Clinical Relevance of [18F]Florbetaben and [18F]FDG PET/CT Imaging on the Management of Patients with Dementia
Abstract
:1. Introduction
2. Results
3. Discussion
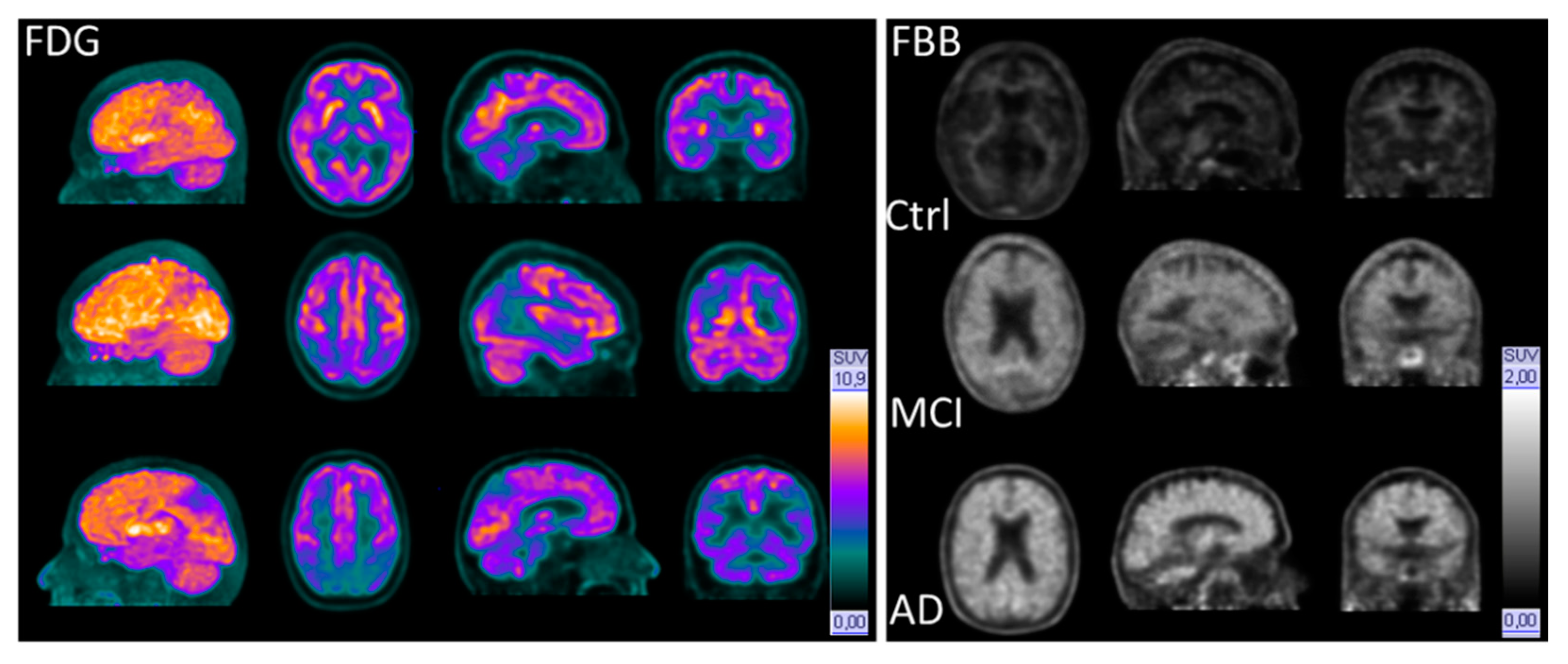
3.1. Glucose Metabolism
3.2. Amyloid PET Positivity/Negativity
4. Materials and Methods
4.1. Cohort
4.2. Neuropsychological Diagnostics
4.3. CSF Diagnostics
4.4. [18F]FBB and [18F]FDG-PET/CT
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, M. World Alzheimer Report 2015, The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer′s Disease International: London, UK, 2015. [Google Scholar]
- Alzheimer’s Disease International. World Alzheimer Report 2018—The State of the Art of Dementia Research: New Frontiers; Alzheimer’s Disease International: London, UK, 2018. [Google Scholar]
- World Health Organization. Alzheimer’s Disease International. Dementia: A Public Health Priority; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Trevisan, K.; Cristina-Pereira, R.; Silva-Amaral, D.; Aversi-Ferreira, T.A. Theories of Aging and the Prevalence of Alzheimer’s Disease. BioMed Res. Int. 2019, 2019, 9171424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, A.; Gao, J.; Xu, L.; Meng, D. Shared mechanisms of neurodegeneration in Alzheimer’s disease and Parkinson’s disease. BioMed Res. Int. 2014, 2014, 648740. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H., III. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzheimer’s Association. Alzheimer’s Association Report 2018. Facts and Figures; Alzheimer’s Association: Chicago, IL, USA, 2018. [Google Scholar]
- Zhang, Y.-W.; Thompson, R.; Zhang, H.; Xu, H. APP processing in Alzheimer’s disease. Mol. Brain 2011, 4, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.; Guo, Z. Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J. Neurochem. 2013, 126, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Delacourte, A. Les diagnostics de la maladie d’Alzheimer. Ann. Biol. Clin. 1998, 56, 133–142. [Google Scholar]
- Takizawa, C.; Thompson, P.L.; van Walsem, A.; Faure, C.; Maier, W.C. Epidemiological and economic burden of Alzheimer’s disease: A systematic literature review of data across Europe and the United States of America. J. Alzheimer Dis. 2015, 43, 1271–1284. [Google Scholar] [CrossRef]
- Johnson, K.A.; Fox, N.C.; Sperling, R.A.; Klunk, W.E. Brain Imaging in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006213. [Google Scholar] [CrossRef]
- Budson, A.E.; Solomon, P.R. New criteria for Alzheimer disease and mild cognitive impairment: Implications for the practicing clinician. Neurologist 2012, 18, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer Dement. J. Alzheimer Assoc. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- European Medicines Agency. Qualification Opinion of Novel Methodologies in the Predementia Stage of Alzheimer’s Disease: Cerebrospinal Fluid Related Biomarkers for Drugs Affecting Amyloid Burden. 14 April 2011, EMA/CHMP/SAWP/102001/2011 Procedure No.: EMEA/H/SAB/005/1/QA/2010. Available online: www.ema.europa.eu/en/documents/regulatory-procedural-guideline/qualification-opinion-novel-methodologies-predementia-stage-alzheimers-disease-cerebrospinal-fluid_en.pdf (accessed on 25 February 2021).
- Blennow, K.; Hampel, H.; Zetterberg, H. Biomarkers in amyloid-β immunotherapy trials in Alzheimer’s disease. Neuropsychopharmacology 2014, 39, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Palmqvist, S.; Zetterberg, H.; Mattsson, N.; Johansson, P.; Minthon, L.; Blennow, K.; Olsson, M.; Hansson, O. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology 2015, 85, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Bürger, K.; Teipel, S.J.; Bokde, A.L.; Zetterberg, H.; Blennow, K. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimer Dement. 2008, 4, 38–48. [Google Scholar] [CrossRef]
- Zetterberg, H.; Tullhög, K.; Hansson, O.; Minthon, L.; Londos, E.; Blennow, K. Low Incidence of Post-Lumbar Puncture Headache in 1,089 Consecutive Memory Clinic Patients. Eur. Neurol. 2010, 63, 326–330. [Google Scholar] [CrossRef]
- Reiman, E.M.; Jagust, W.J. Brain imaging in the study of Alzheimer’s disease. NeuroImage 2012, 61, 505–516. [Google Scholar] [CrossRef] [Green Version]
- Fawaz, M.V.; Brooks, A.F.; Rodnick, M.E.; Carpenter, G.M.; Shao, X.; Desmond, T.J.; Sherman, P.; Quesada, C.A.; Hockley, B.G.; Kilbourn, M.R.; et al. High Affinity Radiopharmaceuticals Based Upon Lansoprazole for PET Imaging of Aggregated Tau in Alzheimer’s Disease and Progressive Supranuclear Palsy: Synthesis, Preclinical Evaluation, and Lead Selection. ACS Chem. Neurosci. 2014, 5, 718–730. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, S.T.; Marek, K. Looking Backward to Move Forward: Early Detection of Neurodegenerative Disorders. Science 2003, 302, 830–834. [Google Scholar] [CrossRef] [Green Version]
- Bailey, D.L.; Maisey, M.N.; Townsend, D.W.; Valk, P.E. Positron Emission Tomography: Basic Sciences; Springer-Verlag London Limited: London, UK, 2005. [Google Scholar]
- van der Born, D.; Pees, A.; Poot, A.J.; Orru, R.V.A.; Windhorst, A.D.; Vugts, D.J. Fluorine-18 labelled building blocks for PET tracer synthesis. Chem. Soc. Rev. 2017, 46, 4709–4773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmqvist, S.; Mattsson, N.; Hansson, O.; Initiative, F.T.A.D.N. Cerebrospinal fluid analysis detects cerebral amyloid-β accumulation earlier than positron emission tomography. Brain 2016, 139, 1226–1236. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, K.A.; Chandra, R.; Zhuang, Z.-P.; Hou, C.; Oya, S.; Kung, M.-P.; Kung, H.F. Fluoro-pegylated (FPEG) imaging agents targeting Abeta aggregates. Bioconjug. Chem. 2007, 18, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Oya, S.; Kung, M.-P.; Hou, C.; Maier, D.L.; Kung, H.F. F-18 Polyethyleneglycol stilbenes as PET imaging agents targeting Abeta aggregates in the brain. Nucl. Med. Biol. 2005, 32, 799–809. [Google Scholar] [CrossRef]
- Kung, H.F.; Choi, S.R.; Qu, W.; Zhang, W.; Skovronsky, D. 18F stilbenes and styrylpyridines for PET imaging of A beta plaques in Alzheimer’s disease: A miniperspective. J. Med. Chem. 2010, 53, 933–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, C.C.; Ackerman, U.; Browne, W.; Mulligan, R.; Pike, K.L.; O’Keefe, G.; Tochon-Danguy, H.; Chan, G.; Berlangieri, S.U.; Jones, G.; et al. Imaging of amyloid β in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: Proof of mechanism. Lancet Neurol. 2008, 7, 129–135. [Google Scholar] [CrossRef]
- Yousefi, B.H.; von Reutern, B.; Scherübl, D.; Manook, A.; Schwaiger, M.; Grimmer, T.; Henriksen, G.; Förster, S.; Drzezga, A.; Wester, H.J. FIBT versus florbetaben and PiB: A preclinical comparison study with amyloid-PET in transgenic mice. EJNMMI Res. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villemagne, V.L.; Ong, K.; Mulligan, R.S.; Holl, G.; Pejoska, S.; Jones, G.; O’Keefe, G.; Ackerman, U.; Tochondanguy, H.J.; Chan, J.G.; et al. Amyloid Imaging with 18F-Florbetaben in Alzheimer Disease and Other Dementias. J. Nucl. Med. 2011, 52, 1210–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabri, O.; Seibyl, J.; Rowe, C.; Barthel, H. Beta-amyloid imaging with florbetaben. Clin. Transl. Imaging 2015, 3, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Guideline on the Clinical Investigation of Medicines for the Treatment of Alzheimer’s Disease. 22 February 2018, CPMP/EWP/553/95 Rev.2. Available online: www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicines-treatment-alzheimers-disease-revision-2_en.pdf (accessed on 25 February 2021).
- Guillén, E.F.; Rosales, J.J.; Lisei, D.; Grisanti, F.; Riverol, M.; Arbizu, J. Current role of 18F-FDG-PET in the differential diagnosis of the main forms of dementia. Clin. Transl. Imaging 2020, 8, 127–140. [Google Scholar] [CrossRef]
- Förster, S.; Yousefi, B.H.; Wester, H.J.; Klupp, E.; Rominger, A.; Förstl, H.; Kurz, A.; Grimmer, T.; Drzezga, A. Quantitative longitudinal interrelationships between brain metabolism and amyloid deposition during a 2-year follow-up in patients with early Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1927–1936. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Minoshima, S. FDG-PET and molecular brain imaging in the movement disorders clinic. Neurology 2012, 79, 1306–1307. [Google Scholar] [CrossRef] [Green Version]
- Ramusino, M.C.; Garibotto, V.; Bacchin, R.; Altomare, D.; Dodich, A.; Assal, F.; Mendes, A.; Costa, A.; Tinazzi, M.; Morbelli, S.D.; et al. Incremental value of amyloid-PET versus CSF in the diagnosis of Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 270–280. [Google Scholar] [CrossRef]
- Klupp, E.; Grimmer, T.; Tahmasian, M.; Sorg, C.; Yakushev, I.; Yousefi, B.H.; Drzezga, A.; Förster, S. Prefrontal Hypometabolism in Alzheimer Disease Is Related to Longitudinal Amyloid Accumulation in Remote Brain Regions. J. Nucl. Med. 2015, 56, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Thientunyakit, T.; Sethanandha, C.; Muangpaisan, W.; Chawalparit, O.; Arunrungvichian, K.; Siriprapa, T.; Vichianin, Y.; Kamal, S.; Suppasilp, C.; Thongpraparn, T.; et al. Relationships between amyloid levels, glucose metabolism, morphologic changes in the brain and clinical status of patients with Alzheimer’s disease. Ann. Nucl. Med. 2020, 34, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Moms, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The Consortium to establish a registry for Alzheimer’s disease (CERAD). part I. clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989, 39, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Nobili, F.; Arbizu, J.; Bouwman, F.; Drzezga, A.; Agosta, F.; Nestor, P.; Walker, Z.; Boccardi, M. European Association of Nuclear Medicine and European Academy of Neurology recommendations for the use of brain 18 F-fluorodeoxyglucose positron emission tomography in neurodegenerative cognitive impairment and dementia: Delphi consensus. Eur. J. Neurol. 2018, 25, 1201–1217. [Google Scholar] [CrossRef] [Green Version]
- Barthel, H.; Meyer, P.T.; Drzezga, A.; Bartenstein, P.; Boecker, H.; Brust, P.; Buchert, R.; Coenen, H.H.; la Fougere, C.; Grunder, G.; et al. German Society of Nuclear Medicine procedure guideline on beta-amyloid brain PET imaging. Nuklearmedizin 2016, 55, 129–137. [Google Scholar]
- Minoshima, S.; Drzezga, A.E.; Barthel, H.; Bohnen, N.; Djekidel, M.; Lewis, D.H.; Mathis, C.A.; McConathy, J.; Nordberg, A.; Sabri, O.; et al. SNMMI Procedure Standard/EANM Practice Guideline for Amyloid PET Imaging of the Brain 1.0. J. Nucl. Med. 2016, 57, 1316–1322. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria Version 3.6.2. Available online: https://www.R-project.org (accessed on 31 December 2019).
| [18F]FBB+ (N = 21) | [18F]FBB− (N = 19) | Total (N = 40) | |
|---|---|---|---|
| Age, mean (SD) | 71.0 (±9.79) | 70.6 (±8.23) | 70.8 (±8.97) |
| Sex, N (%) | |||
| Female | 11 (52.4%) | 6 (31.6%) | 17 (42.5%) |
| Male | 10 (47.6%) | 13 (68.4%) | 23 (57.5%) |
| MMSE *, mean (SD) | 19.9 (±4.34) | 22.8 (±5.01) | 21.2 (±4.83) |
| Aβ1–42, N (%) | |||
| Pathological | 2 (9.5%) | 2 (10.5%) | 4 (10.0%) |
| Normal | 13 (61.9%) | 14 (73.7%) | 27 (67.5%) |
| Missing | 6 (28.6%) | 3 (15.8%) | 9 (22.5%) |
| Aβ Ratio, N (%) | |||
| Pathological | 9 (42.9%) | 11 (57.9%) | 20 (50.0%) |
| Normal | 6 (28.6%) | 5 (26.3%) | 11(27.5%) |
| Missing | 6 (28.6%) | 3(15.8%) | 9 (22.5%) |
| Total tau, N (%) | |||
| Pathological | 12 (57.1%) | 5 (26.3%) | 17 (42.5%) |
| Normal | 3 (14.3%) | 11 (57.9%) | 14 (35.0%) |
| Missing | 6 (28.6%) | 3 (15.8%) | 9 (22.5%) |
| p-tau, N (%) | |||
| Pathological | 11 (52.4%) | 8 (42.1%) | 19 (47.5%) |
| Normal | 4 (19.0) | 8 (42.1%) | 12 (30.0%) |
| Missing | 6(28.6) | 3(15.8%) | 9(22.5%) |
| Treatment before beta-amyloid imaging | |||
| Antidementia | 4 (19.0) | 0 (0.0%) | 4 (10.0%) |
| No Antidementia | 17 (81.0) | 19 (100.0%) | 36 (90.0%) |
| [18F]FBB+ N (%) | [18F]FBB− N (%) | |
|---|---|---|
| Antidementia | 17 (81.0) | 3 (15.8) |
| No antidementia | 4 (19.0) | 16 (84.2) |
| Sum | 21 | 19 |
| Odds ratio * | 22.67 | |
| (95% confidence interval) * | (4.96; 141.14) | |
| p-value * | 0.0002 | |
| N (%) of [18F]FBB+ | N (%) of [18F]FBB− | Total (%) | |
|---|---|---|---|
| Antidepressant * | 1 (4.8) | 7 (36.8) | 8 (20.0) |
| No antidepressant | 20 (95.2) | 12 (63.2) | 32 (80.0) |
| Sum | 21 | 19 | 40 |
| [18F]FBB+ (N = 21) | [18F]FBB− (N = 19) | Total (N = 40) | ||||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| [18F]FDG+ | 4 | (19.0) | 2 | (10.5) | 6 | (15.0) |
| [18F]FDG− | 0 | (0.0) | 4 | (21.1) | 4 | (10.0) |
| No [18F]FDG PET performed | 17 | (81.0) | 13 | (68.4) | 30 | (75.0) |
| Age, y, Median (SD) | 68.6 (±10.4), Female 40 % |
|---|---|
| CSF t-tau, median (range) (in pg/mL) | 876 (555–2200) |
| CSF p-tau, median (range) (in pg/mL) | 121 (63–210) |
| CSF Aβ42, median (range) (in pg/mL) | 501 (427–571) |
| Neocortical FBB-PET SUVR * (cerebellar), median (range) | 1.80 (1.3–2.5) |
| FBB-PET SUVR (cerebellar), frontal lobe, median (range) | 1.78 (1.3–2.6) |
| FBB-PET SUVR (cerebellar), parietal lobe, median (range) | 1.85 (1.3–2.3) |
| FBB-PET SUVR (cerebellar), temporal lobe, median (range) | 1.72 (1,2–2.2) |
| FBB-PET SUVR (cerebellar), occipital lobe, median (range) | 1.83 (1.2–2.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Librizzi, D.; Cabanel, N.; Zavorotnyy, M.; Riehl, E.; Kircher, T.; Luster, M.; Hooshyar Yousefi, B. Clinical Relevance of [18F]Florbetaben and [18F]FDG PET/CT Imaging on the Management of Patients with Dementia. Molecules 2021, 26, 1282. https://doi.org/10.3390/molecules26051282
Librizzi D, Cabanel N, Zavorotnyy M, Riehl E, Kircher T, Luster M, Hooshyar Yousefi B. Clinical Relevance of [18F]Florbetaben and [18F]FDG PET/CT Imaging on the Management of Patients with Dementia. Molecules. 2021; 26(5):1282. https://doi.org/10.3390/molecules26051282
Chicago/Turabian StyleLibrizzi, Damiano, Nicole Cabanel, Maxim Zavorotnyy, Elisabeth Riehl, Tilo Kircher, Markus Luster, and Behrooz Hooshyar Yousefi. 2021. "Clinical Relevance of [18F]Florbetaben and [18F]FDG PET/CT Imaging on the Management of Patients with Dementia" Molecules 26, no. 5: 1282. https://doi.org/10.3390/molecules26051282
APA StyleLibrizzi, D., Cabanel, N., Zavorotnyy, M., Riehl, E., Kircher, T., Luster, M., & Hooshyar Yousefi, B. (2021). Clinical Relevance of [18F]Florbetaben and [18F]FDG PET/CT Imaging on the Management of Patients with Dementia. Molecules, 26(5), 1282. https://doi.org/10.3390/molecules26051282








