mix ( + ) | Positive | 2-AA | 2-AA | 2-AA | BP | 2-AA |
| Dose | 1.0 | 2.0 | 10.0 | 10.0 | 2.0 | |
| Mean ± SD | 309 ± 76.4 *** | 193 ± 6.4 *** | 263 ± 52.6 *** | 522 ± 90.3 *** | 143 ± 7.5 *** | |
| Condition | Dose (μg/mL) | Aberration (No. in 300 cells) | Aberration Rate (%) | ||||
|---|---|---|---|---|---|---|---|
| Chromatid | Chromosome | PP+ER | |||||
| Breaks | Exchange | Breaks | Exchange | ||||
| 0–24 h S9 mix( − ) | 0 | 0 | 2 | 0 | 0 | 0 | 0.67 |
| 20.58 | 1 | 0 | 0 | 0 | 0 | 0.33 | |
| 61.73 | 0 | 1 | 0 | 0 | 0 | 0.33 | |
| 185.19 | 2 | 1 | 0 | 0 | 0 | 1.00 | |
| MMC 0.04 | 9 | 62 | 0 | 0 | 0 | 23.67 *** | |
| 6–18 h S9 mix( − ) | 0 | 1 | 0 | 0 | 0 | 0 | 0.33 |
| 20.58 | 1 | 1 | 0 | 0 | 0 | 0.67 | |
| 61.73 | 1 | 1 | 0 | 0 | 0 | 0.67 | |
| 185.19 | 1 | 2 | 0 | 0 | 0 | 1.00 | |
| MMC 0.04 | 12 | 54 | 0 | 0 | 0 | 22.00 *** | |
| 6–18 h S9 mix( + ) | 0 | 0 | 2 | 0 | 0 | 0 | 0.67 |
| 61.73 | 0 | 1 | 0 | 0 | 0 | 0.33 | |
| 185.19 | 1 | 0 | 0 | 0 | 0 | 0.33 | |
| 555.56 | 1 | 2 | 0 | 0 | 0 | 1.00 | |
| CPA 10 | 7 | 64 | 0 | 0 | 0 | 23.67 *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-B.; Lee, J.-S.; Wang, J.-H.; Kim, M.-Y.; Choi, Y.-H.; Lee, H.-D.; Son, C.-G. Genotoxicity of Water Extract from Bark-Removed Rhus verniciflua Stokes. Molecules 2021, 26, 896. https://doi.org/10.3390/molecules26040896
Lee S-B, Lee J-S, Wang J-H, Kim M-Y, Choi Y-H, Lee H-D, Son C-G. Genotoxicity of Water Extract from Bark-Removed Rhus verniciflua Stokes. Molecules. 2021; 26(4):896. https://doi.org/10.3390/molecules26040896
Chicago/Turabian StyleLee, Sung-Bae, Jin-Seok Lee, Jing-Hua Wang, Min-Young Kim, Yung-Hyun Choi, Hwa-Dong Lee, and Chang-Gue Son. 2021. "Genotoxicity of Water Extract from Bark-Removed Rhus verniciflua Stokes" Molecules 26, no. 4: 896. https://doi.org/10.3390/molecules26040896