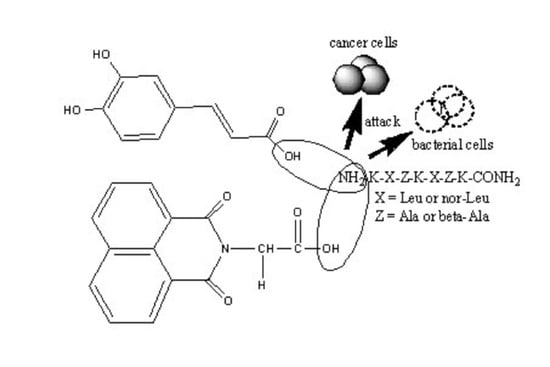
Synthesis, Antitumor and Antibacterial Studies of New Shortened Analogues of (KLAKLAK)2-NH2 and Their Conjugates Containing Unnatural Amino Acids
Abstract
:1. Introduction
2. Results
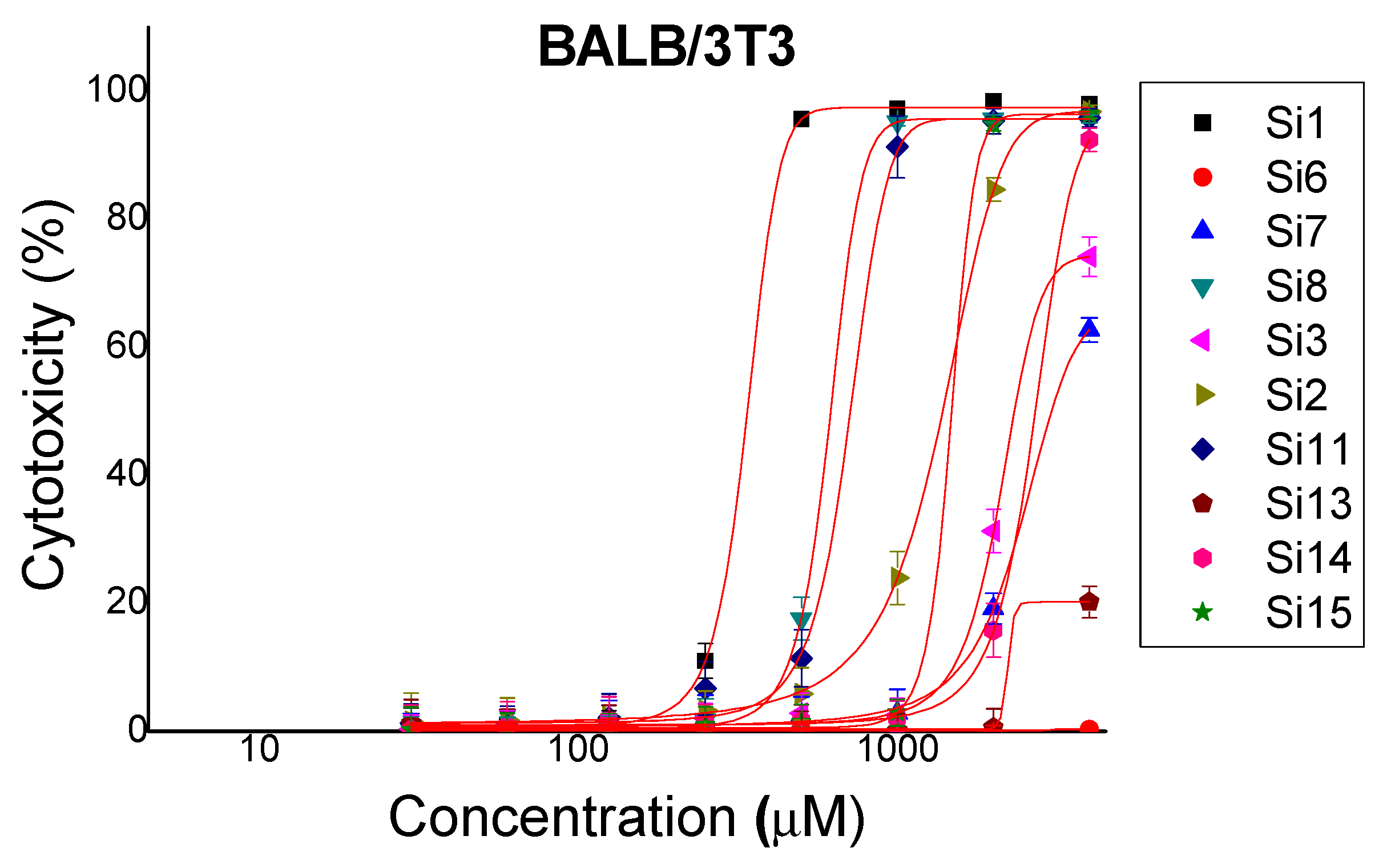
2.1. Cytotoxicity
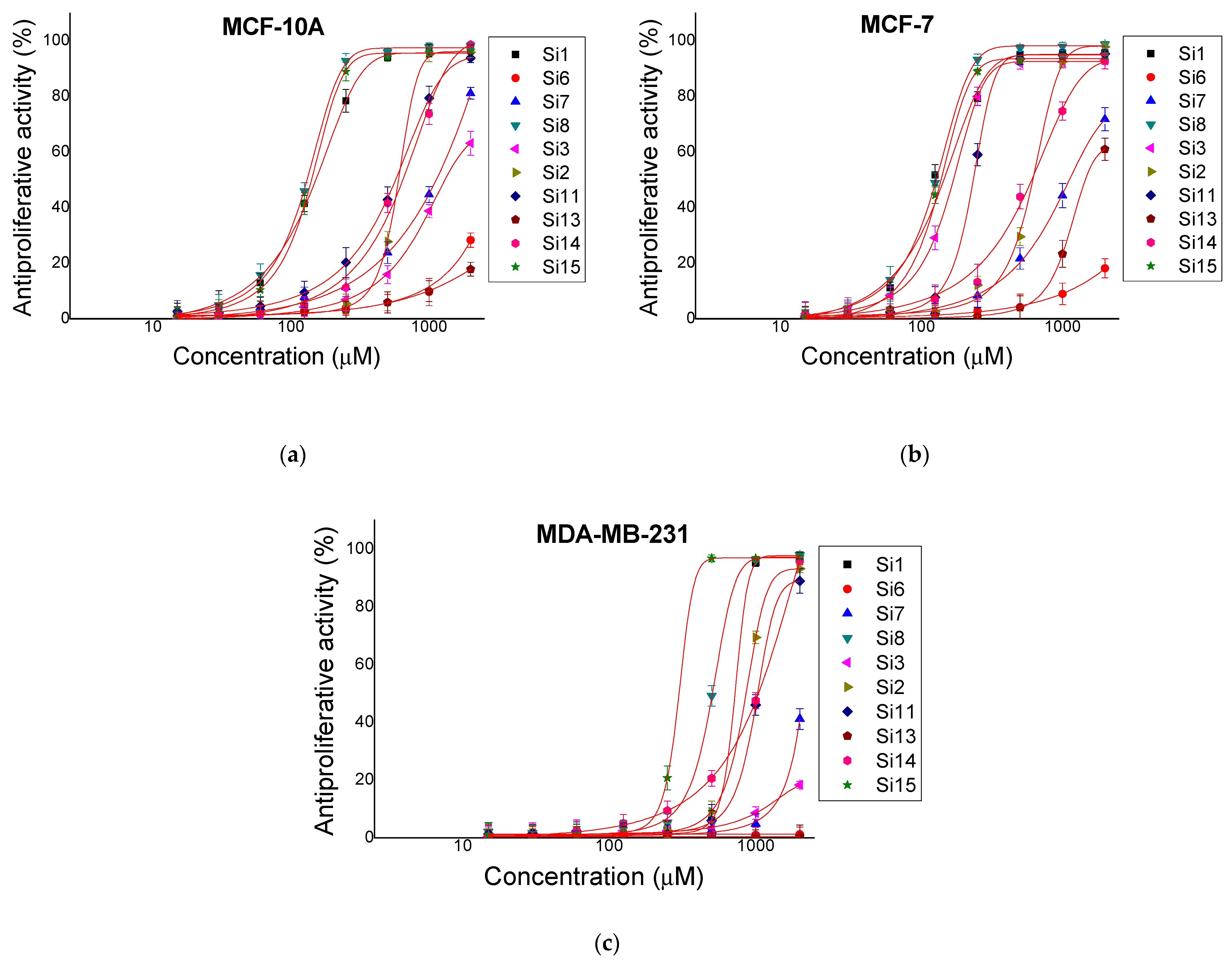
2.2. Antiproliferative Activity
2.3. Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Chemical Part
4.2. Biological Part
4.2.1. In Vitro Cytotoxicity Testing (3T3 NRU Test)
4.2.2. In Vitro Antiproliferative Activity
4.2.3. Antibacterial Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Deslouches, B.; Peter, Y. Antimicrobial peptides with selective antitumor mechanisms: Prospect for anticancer applications. Oncotarget 2017, 8, 46635–46651. [Google Scholar] [CrossRef] [Green Version]
- Javadpour, M.; Juban, M.; Lo, W.; Bishop, S.; Alberti, J.; Cowell, S.; Becker, C.; McLaughlin, M. De novo antimicrobial peptides with low mammalian cell toxicity. J. Med. Chem. 1996, 39, 3107–3113. [Google Scholar] [CrossRef]
- Mai, J.; Mi, Z.; Kim, S.; Ng, B.; Robbins, P. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 2001, 61, 7709–7712. [Google Scholar]
- Oelkrug, C.; Hartke, M.; Schubert, A. Mode of Action of Anticancer Peptides (ACPs) from Amphibian Origin. Anticancer Res. 2015, 35, 635–644. [Google Scholar] [PubMed]
- Schweizer, F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef]
- Mader, J.S.; Hoskin, D.W. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Investig. Drugs 2006, 15, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Mistry, N.; Drobni, P.; Näslund, J.; Sunkari, V.G.; Jenssen, H.; Evander, M. The anti-papillomavirus activity of human and bovine lactoferricin. Antivir. Res. 2007, 75, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Marqus, S.; Pirogova, E.; Piva, T. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef] [Green Version]
- Thundimadathil, J. Cancer Treatment Using Peptides: Current Therapies and Future Prospects. J. Amino Acids 2012, 2012, 967347. [Google Scholar] [CrossRef] [Green Version]
- Ellerby, H.M.; Arap, W.; Ellerby, L.M.; Kain, R.; Andrusiak, R.; Del Rio, G.; Krajewski, S.; Lombardo, C.R.; Rao, R.; Ruoslahti, E.; et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat. Med. 1999, 5, 1032–1038. [Google Scholar] [CrossRef]
- Pollak, M. The potential role of somatostatin analogues in breast cancer treatment. Yale J. Biol. Med. 1997, 70, 535–539. [Google Scholar]
- Pollak, M.N.; Shally, A.V. Mechanisms of antineoplastic action of somatostatin analogs. Proc. Soc. Exp. Biol. Med. 1998, 217, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appetecchia, M.; Baldelli, R. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives. J. Exp. Clin. Cancer Res. 2010, 29, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strosberg, J.; Kvols, L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J. Gastroenterol. 2010, 16, 2963–2970. [Google Scholar] [CrossRef]
- Angliker, H.; Stone, S.; Shaw, E. Thrombin inhibitors based on peptidyl halomethanes with a long peptide sequence. In Peptides; Springer: Berlin/Heidelberg, Germany, 1990; pp. 772–773. [Google Scholar]
- Kettner, C.; Mersinger, L.; Knabb, R. The selective inhibition of thrombin by peptides of boroarginine. J. Biol. Chem. 1990, 265, 18289–18297. [Google Scholar] [CrossRef]
- Cheng, L.; Goodwin, C.; Scully, M.; Kakkar, V.V.; Claeson, G. Substrate-related phosphonopeptides, a new class of thrombin inhibitors. Tetrahedron Lett. 1991, 32, 7333–7336. [Google Scholar] [CrossRef]
- Rupin, A.; Mennecier, P.; Lila, C.; de Nanteuil, G.; Verbeuren, T.J. Selection of S18326 as a new potential and selective boronic acid direct thrombin inhibitor. Thromb. Haemost. 1997, 78, 1221–1227. [Google Scholar] [PubMed]
- Chayrov, R.; Stylos, E.; Chatziathanasiadou, M.; Chuchkov, K.; Tencheva, A.; Kostagianni, A.; Milkova, T.; Angelova, A.; Galabov, A.; Shishkov, S.; et al. Tailoring acyclovir prodrugs with enhanced antiviral activity: Rational design, synthesis, human plasma stability and in vitro evaluation. Amino Acids 2018, 50, 1131–1143. [Google Scholar] [CrossRef]
- Chuchkov, K.; Nakova, C.; Mukova, L.; Galabov, A.; Stankova, I. New derivatives of oseltamivir with bile acids. Chemistry 2015, 24, 355–362. [Google Scholar]
- Chayrov, R.; Mukova, L.; Galabov, A.; Mitrev, Y.; Stankova, I. Amantadine analogues—Synthesis and biological activity. Bulg. Chem. Commun. 2017, 49, 61–63. [Google Scholar]
- Snyder, E.L.; Dowdy, S.F. Cell Penetrating Peptides in Drug Delivery. Pharm. Res. 2004, 21, 389–393. [Google Scholar] [CrossRef]
- Tréhin, R.; Merkle, H.P. Chances and pitfalls of cell penetrating peptides for cellular drug delivery. Eur. J. Pharm. Biopharm. 2004, 58, 209–223. [Google Scholar] [CrossRef]
- Vives, E. Present and future of cell-penetrating peptide mediated delivery systems: “Is the Trojan horse too wild to go only to Troy?”. J. Control. Release 2005, 109, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Foerg, C.; Merkle, H.P. On the biomedical promise of cell penetrating peptides: Limits versus prospects. J. Pharm. Sci. 2008, 97, 144–162. [Google Scholar] [CrossRef]
- Morris, M.C.; Deshayes, F.; Simeoni, F.; Adrian-Herrada, G.; Heitz, F.; Divita, G. A noncovalent peptide-based strategy for peptide and short interfering RNA delivery. In Cell Penetrating Peptides; Langel, Ü., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 387–408. [Google Scholar]
- Crombez, L.; Gudrun, A.; Konate, K.; Nguyen, Q.N.; McMaster, G.; Brasseur, R.; Heitz, F.; Divita, G. A New Potent Secondary Amphipathic Cell–penetrating Peptide for siRNA Delivery Into Mammalian Cells. Mol. Ther. 2009, 17, 95–107. [Google Scholar] [CrossRef]
- Shikolenko, I.N.; Alexeyev, M.F.; LeDoux, S.P.; Wilaon, G.L. Tat-mediated protein transduction and targeted delivery of fusion proteins into mitochondria of breast cancer cells. DNA Repair 2005, 4, 511–518. [Google Scholar] [CrossRef]
- Moghaddam, M.M.; Aghamollaei, H.; Kooshki, H.; Barjini, K.A.; Mirnejad, R.; Choopani, A. The development of antimicrobial peptides as an approach to prevention of antibiotic resistance. Rev. Med. Microbiol. 2015, 26, 98–110. [Google Scholar] [CrossRef]
- Gestin, M.; Dowaidar, M.; Langel, Ü. Uptake Mechanism of Cell-Penetrating Peptides. In Peptides and Peptide-Based Biomaterials and Their Biomedical Applications. Advances in Experimental Medicine and Biology; Sunna, A., Care, A., Bergquist, P., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Roudi, R.; Syn, N.L.; Roudbary, M. Antimicrobial Peptides as Biologic and immunotherapeutic Agents against Cancer: A Comprehensive Overview. Front. Immunol. 2017, 8, 1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
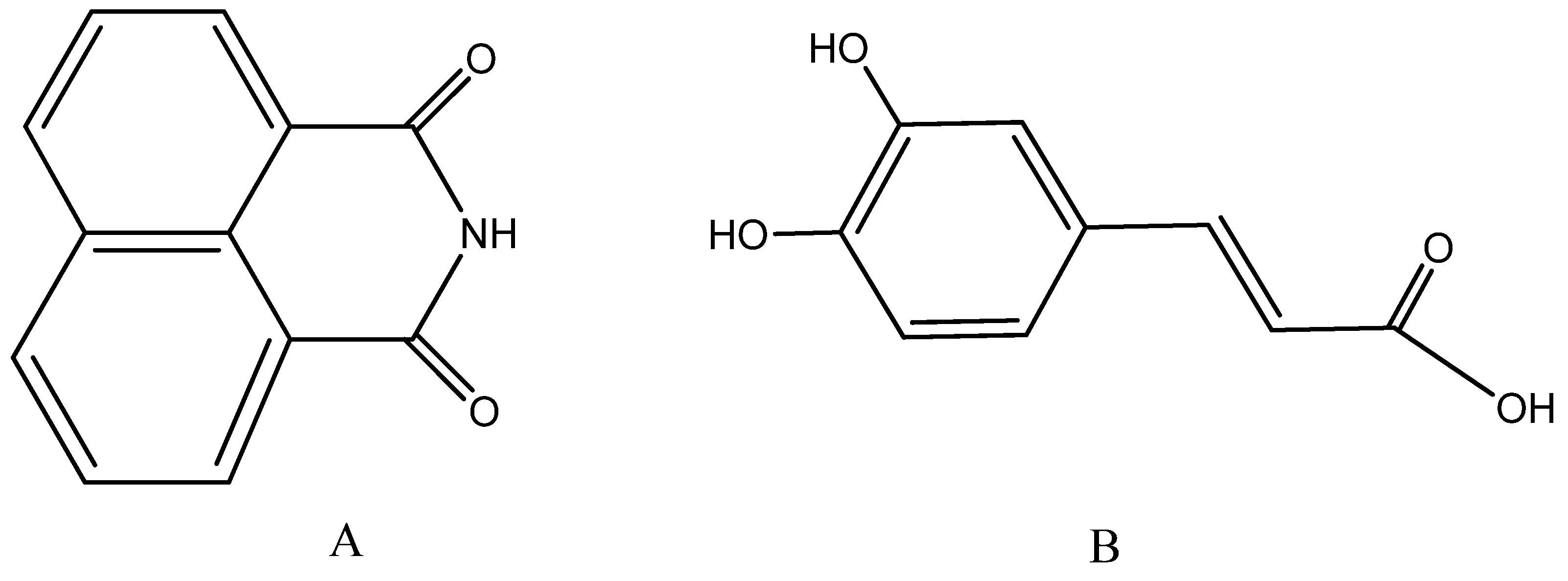
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Maurya, D.K.; Devasagayam, T.P. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem. Toxicol. 2010, 48, 3369–3373. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-W.; Moon, S.-K.; Chang, Y.-C.; Ko, J.-H.; Lee, Y.-C.; Cho, G.; Kim, S.-H.; Kim, J.-G.; Kim, C.-H. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: Complete regression of hepatoma growth and metastasis by dualmechanism. FASEB J. 2004, 18, 1670–1681. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.-C.; Hsieh, C.-H.; Hsiao, M.-W.; Lin, W.-C.; Hung, Y.-C.; Ye, J.-C. Caffeic acid induces apoptosis in human cervical cancer cells through the mitochondrial pathway. Taiwan J. Obstet. Gynecol. 2010, 49, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Prasad, N.R.; Karthikeyan, A.; Karthikeyan, S.; Reddy, B.V. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Murad, L.D.; Soares, N.D.C.P.; Brand, C.; Monteirod, M.C.; Teodoro, A.J. Effects of caffeic and 5-caffeoylquinic acids on cell viability and cellular uptake in human colon adenocarcinoma cells. Nutr. Cancer 2015, 67, 532–542. [Google Scholar] [CrossRef]
- Rosendahl, A.H.; Perks, C.M.; Zeng, L.; Markkula, A.; Simonsson, M.; Rose, C.; Ingvar, C.; Holly, J.M.P.; Jernström, H. Caffeine and caffeic acid inhibit growth and modify estrogen receptor and insulin-like growth factor I receptor levels in human breast cancer. Clin. Cancer Res. 2015, 21, 1877–1887. [Google Scholar] [CrossRef] [Green Version]
- Ignatova, M.G.; Manolova, N.I.; Rashkov, I.B.; Markova, N.D.; Toshkova, R.A.; Georgieva, A.K.; Nikolova, E.B. Poly(3-hydroxybutyrate)/caffeic acid electrospun fibrous materials coated with polyelectrolyte complex and their antibacterial activity and in vitro antitumor effect against HeLa cells. Mater. Sci. Eng. C 2016, 65, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Braña, M.F.; Ramos, A. Naphthalimides as anticancer agents: Synthesis and biological activity. Anti-Cancer Agents Med. Chem. 2001, 1, 237–255. [Google Scholar] [CrossRef]
- Banerjee, S.; Veale, E.B.; Phelan, C.M.; Murphy, S.A.; Tocci, G.M.; Gillespie, L.J.; Frimannsson, D.O.; Kelly, J.M.; Gunnlaugsson, T. Recent advances in the development of 1,8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem. Soc. Rev. 2013, 42, 1601–1618. [Google Scholar] [CrossRef] [Green Version]
- Kamal, A.; Bolla, N.R.; Srikanth, P.S.; Srivastava, A.K. Naphthalimide derivatives with therapeutic characteristics: A patent review. Expert Opin. Ther. Pat. 2013, 23, 299–317. [Google Scholar] [CrossRef]
- Wang, K.-R.; Qian, F.; Wang, X.-M.; Tan, G.-H.; Rong, R.-X.; Cao, Z.-R.; Chen, H.; Zhang, P.-Z.; Li, X.-L. Cytotoxic activity and DNA binding of naphthalimide derivatives with amino acid and dichloroacetamide functionalizations. Chin. Chem. Lett. 2014, 25, 1087–1093. [Google Scholar] [CrossRef]
- Marinov, M.N.; Naydenova, E.D.; Momekov, G.T.; Prodanova, R.Y.; Markova, N.V.; Voynikov, Y.T.; Stoyanov, N.M. Synthesis, Characterization, Quantum-Chemical Calculations and Cytotoxic Activity of 1,8-Naphthalimide Derivatives with Non-Protein Amino Acids. Anti-Cancer Agents Med. Chem. 2019, 19, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Braña, M.F.; Castellano, J.M.; Jiménez, A.; Lombart, A.; Rabadan, F.P.; Roldán, M.; Roldán, C.; Santos, A.; Vázquez, D. Synthesis, cytostatic activity and mode of action of a new series of imide derivatives of 3-nitro-11α naphtalic acid. Curr. Chemother. 1978, 2, 1216–1217. [Google Scholar]
- Braña, M.F.; Castellano, J.M.; Roldán, C.M.; Santos, A.; Vázquez, D.; Jiménez, A. Synthesis and mode(s) of action of a new series of imide derivatives of 3-nitro-1,8 naphthalic acid. Cancer Chemother. Pharmacol. 1980, 4, 61–66. [Google Scholar] [CrossRef]
- Chan, K.H.; Xue, B.; Robinson, R.C.; Hauser, C.A.E. Systematic single moiety variations of ultrashort peptides produce profound effects on self-assembly, nanostructure formation, hydrogelation, and phase transition. Sci. Rep. 2017, 7, 12897. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.H.; Lee, W.H.; Ni, M.; Loo, Y.; Hauser, C.A.E. C-Terminal residue of ultrashort peptides impacts on molecular self-assembly, hydrogelation, and interaction with small-molecule drugs. Sci. Rep. 2018, 8, 17127. [Google Scholar] [CrossRef] [PubMed]
- Cabrele, C.; Martinek, T.A.; Reiser, O.; Berlicki, Ł. Peptides Containing β-Amino Acid Patterns: Challenges and Successes in Medicinal Chemistry. J. Med. Chem. 2014, 57, 9718–9739. [Google Scholar] [CrossRef]
- Johnson, G.; Ellis, E.; Kim, H.; Muthukrishnan, N.; Snavely, T.; Pellois, J.-P. Photoinduced Membrane Damage of E. coli and S. aureus by the Photosensitizer-ntimicrobial Peptide Conjugate Eosin-(KLAKLAK)2. PLoS ONE 2014, 9, e91220. [Google Scholar]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and Neutral Red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Spielmann, H.; Balls, M.; Dupuis, J.; Pape, W.J.W.; Pechovitch, G.; de Silva, O. The international EU/COLIPA in vitro phototoxicity validation study: Results of Phase II (blind trial). Part I: The 3T3 NRU phototoxicity test. Toxicol. In Vitro 1998, 12, 305–327. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]

| Abb | Peptide Structure | Molecular Formula | Mwexact | [M + H]+ Observed | [M + Na]+ Observed | tR (min) | M.p. [°C] | Chromatographic Purity (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Si1 | (KLAKLAK)2-NH2 | C71H135N21O15 | 1522.05 | 1523.30 | - | 3.53 | 118 | −85 | 100.00 |
| Si6 | KLAKLAK-NH2 | C35H67N11O8 | 769.52 | 770.65 | - | 2.49 | 136 | −33 | 100.00 |
| Si7 | 1,8-naphthalimideG-KLAKLAK-NH2 | C49H74N12O11 | 1006.56 | 1007.70 | - | 3.44 | 89 | −98 | 98.07 |
| Si8 | Caf-KLAKLAK-NH2 | C45H77N11O10 | 931.59 | 932.70 | 956.70 | 3.72 | 154 | −35 | 82.37 |
| Si3 | KLβAKLβAK-NH2 | C35H67N11O8 | 769.52 | 770.70 | - | 2.48 | 123 | −98 | 99.14 |
| Si2 | 1,8-naphthalimideG-KLβAKLβAK-NH2 | C49H74N12O11 | 1006.56 | 1007.80 | 1029.75 | 1.48 | 98 | −156 | 100.00 |
| Si11 | Caf-KLβAKLβAK-NH2 | C45H77N11O10 | 931.59 | 932.65 | - | 3.65 | 125 | −23 | 82.17 |
| Si13 | KnLAKnLAK-NH2 | C35H67N11O8 | 769.52 | 770.80 | - | 1.25 | 93 | −34 | 100.00 |
| Si14 | 1,8-naphthalimideG-KnLAKnLAK-NH2 | C49H74N12O11 | 1006.56 | 1007.75 | 1029.75 | 3.42 | a ** | −12 | 87.48 |
| Si15 | Caf-KnLAKnLAK-NH2 | C45H77N11O10 | 931.59 | 932.75 | - | 1.33 | 92 | −64 | 100.00 |
| Abb | Peptide Structure | IC50 of Mean ± SD (μM) | |||
|---|---|---|---|---|---|
| Cytotoxicity | Antiproliferative Activity | ||||
| BALB/3T3 | MCF-10A | MCF-7 | MDA-MB-231 | ||
| Si1 | (KLAKLAK)2-NH2 | 365.3 ± 4.08 | 154 ± 6.53 | 124.1 ± 8.12 | 746.5 ± 7.6 |
| Si6 | KLAKLAK-NH2 | >4000 | >2000 | >2000 | >2000 |
| Si7 | 1,8-naphthalimideG-KLAKLAK-NH2 | 3422 ± 51.26 | 1144 ± 64.53 | 1195 ± 131.5 | >2000 |
| Si8 | Caf-KLAKLAK-NH2 | 710.3 ± 11.91 | 135.6 ± 7.09 | 128.6 ± 8.03 | 514.3 ± 26.82 |
| Si3 | KLβAKLβAK-NH2 | 2874 ± 129.5 | 1469 ± 103.8 | 176.3 ± 4.66 | >2000 |
| Si2 | 1,8-naphthalimideG-KLβAKLβAK-NH2 | 1429 ± 48.38 | 666 ± 20.89 | 662.9 ± 20.02 | 840 ± 21.18 |
| Si11 | Caf-KLβAKLβAK-NH2 | 742.5 ± 18.49 | 597.2 ± 53.05 | 228.8 ± 7.18 | 1087 ± 70.71 |
| Si13 | KnLAKnLAK-NH2 | >4000 | >2000 | 1704 ± 112 | >2000 |
| Si14 | 1,8-naphthalimideG-KnLAKnLAK-NH2 | 2893 ± 61.38 | 630.8 ± 51.16 | 593.3 ± 60.3 | 1049 ± 49.77 |
| Si15 | Caf-KnLAKnLAK-NH2 | 1514 ± 12.16 | 146.8 ± 7.96 | 140.3 ± 7.12 | 346.3 ± 7.91 |
| Cisplatin * | >100 | 46.89 ± 19.85 | 19.85 ± 3.74 | 1.833 ± 0.13 | |
| Abb | Peptide Structure | Average Value [mm] * |
|---|---|---|
| Si1 | (KLAKLAK)2-NH2 (control compound) | 5 ± 0.15 |
| Si6 | KLAKLAK-NH2 | 9.7 ± 0.25 |
| Si7 | 1,8-naphthalimideG-KLAKLAK-NH2 | 0 |
| Si8 | Caf-KLAKLAK-NH2 | 5 ± 0.15 |
| Si3 | KLβAKLβAK-NH2 | 0 |
| Si2 | 1,8-naphthalimideG-KLβAKLβAK-NH2 | 0 |
| Si11 | Caf-KLβAKLβAK-NH2 | 0 |
| Si13 | KnLAKnLAK-NH2 | 0 |
| Si14 | 1,8-naphthalimideG-KnLAKnLAK-NH2 | 11.7 ± 0.3 |
| Si15 | Caf-KnLAKnLAK-NH2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaber, S.; Iliev, I.; Angelova, T.; Nemska, V.; Sulikovska, I.; Naydenova, E.; Georgieva, N.; Givechev, I.; Grabchev, I.; Danalev, D. Synthesis, Antitumor and Antibacterial Studies of New Shortened Analogues of (KLAKLAK)2-NH2 and Their Conjugates Containing Unnatural Amino Acids. Molecules 2021, 26, 898. https://doi.org/10.3390/molecules26040898
Jaber S, Iliev I, Angelova T, Nemska V, Sulikovska I, Naydenova E, Georgieva N, Givechev I, Grabchev I, Danalev D. Synthesis, Antitumor and Antibacterial Studies of New Shortened Analogues of (KLAKLAK)2-NH2 and Their Conjugates Containing Unnatural Amino Acids. Molecules. 2021; 26(4):898. https://doi.org/10.3390/molecules26040898
Chicago/Turabian StyleJaber, Sirine, Ivan Iliev, Tsvetelina Angelova, Veronica Nemska, Inna Sulikovska, Emilia Naydenova, Nelly Georgieva, Ivan Givechev, Ivo Grabchev, and Dancho Danalev. 2021. "Synthesis, Antitumor and Antibacterial Studies of New Shortened Analogues of (KLAKLAK)2-NH2 and Their Conjugates Containing Unnatural Amino Acids" Molecules 26, no. 4: 898. https://doi.org/10.3390/molecules26040898
APA StyleJaber, S., Iliev, I., Angelova, T., Nemska, V., Sulikovska, I., Naydenova, E., Georgieva, N., Givechev, I., Grabchev, I., & Danalev, D. (2021). Synthesis, Antitumor and Antibacterial Studies of New Shortened Analogues of (KLAKLAK)2-NH2 and Their Conjugates Containing Unnatural Amino Acids. Molecules, 26(4), 898. https://doi.org/10.3390/molecules26040898